Watch a video presentation of this article
Watch the interview with the author
Although effective therapies for chronic hepatitis B (CHB) are available, long‐term treatment is required in most cases, and antiviral drug resistance represents a serious potential complication. The particular characteristics of the hepatitis B virus (HBV) underlie its susceptibility to develop resistance, namely the lack of proofreading function on its DNA polymerase and its high replication rate. Viral fitness, drug potency, genetic barrier of the antiviral agents, and compliance are important factors that are associated with the development of antiviral resistance.
Abbreviations:
- CHB
chronic hepatitis B
- HBeAg
hepatitis B e antigen
- HBV
hepatitis B virus
Nomenclature, Rates of Genotypic Resistance, and Clinical Features of Resistance
The acquisition of specific mutations that confer a replicative advantage to the virus in the presence of the antiviral agent is referred to as antiviral resistance. Several definitions have been developed to describe antiviral resistance and are highlighted in Table 1. 1 The detection of HBV polymerase mutations during therapy that have been shown to decrease susceptibility to treatment in a phenotypic assay is referred to as genotypic resistance. Clinically, antiviral drug resistance usually manifests first as an increase in HBV DNA levels (virological breakthrough) and then as an increase in alanine aminotransferase levels (biochemical breakthrough) (Table 1 and Fig. 1). Most mutations that confer resistance have an adverse effect on viral fitness but are tolerated because they provide a survival advantage to the virus in the presence of the antiviral agent. Over time, compensatory mutations develop that restore viral fitness leading to virological rebound.
Table 1.
Antiviral Resistance Nomenclature
| Term | Definition |
|---|---|
| Primary treatment failure (nonresponse) | <1 log decline in HBV DNA 12 weeks after starting therapy |
| Secondary treatment failure (virological breakthrough) | ≥1 log increase in HBV DNA from nadir in a compliant patient, confirmed with repeat testing at least 1 month later |
| Biochemical breakthrough | Rise in ALT while on treatment, after having achieved normalization in a compliant patient |
| Genotypic resistance | Detection of viral populations bearing reverse transcriptase mutations previously shown to confer resistance to antiviral drugs in a phenotypic assay |
| Phenotypic resistance | Decreased susceptibility of an HBV polymerase to antiviral treatment in vitro |
| Cross‐resistance | Decreased susceptibility to more than one antiviral drug conferred by the same mutation or combination of mutations |
Adapted with permission from Hepatology.1 Copyright 2007, Wiley.
Figure 1.
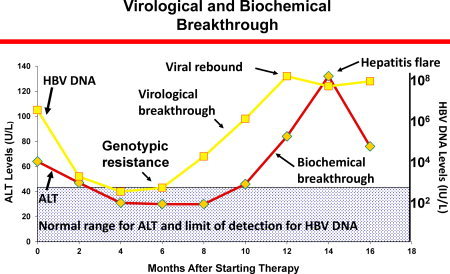
Schematic of genotypic resistance and virological and biochemical breakthrough.
Rates of antiviral drug resistance vary with the agent used and the duration of therapy (Fig. 2). The highest rates of resistance are associated with lamivudine, telbivudine, and adefovir, whereas much lower rates occur with entecavir and tenofovir. The 5‐year rate of resistance to entecavir in previously untreated patients was reported as 1.2%. In contrast, the rate of resistance in lamivudine‐resistant patients was approximately 50% at year 5. 2 There has been no confirmed resistance to tenofovir in nucleos(t)ide‐naïve patients at year 5. 3 These low rates of resistance may be somewhat misleading, because patients with incomplete viral suppression in these phase 3 studies were either withdrawn from therapy or changed therapy. Nevertheless, for subjects who do achieve an initial response, rates of resistance are low with entecavir and tenofovir. Consequently, practice guidelines recommend entecavir, tenofovir, or pegylated interferon as first‐line agents for the treatment of CHB. Risk factors for the development of resistance include a high baseline viral load, immune suppression, inadequate viral suppression during therapy, and prior antiviral therapy.
Figure 2.
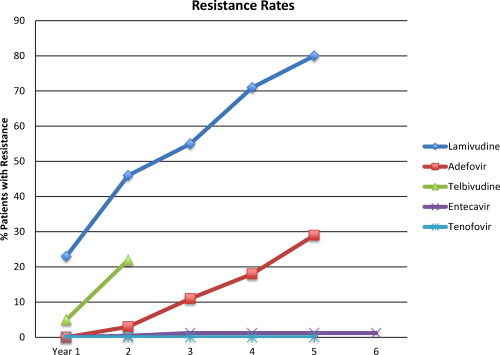
Rates of genotypic resistance to the approved agents in nucleoside‐naïve subjects.
Patients who develop antiviral drug resistance and do not change therapy usually lose the clinical benefit of treatment. Of concern, hepatitis flares, decompensation, and even death have been associated with the development of antiviral resistance. 4, 5, 6, 7 Development of antiviral resistance may limit future treatment options, and transmission of drug‐resistant mutants to treatment‐naïve subjects may pose a potential public health problem.
Monitoring for Antiviral Resistance
Most expert guidelines recommend following HBV DNA levels every 3 months using a sensitive polymerase chain reaction–based assay to monitor for antiviral resistance. Monitoring should be continued at this frequency until HBV DNA levels become undetectable, after which the testing period should be tailored based on the genetic barrier to resistance of the drug used, i.e. longer intervals (approximately every 3‐6 months) for entecavir or tenofovir, shorter intervals (approximately 3 months) if lamivudine, telbivudine or adefovir are used. 8, 9 If HBV DNA levels are noted to rise, it is important to repeat the test and review compliance with the patient. If confirmed, testing for antiviral resistance is advised.
An international group of experts developed a management roadmap (Fig. 3) as a guideline to limit the development of resistance by monitoring HBV DNA levels at specific time points and altering therapy if certain criteria were not met. Although not evidenced‐based, this approach is a logical one based on the rationale that inadequate viral suppression while on treatment is associated with a greater risk of developing antiviral resistance. A major limitation of the roadmap is that it was developed prior to the availability of more potent agents with high barriers to resistance, and several of the key recommended time points for testing HBV DNA may not be relevant with the newer agents. For example, when using drugs with a low genetic barrier to resistance such as lamivudine and telbivudine, even in patients with complete virologic response at week 24, resistance can still develop subsequently, albeit at lower rates. Furthermore, up to 50% of hepatitis B e antigen (HBeAg)‐positive patients would be considered as having an inadequate response at week 24, necessitating addition of a second agent that should be more potent and share no cross‐resistance. On the other hand, several studies have shown that in patients on entecavir or tenofovir, even if HBV DNA is incompletely suppressed at week 48, continuation of the same drug as monotherapy is rarely associated with drug resistance.
Figure 3.
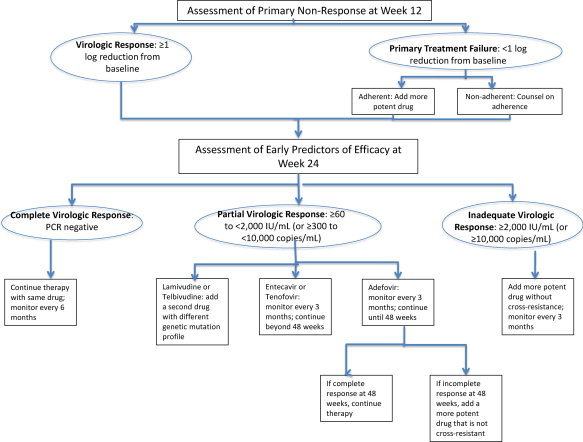
Modified HBV roadmap concept. Abbreviation: PCR, polymerase chain reaction.
Role of Resistance Mutation Testing
The role of genotypic testing in clinical practice is controversial. When testing should be performed and with what assay are unresolved issues. Currently, expert opinion recommends resistance testing at week 12 of therapy if there is primary treatment failure (defined as a <1 log decline in HBV DNA from baseline) and in any patient in whom virological breakthrough is observed and confirmed. Typically, the rise in HBV DNA should be confirmed with repeat testing within 4 weeks. However, in immunosuppressed patients or patients with cirrhosis, this interval should be shorter because of risk of serious flares if therapy is not changed rapidly. Genotypic testing is not recommended prior to initiating therapy in a previously untreated subject, because even though resistance mutations may exist in up to 10% to 15% of treatment‐naïve subjects, they are present at such low amounts that they have a minimal impact on response to therapy. 10 Resistance testing should be considered in all treatment‐experienced patients prior to initiation of therapy, particularly if it is likely to influence choice of therapy.
Two assays are commercially available for resistance testing–direct sequencing and reverse hybridization assays. Each has advantages and limitations. Sequencing assays can detect all known and emerging mutations, but they have low sensitivity for detecting resistant mutants present in <20% of the viral population and may require correlation with clinical or phenotypic data for proper interpretation. Hybridization assays are more sensitive but can only detect known mutations, and single nucleotide polymorphisms can lead to false negative results. Mass spectroscopy, microchip, and deep pyrosequencing are research assays with enhanced sensitivity, but the clinical use of such ultrasensitive assays remains to be determined.
Management of Resistance
The basic principles of management of antiviral resistance are to change therapy early once virological breakthrough is observed, select an add‐on approach over switching, and choose rescue therapy based on the cross‐resistance profile and potency of the rescue drug and presence of comorbid conditions. The management of specific resistance mutations is shown in Table 2.
Table 2.
Management of Established Antiviral Resistance
| Drug | Resistant Mutation | Management Strategy |
|---|---|---|
| Lamivudine | rtV173L, rtL180M; rtM204V/I; rtA181V/T | Switch to tenofovir ± emtricitabine |
| Adefovir 18 | rtN236T | Add entecavir |
| Switch to tenofovir ± emtricitabine | ||
| rt181T/V | Add entecavir | |
| Switch to entecavir plus tenofovir | ||
| Switch to tenofovir/emtricitabine | ||
| Telbivudine | rtM204I | Add tenofovir (or adefovir) |
| Switch to tenofovir ± emtricitabine | ||
| Entecavir | rtS184G; rtS202I; rtM204V/I, rtM250V | Add tenofovir |
| Switch to tenofovir/emtricitabine | ||
| Tenofovir | ? | Unknown |
Prevention is always better than cure. In this regard, the best way to prevent the development of antiviral resistance is through judicious use of antiviral therapy with avoidance of inappropriate treatment. If treatment is required in a nucleos(t)ide‐naïve patient, one should select a potent agent with a high genetic barrier to resistance (e.g, entecavir or tenofovir) and monitor the virological response. If the response is suboptimal (a rare occurrence with these first‐line agents), therapy should be promptly changed. It is important to counsel the patient on adherence with the prescribed regimen before starting therapy, and patients with lower response rates to monotherapy—those with cirrhosis, high baseline viral loads (>108 IU/mL), HIV/HBV coinfection—may benefit from de novo combination therapy. De novo combination therapy has also been suggested if drugs with low barrier to resistance are used, but there is no evidence to support such a strategy if entecavir or tenofovir are used. 11
Managing Multidrug Resistance
Multidrug resistance may develop due to multiple mutations conferring resistance that reside on a single viral genome or a diverse viral population with different mutations on multiple genomes. Cycling patients through repeated courses of monotherapy, particularly agents with low potency and barrier to resistance, may give rise to multidrug resistance. The prevalence of multidrug resistance is unknown, but a large series from China found a rate of approximately 0.5% among treated patients. 12 Management of multidrug resistance is challenging, particularly because all the approved oral agents have the same viral target—the HBV polymerase—and information on how to manage these difficult cases is sparse. At present, three options are available. One option is to treat with two potent agents, such as entecavir and tenofovir. 13 However, the success of this approach may depend on the particular combination of resistant mutations. 14, 15, 16 A second option is to treat the subject with pegylated interferon, provided there are no contraindications to using this drug. One limitation is that if a durable endpoint such as HBeAg or HBsAg seroconversion is not achieved, pegylated interferon cannot be used long‐term to suppress HBV replication. 17 Finally, in some circumstances, withdrawal of therapy can be considered if the patient did not have a strong indication for treatment, does not have advanced disease, and can be monitored closely.
Conclusions
Currently, we do not have the ability to cure CHB, and our therapeutic armamentarium, while effective, is subject to the development of antiviral resistance. With very few new drugs in the development pipeline, we must make every effort to prevent resistance by following clear indications for therapy, counseling patients repeatedly on adherence, and monitoring closely for the development of virologic breakthrough.
This study was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute, National Institutes of Health.
Potential conflict of interest: Nothing to report.
References
- 1. Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, et al. Antiviral drug‐resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology 2007; 46: 254–265. [DOI] [PubMed] [Google Scholar]
- 2. Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long‐term monitoring shows hepatitis B virus resistance to entecavir in nucleoside‐naive patients is rare through 5 years of therapy. Hepatology 2009; 49: 1503–1514. [DOI] [PubMed] [Google Scholar]
- 3. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5‐year open‐label follow‐up study. Lancet; doi: 10.1016/S0140–6736(12)61425–1. [DOI] [PubMed] [Google Scholar]
- 4. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351: 1521–1531. [DOI] [PubMed] [Google Scholar]
- 5. Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long‐term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125: 1714–1722. [DOI] [PubMed] [Google Scholar]
- 6. Nafa S, Ahmed S, Tavan D, Pichoud C, Berby F, Stuyver L, et al. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology 2000; 32: 1078–1088. [DOI] [PubMed] [Google Scholar]
- 7. Di Marco V, Marzano A, Lampertico P, Andreone P, Santantonio T, Almasio PL, et al. Clinical outcome of HBeAg‐negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40: 883–891. [DOI] [PubMed] [Google Scholar]
- 8. Pawlotsky JM, Dusheiko G, Hatzakis A, Lau D, Lau G, Liang TJ, et al. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. Gastroenterology 2008; 134: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45: 507–539. [DOI] [PubMed] [Google Scholar]
- 10. Tan Y, Ding K, Su J, Trinh X, Peng Z, Gong Y, et al. The naturally occurring YMDD mutation among patients chronically infected HBV and untreated with lamivudine: a systematic review and meta‐analysis. PLoS One 2012; 7: e32789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghany MG, Feld JJ, Zhao X, Heller T, Doo E, Rotman Y, et al. Randomised clinical trial: the benefit of combination therapy with adefovir and lamivudine for chronic hepatitis B. Aliment Pharmacol Ther; doi: 10.1111/j.1365–2036.2012.05059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Wang C, Zhong Y, Li X, Dai J, Ren X, et al. Genotypic resistance profile of hepatitis B virus (HBV) in a large cohort of nucleos(t)ide analogue‐experienced Chinese patients with chronic HBV infection. J Viral Hepat 2011; 18: e29–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scotto G, D'Addiego G, Giammario A, Campanale F, Fazio V. Tenofovir plus entecavir as rescue therapy for multidrug‐resistant chronic hepatitis B. Liver Int 2012; 32: 171–172. [DOI] [PubMed] [Google Scholar]
- 14. Petersen J, Ratziu V, Buti M, Janssen HL, Brown A, Lampertico P, et al. Entecavir plus tenofovir combination as rescue therapy in pre‐treated chronic hepatitis B patients: an international multicenter cohort study. J Hepatol 2012; 56: 520–526. [DOI] [PubMed] [Google Scholar]
- 15. Jeon JW, Shin HP, Lee JI, Joo KR, Cha JM, Park JJ, et al. Efficacy of entecavir and adefovir combination therapy for patients with lamivudine‐ and entecavir‐resistant chronic hepatitis B. Dig Dis Sci 2012; 57: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 16. Yang HJ, Lee JH, Kim YJ, Yoon JH, Lee HS. Antiviral efficacy of combination therapy with entecavir and adefovir for entecavir/lamivudine‐resistant hepatitis B virus with or without adefovir resistance. J Med Virol 2012; 84: 424–430. [DOI] [PubMed] [Google Scholar]
- 17. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon alfa‐2a, lamivudine, and the combination for HBeAg‐positive chronic hepatitis B. N Engl J Med 2005; 352: 2682–2695. [DOI] [PubMed] [Google Scholar]
- 18. Berg T, Marcellin P, Zoulim F, Moller B, Trinh H, Chan S, et al. Tenofovir is effective alone or with emtricitabine in adefovir‐treated patients with chronic‐hepatitis B virus infection. Gastroenterology 2010; 139: 1207–1217. [DOI] [PubMed] [Google Scholar]


