Watch a video presentation of this article
Watch the interview with the author
Patients with cirrhosis have an increased risk of developing bacterial infection, sepsis, and death.1, 2, 3 Infection is present at admission or develops during hospitalization in about 25% to 35% of patients.1, 4 Spontaneous bacterial peritonitis (SBP) and urinary infections are the most frequent infections followed by pneumonia, cellulitis, and bacteremia. Approximately 30% of bacterial infections are community‐acquired (CA), 30% are health care–associated (HCA), and 35% to 40% are nosocomial.1, 4 Clinical risk factors include poor liver function, variceal bleeding, low protein ascites, prior episode of SBP, and hospitalization.1, 2 A genetic immune defect could contribute to an increase in the risk of bacterial infections in cirrhosis. In patients with advanced cirrhosis, infection induces a systemic inflammatory response characterized by high circulating levels of proinflammatory cytokines. This excessive proinflammatory response contributes to the development of sepsis‐related organ failures (acute‐on‐chronic liver failure) and septic shock in cirrhosis.1
Abbreviations.
HCA, health care–associated; MRSA, methicillin‐resistant Staphylococcus aureus; SBP, spontaneous bacterial peritonitis; VRE, vancomycin‐resistant Enterococcus; VSE, vancomycin‐susceptible Enterococcus.
Diagnosis of Bacterial Infections
Early diagnosis and treatment of infection is pivotal in the management of patients with decompensated cirrhosis and is based on clinical and analytical grounds.1 Complete workup (paracentesis, urinary sediment, chest X‐ray, and blood, ascitic fluid, and urine cultures) should be performed at admission and whenever a hospitalized patient deteriorates clinically (Fig. 1). Common clinical and analytical markers of infection have limitations in cirrhosis. Systemic inflammatory response syndrome criteria and serum C‐reactive protein and procalcitonin levels have less diagnostic capacity in the cirrhosis patient population. Cutoff values proposed for the diagnosis of infection in patients with advanced cirrhosis are 2 mg/dL for C‐reactive protein (lower than that recommended in the general population) and 0.5 ng/mL for procalcitonin.1
Figure 1.
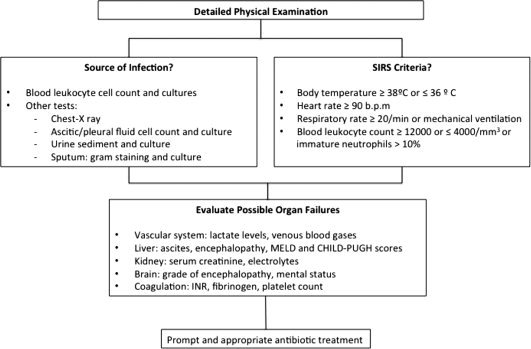
Suggested algorithm for the diagnosis of bacterial infections in cirrhosis. The initial workup includes a detailed physical examination and different diagnostic tests to clarify the source of the infection. Severity of infection should also be assessed (systemic inflammatory response syndrome criteria and organ failures). Abbreviations: INR, international normalized ratio; MELD, model for end‐stage liver disease.
Diagnosis of SBP is based on ascitic fluid analysis (PMN count ≥250 cells/mm3). Leukocyte reagent strips are not useful as screening test considering their variable sensitivity (45%‐100%). Secondary peritonitis constitutes the main differential diagnosis of SBP and is often due to a perforated viscus or infection in adjacent organs. Runyon's criteria (glucose levels <50 mg/dL, protein concentration >10 g/L, lactate dehydrogenase concentration > normal serum levels) are clues to this diagnosis and prompt computed tomography examination of the abdomen and early surgery in the management of patients with secondary bacterial peritonitis.1, 5
Infections by Multiresistant Bacteria
Growing data from different geographical areas show an increased prevalence of infections caused by multiresistant bacteria in cirrhosis (pathogens resistant to the main antibiotics, including β‐lactams).1, 4 The most common are extended‐spectrum β‐lactamase–producing Enterobacteriaceae, nonfermentable gram‐negative bacilli (e.g., Pseudomonas aeruginosa), methicillin‐resistant Staphylococcus aureus (MRSA), vancomycin‐susceptible Enterococcus (VSE), and vancomycin‐resistant Enterococcus (VRE). Epidemiological patterns of multiresistance are different among geographical areas. Multiresistant bacteria are more frequently isolated in nosocomial infections (35%‐39%) compared with HCA (14%‐20%) or community‐acquired episodes (0%‐4%). Type of multiresistant bacteria also varies among infections. Risk factors of multiresistant bacterial infection include current or recent hospitalization, health care support (outpatient clinic), and previous exposition to β‐lactams or fluoroquinolones, including long‐term norfloxacin prophylaxis. Multiresistant bacterial infections present a lower resolution rate, higher probability of severe sepsis, and higher mortality.4
Empirical Antibiotic Treatment
Prompt and appropriate antibiotic treatment is essential in the management of patients with cirrhosis who have infection. Meanwhile, third‐generation cephalosporins continue to be the gold standard antibiotic treatment of many of the infections acquired in the community.6, 7 The empirical treatment of nosocomial and HCA infections should be adapted to the local epidemiological pattern of antibiotic resistance. Patients with cirrhosis and severe infections should receive intravenous antibiotics immediately after diagnosis.1, 2
Community‐Acquired Infections
β‐Lactams (third‐generation cephalosporins or amoxicillin‐clavulanic acid) are still effective in the treatment of spontaneous infections acquired in the community (Fig. 2). Quinolones are not recommended in patients submitted to long‐term norfloxacin prophylaxis.1, 6, 7 Empirical treatment of community‐acquired urinary infections includes β‐lactams, quinolones, or trimethoprim‐sulfamethoxazole. β‐Lactams are also the base of the treatment of community‐acquired pneumonia (in combination with levofloxacin, moxifloxacin, or a macrolide) and cellulitis.1
Figure 2.
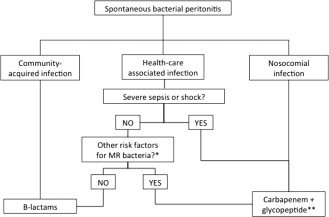
Proposed algorithm for the empirical treatment of SBP in cirrhosis. Site of acquisition of infection and presence of risk factors for multiresistant (MR) bacteria are considered. *Infection by multiresistant bacteria in the last 6 months, use of β‐lactams in the last 3 months, and long‐term norfloxacin prophylaxis. **Linezolid or daptomycin should replace glycopeptides in areas with a high prevalence of VRE.
Nosocomial Infections
New guidelines for the treatment of nosocomial SBP are needed. Empirical antibiotic strategies should consider the local epidemiological patterns of multiresistance (Table 1 and Fig. 2).1, 4 In areas with a high prevalence of Enterobacteriaceae‐producing Enterobacteriaceae, carbapenems should be used in the empirical treatment of SBP and of other spontaneous infections in combination with antibiotics active against VSE/VRE and MRSA (glycopeptide, linezolid, or daptomycin). Urinary infections acquired during hospitalization should be treated with oral nitrofurantoin or fosfomycin (in uncomplicated infections) or carbapenems plus glycopeptides (VSE) or linezolid/daptomycin (VRE) in patients with sepsis (Table 1). Cellulitis should be treated with antibiotics active against MRSA and Pseudomonas aeruginosa. Empirical treatment of nosocomial pneumonia should follow the local guidelines for general population. In line with a policy of antibiotic restriction, de‐escalation to the most appropriate antibiotic should be done early after knowing the results of microbiological tests.1
Table 1.
Suggested Empirical Antibiotic Treatment for Nosocomial Bacterial Infections in Cirrhosis
| Type of Infection | Empirical Antibiotic Treatmenta |
|---|---|
| SBP or other spontaneous infections | Carbapenem (to cover ESBL‐producing Enterobacteriaceae) + a glycopeptide (to cover MRSA and VSE)b |
| Urinary infectionsc | Uncomplicated infections: nitrofurantoin (50 mg/6 hours by mouth) |
| Complicated infections (sepsis, severe sepsis, or shock): carbapenem + glycopeptideb | |
| Pneumoniac | Antibiotics active against Pseudomonas aeruginosa (i.e., meropenem or ceftazidime + ciprofloxacin) |
| A glucopeptide or linezolid should be added in patients with risk factors for MRSAd | |
| Cellulitis | Antibiotics active against Pseudomonas aeruginosa + glycopeptideb |
Abbreviation: ESBL, extended‐spectrum β‐lactamase.
Empirical antibiotic therapy should be adapted to the local epidemiological patterns of resistant bacteria.
In areas with a high prevalence of VRE, glycopeptides must be replaced with intravenous linezolid or daptomycin.
Nosocomial and HCA infections.
Ventilator‐associated pneumonia, previous antibiotic therapy, nasal MRSA carriage.
HCA Infections
β‐Lactams are still effective in the treatment of HCA SBP and cellulitis in some geographical areas. Efficacy is lower in pneumonia and urinary infections.4 Empirical antibiotic strategies for these two latter infections and for patients with HCA SBP and risk factors of multiresistant bacterial infections and/or severe sepsis or shock should also follow that described for nosocomial infections (Table 1 and Fig. 2).
Albumin Administration
Bacterial infections can accentuate circulatory dysfunction in patients with cirrhosis and ascites and induce renal failure.6, 7 SBP is the most frequent infection causing hepatorenal syndrome but it can also be induced by biliary, gastrointestinal, and complicated urinary infections.1 Treatment with intravenous albumin (1.5 g/kg at diagnosis and 1 g/kg on day 3) reduces the incidence of hepatorenal syndrome (from 33% to 10%) and improves short‐term survival in patients with SBP.8 Patients with bilirubin >4 mg/dL or creatinine >1.0 mg/dL clearly benefit from intravenous albumin. The administration of albumin in unselected patients with cirrhosis who have non‐SBP infections is not associated with clinically relevant effects.1
Prevention of Infection in Cirrhosis
Antibiotic prophylaxis must be restricted to selected patients at a very high risk for the development of bacterial infections. This restriction to specific subpopulations is essential to prevent the development of antibiotic resistance in cirrhosis and to make these prophylactic strategies cost‐effective. Current indications of antibiotic prophylaxis in cirrhosis are gastrointestinal bleeding, low protein ascites in advanced cirrhosis, and previous episode of SBP1, 6, 7, 9, 10 (Table 2).
Table 2.
Current Indications of Antibiotic Prophylaxis in Cirrhosis
| Indication | Antibiotic Regimen | Duration |
|---|---|---|
| Gastrointestinal bleeding | Norfloxacin 400 mg/12 hours by mouth | Seven days |
| Intravenous ceftriaxone 1 g/day in patients with advanced cirrhosis (at least two of the following: ascites, jaundice, hepatic encephalopathy, malnutrition) | ||
| Low protein ascites (<15 g/L) and advanced cirrhosis | Norfloxacin 400 mg/day PO in patients with renal dysfunction (serum creatinine ≥1.2 mg/dL, blood urea nitrogen ≥25 mg/dL, or serum sodium ≤130 mEq/L) and/or poor liver function (Child‐Pugh score ≥9 with serum bilirubin ≥3 mg/dL) | Until liver transplantation, disappearance of ascites, or death |
| Secondary prophylaxis for SBP | Norfloxacin 400 mg/day by mouth | Until liver transplantation or death |
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Fernandez J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol 2012: S1– S12. [DOI] [PubMed] [Google Scholar]
- 2. Tandon P, Garcia‐Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 2008; 28: 26–42. [DOI] [PubMed] [Google Scholar]
- 3. Arvaniti V, D'Amcio G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four‐fold and should be used in determining prognosis. Gastroenterology 2010; 139: 1246–1256. [DOI] [PubMed] [Google Scholar]
- 4. Fernández J, Acevedo J, Castro M, Garcia O, Rodriguez de Lopez C, Roca D, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 2012; 55: 1551–1561. [DOI] [PubMed] [Google Scholar]
- 5. Soriano G, Castellote J, Alvarez C, Girbau A, Gordillo J, Baliellas C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52: 39–44. [DOI] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53: 397–417. [DOI] [PubMed] [Google Scholar]
- 7. Runyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49: 2087– 2107. [DOI] [PubMed] [Google Scholar]
- 8. Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz del Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999; 5: 403–409. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez J, Ruiz del Arbol L, Gomez C, Durandez R, Serradilla R, Guarner C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 10. Fernandez J, Navasa N, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 2007; 133: 818–824. [DOI] [PubMed] [Google Scholar]


