Watch a video presentation of this article
Watch the interview with the author
Alcohol has been the most frequently abused drug for centuries, but it was not until the 1960s that it was recognized as a direct hepatotoxin.1 Chronic excessive use of alcohol can lead to fatty liver, alcoholic hepatitis, alcoholic cirrhosis, and liver cancer. Whereas fatty liver is a reversible process upon cessation of drinking alcohol, other pathologies are usually progressive despite abstinence. In the United States, approximately 60% of people in the general population report some drinking of alcohol with approximately 8% to 10% reporting heavy drinking (two or more drinks per day) where one drink is equivalent to 12 ounces of beer (4%‐5% wt/vol), 6 ounces of wine (8%‐10% wt/vol), and 2 ounces of hard liquor or whiskey (45% wt/vol).2
Abbreviations.
DALY, disability adjusted life years; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Alcohol remains the second most common cause of liver cirrhosis after hepatitis C virus (HCV) infection in the United States, contributing to approximately 20% to 25% cases of liver cirrhosis and about half of all admissions among patients with cirrhosis. Of the various factors responsible for liver disease, duration and amount of drinking alcohol is the most important factor. Pooled data from many epidemiological studies show a minimum intake of 30 g/day of alcohol in women and 50 g/day in men consumed over at least 5 years to cause liver cirrhosis.3 Prevalence and mortality rates from cirrhosis parallel alcohol consumption prevalence rates in the population globally. Within Europe, which has the highest rate of consumption per capita in the implementation of policies on alcohol sale has resulted in changing epidemiology in different parts of Europe. Alcohol consumption rates have decreased in southern Europe, whereas they have increased in eastern Europe and some areas of northern Europe (including Ireland and the United Kingdom).4 Because only 10% to 15% of people engaged in heavy drinking develop liver cirrhosis, other factors such as host factors and comorbidities are clearly important (Fig. 1). Women are more prone to the hepatotoxic effect of alcohol compared with men and are prone to develop liver cirrhosis at a lower amount of alcohol consumption. Type of alcohol consumed, binge drinking (five or more drinks at one time), drinking on an empty stomach, and participation in alcohol rehabilitation programs such as Alcoholic Anonymous are some of the other factors. Alcohol use and concomitant HCV infection act synergistically to cause more frequent and faster progression of fibrosis.5 With the epidemic of obesity in the United States, prevalence rates of metabolic syndrome and visceral adiposity are increasing among alcoholics contributing to more prevalent and severe liver disease.6
Figure 1.
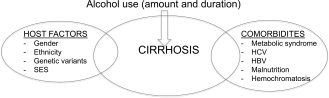
Interaction of alcohol consumption with host factors and comorbidities in causing liver disease. Abbreviation: HBV, hepatitis B virus; SES, socioeconomic status.
Alcohol remains the third most common preventable cause of death after smoking and hypertension. Alcohol‐related mortality affects young and middle‐aged population with loss of productive life years. Liver‐related mortality from alcohol contributes to 4% of mortality and 5% of disability adjusted life years (DALY) globally, with highest impact in Europe, where these same figures are 7% and 12%, respectively (Fig. 2).7 This huge disease burden has an economic impact of about €125 billion annually in Europe, about 1.3% of the gross domestic product. These figures are probably underestimates due to inaccuracies in death certificate reports, because mentioning alcohol as a contributing cause of death may have social and legal implications. Fortunately, over the last two to three decades, the mortality rates from liver cirrhosis have decreased from 20 to 25 per 100,000 population in the early twentieth century to less than 10/100,000 currently (Fig. 3).8 Improved management of cirrhosis patients combined with the implementation of policies to control the sale and availability of alcohol and promote public education regarding the harmful effects of alcohol have all resulted in this change.
Figure 2.
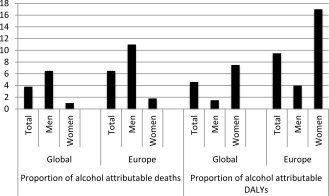
Proportion of deaths and DALY contributed by alcohol globally and within Europe. Adapted with permission from the World Health Organization. Copyright 2011.
Figure 3.
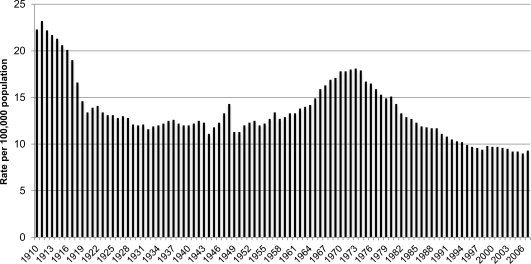
Trends on liver cirrhosis mortality in the United States (1910‐2006). Adapted with permission from the National Institute of Alcohol Abuse and Alcoholism. Copyright 2008.
Alcoholic hepatitis, a common and distinct subset of patients with alcoholic liver disease, occurs in 35% to 40% of patients with chronic excessive alcohol abuse, has a high mortality of about 40% to 50% in untreated subjects with severe disease, and represents about 0.2% (20 out of every 1,000) hospital admissions within the United States (Fig. 4).9, 10 Although mortality from alcoholic hepatitis has decreased over the last decade, similar to alcoholic cirrhosis, patients with alcoholic hepatitis and concomitant HCV infection remain at a higher risk of death, having 20% to 25% higher mortality compared with alcoholic hepatitis patients without HCV infection (Fig. 4).10 One of the possible reasons may be fear among physicians and gastroenterologists to use corticosteroids for treatment of alcoholic hepatitis in the presence of HCV infection.11 The development of guidelines for managing alcoholic hepatitis patients who also have HCV infection is needed.
Figure 4.
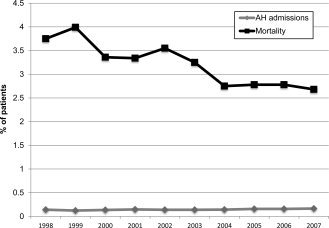
Trends in hospital admissions and mortality (1998‐2007) from alcoholic hepatitis in the United States. Adapted with permission from the European Journal of Gastroenetrology & Hepatology.10 Copyright 2012, Lippincott Williams and Wilkins.
As with any other form of cirrhosis, alcoholic cirrhosis is a risk factor for hepatocellular carcinoma (HCC). However, a recent epidemiologic population‐based study from Denmark showed a low incidence of and mortality from HCC in pure alcoholic cirrhosis.12 The authors reported that the risk of HCC in pure alcoholic cirrhosis does not warrant screening alcoholic patients with cirrhosis for HCC, because the cost efficacy benefit requires an incidence of HCC of at least 1.5% per year as per American Association for Study of Liver Diseases guidelines.13
Alcoholic cirrhosis remains the second most common indication for liver transplantation, a definitive treatment option for patients with end‐stage liver disease. Encouraging reports of posttransplantation outcomes for alcoholic cirrhosis as good as any other indication and better than HCV infection have resulted in an increase in the number of transplantations performed for alcoholic cirrhosis over the last two decades (Fig. 5). However, posttransplantation outcomes of alcoholic patients with cirrhosis who are concomitantly infected with HCV are conflicting, because reports using large databases (such as the United Network for Organ Sharing and the European Liver Transplant Registry) have shown worse survival among HCV‐infected drinkers compared with HCV‐negative drinkers, but similar survival rates have been reported in a single‐center database in a European study.14, 15, 16 These differences could be due to a lack of details on alcohol use and anti‐HCV treatment in large databases compared with single‐center databases.17 Patients with alcohol‐related liver disease require 6 months of abstinence before they can be listed for liver transplantation. However, this rule cannot be applied to patients with alcoholic hepatitis who do not respond to corticosteroid treatment. Recent reports of the beneficial effects of liver transplantation in this group of patients are encouraging and provide hope for selected alcoholic hepatitis patients who meet the criteria for liver transplantation. However, these data need confirmation in larger prospective studies before implementing this into routine clinical practice.18, 19
Figure 5.
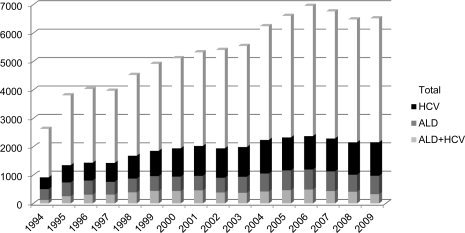
Liver transplantation trends for alcoholic cirrhosis, HCV infection, and combined alcohol and HCV. Abbreviation: ALD, alcoholic liver disease. Adapted with permission from the United Network for Organ Sharing. Copyright 2010.
Alcohol‐related liver disease remains an important public health problem. The World Health Organization's goal is to reduce the mortality rate from alcoholic cirrhosis to below 3.2 per 100,000 population by the year 2020, yet we are far away from this number. Further, reduction of the mortality rate in alcoholic cirrhosis is much lower compared with other etiologies of liver disease (30% versus 47%). Abstinence remains the cornerstone of treatment, with an additional need for public awareness on the toxic effects of alcohol use and the implementation of policies to restrict availability of alcohol. In this regard, there is an unmet need for the development of more effective treatment options for patients with alcoholic liver disease. This is more pertinent in light of the lack of research in this area, with the death to trial ratio (age‐adjusted liver disease deaths per 100,000/number of clinical trials as reported at ClinicalTrials.gov) exceeding 350 for alcoholic liver disease compared with <50 for NASH and <5 for HCV.20 In light of these facts, the decision to preserve alcohol‐related liver research by the National Institute of Alcohol Abuse and Alcoholism is timely and encouraging for clinical and translational researchers in this field. ▪
Potential conflict of interest: Nothing to report.
References
- 1. Rubin E, Lieber CS. Relation of alcoholic liver injury to cirrhosis. Clin Gastroenterol 1975; 4: 247‐272. [PubMed] [Google Scholar]
- 2. Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis 2004; 24: 217‐232. [DOI] [PubMed] [Google Scholar]
- 3. Becker U, Deis A, Sørensen TI, Grønbaek M, Borch‐Johnsen K, Müller CF, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996; 23: 1025‐1029. [DOI] [PubMed] [Google Scholar]
- 4. Jewell J, Sheron N. Trends in European liver death rates: implications for alcohol policy. Clin Med 2010; 10: 259‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol 2007; 41: 761‐772. [DOI] [PubMed] [Google Scholar]
- 6. Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, et al. Risk factors of fibrosis in alcohol‐induced liver disease. Hepatology 2002; 35: 635‐638. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 8. Yoon Y‐H, Yi H‐y. Liver Cirrhosis Mortality in the United States 1970‐2007. Bethesda, MD: National Institute on Alcohol and Alcoholism; 2010. [PMC free article] [PubMed] [Google Scholar]
- 9. Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360: 2758‐2769. [DOI] [PubMed] [Google Scholar]
- 10. Singal AK, Kuo YF, Anand BS. Hepatitis C virus infection in alcoholic hepatitis: prevalence patterns and impact on in‐hospital mortality. Eur J Gastroenterol Hepatol 2012; 24: 1178‐1184. [DOI] [PubMed] [Google Scholar]
- 11. Singal AK, Sagi S, Kuo YF, Weinman S. Impact of hepatitis C virus infection on the course and outcome of patients with acute alcoholic hepatitis. Eur J Gastroenterol Hepatol 2011; 23: 204‐209. [DOI] [PubMed] [Google Scholar]
- 12. Singal AK, Singal A, Jampana S, Freeman D, Anderson K, Brunder D. Management of alcoholic hepatitis in the presence of concomitant hepatitis c virus infection: a survey of practising gastroenterologists and hepatologists. Am J Gastroenterol 2011; 106: S109. [Google Scholar]
- 13. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucey MR, Schaubel DE, Guidinger MK, Tome S, Merion RM. Effect of alcoholic liver disease and hepatitis C infection on waiting list and posttransplant mortality and transplant survival benefit. Hepatology 2009; 50: 400‐406. [DOI] [PubMed] [Google Scholar]
- 15. Aguilera V, Berenguer M, Rubín A, San‐Juan F, Rayón JM, Prieto M, et al. Cirrhosis of mixed etiology (hepatitis C virus and alcohol): posttransplantation outcome‐comparison with hepatitis C virus‐related cirrhosis and alcoholic‐related cirrhosis. Liver Transpl 2009; 15: 79‐87. [DOI] [PubMed] [Google Scholar]
- 16. Singal AS, Hmoud B, Kuo YF, Salameh H, Wiesner RH. Evolving frequency and outcomes of liver transplantation based on etiology. Transplantation 2013; Jan 30 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17. Anand BS, Currie S, Dieperink E, Bini EJ, Shen H, Ho SB, et al. Alcohol use and treatment of hepatitis C virus: results of a national multicenter study. Gastroenterology 2006; 130: 1607‐1616. [DOI] [PubMed] [Google Scholar]
- 18. Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365: 1790‐1800. [DOI] [PubMed] [Google Scholar]
- 19. Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55: 1398‐1405. [DOI] [PubMed] [Google Scholar]
- 20. Shah VH. Alcoholic liver disease: the buzz may be gone, but the hangover remains. Hepatology 2010; 51: 1483‐1484. [DOI] [PubMed] [Google Scholar]


