Watch a video presentation of this article
Watch the interview with the author
Hyponatremia in the setting of cirrhosis was first described decades ago,1 and it has become very clear that the presence of hyponatremia is a poor prognostic marker in patients with advanced cirrhosis.2 Although it is uncertain that reversing hyponatremia will improve survival, the development of the vaptan drugs, which increase solute‐free water excretion within the renal tubules by antagonizing the effects of arginine vasopressin (AVP), has brought further attention to the management of this difficult‐to‐treat condition.3
Abbreviations.
AVP, arginine vasopressin; MELD, Model for End‐Stage Liver Disease; SNS, sympathetic nervous system; V2, vasopressin 2.
Types of Hyponatremia
Hyponatremia in the setting of cirrhosis has been arbitrarily defined as a serum sodium level equal to or less than 130 mEq/L and has been found to occur in 22% of patients with cirrhosis and ascites.4 However, even sodium levels less than 135 mEq/L (the lower limit of normal) have been found to have significant prognostic value2 and occur in up to 50% of patients with ascites.4 In the setting of cirrhosis, patients can have two types of hyponatremia: hypovolemic hyponatremia and hypervolemic hyponatremia.
Hypovolemic hyponatremia is a result of fluid losses either from the kidneys (most commonly due to iatrogenic overdiuresis) or from the gastrointestinal tract (i.e., diarrhea). Patients typically will have signs of dehydration and findings of prerenal azotemia due to the contraction of the total plasma volume.5 They will not usually have ascites or edema.
In contrast, the majority of patients with cirrhosis present with hypervolemic hyponatremia. This type of hyponatremia is the main focus of this overview. Hypervolemic hyponatremia is characterized by a pronounced deficit of free water excretion and leads to inappropriate water retention in comparison with the sodium concentration. This imbalance results in an expanded extracellular volume and dilutional hyponatremia. Patients with hypervolemic hyponatremia usually have ascites and/or edema and may have concurrent kidney injury.5
Pathophysiology
Arterial (splanchnic and systemic) vasodilatation is the most likely mechanism leading to hyponatremia, and the severity of vasodilatation represents a continuum with the pathophysiology of ascites on one extreme and hepatorenal syndrome on the other extreme (Fig. 1).6 Arterial vasodilatation results in a reduction of the effective arterial blood volume, which in turn leads to the stimulation of several neurohumoral systems [specifically the renin‐angiotensin‐aldosterone system and the sympathetic nervous system (SNS)] and the nonosmotic release of an antidiuretic hormone (or AVP). The activation of the renin‐angiotensin‐aldosterone system and SNS results in sodium retention and, in extreme cases, in renal vasoconstriction. Increased levels of AVP influence the activation of vasopressin 2 (V2) receptors within the renal tubules. These receptors play a major role in the rate of solute‐free water excretion. Therefore, depending on the daily water intake, these patients cannot excrete enough free water and thus develop water retention, which generates serum dilution and hypo‐osmolality.5
Figure 1.
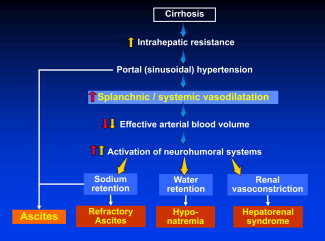
Pathophysiology of hypervolemic hyponatremia in the setting of cirrhosis. In the setting of decompensated cirrhosis, significant vasodilatation of the splanchnic and systemic circulation leads to a reduction in the effective arterial blood volume. Consequently, multiple neurohumoral systems are activated and cause sodium and water retention as well as renal vasoconstriction. As a result, patients will develop refractory ascites, hyponatremia, and eventually hepatorenal syndrome.
In a prospective cohort study of patients with an initial presentation of ascites, the patients first developed hyponatremia, and this was followed by the development of refractory ascites, which in turn preceded the development of hepatorenal syndrome.7 Each of these stages was associated with lower mean arterial pressure values indicative of worsening vasodilatation and with progressively higher Child‐Pugh and Model for End‐Stage Liver Disease (MELD) scores indicative of worsening liver function. In this context, hyponatremia represents an intermediate stage in the progression from ascites to hepatorenal syndrome.
Clinical Importance
The presence of hyponatremia has been appreciated as a significant marker for advanced hepatic dysfunction and has been associated with significant morbidity and mortality. In fact, as mentioned previously, hyponatremia has been found to be a predictor of death independent of the MELD score in patients waiting on the liver transplant list.2 A predictive model combining these two variables, the MELD‐Na model, has been proposed to improve the assignment of priority for liver transplantation. Additionally, several studies have associated decreased sodium levels with the development of hepatic encephalopathy and a poor quality of life.4, 8, 9 Because of its common pathophysiology, hyponatremia has been associated with a higher risk for acute kidney injury, especially in the setting of infections such as spontaneous bacterial peritonitis.10 In fact, hyponatremia is present in most patients with hepatorenal syndrome.11
Patients with hyponatremia are also at risk for complications after liver transplantation. Cases of central pontine myelinolysis after liver transplantation have been reported among patients with hyponatremia and have led to significant morbidity, including prolonged intensive care unit and hospital stays.12 Therefore, careful monitoring of electrolytes and fluid management are required to prevent rapid changes in serum sodium levels, which can cause astrocyte swelling and cellular death.
Management
Early determination of the volume status is critical for the management of hyponatremia in patients with cirrhosis. Patients with hypovolemic hyponatremia should be treated with the withdrawal of diuretics and the infusion of isotonic solutions to normalize the total body sodium level. On the other hand, hypervolemic hyponatremia should be managed by restricting free water ingestion, by increasing renal excretion of solute‐free water, and, ultimately, by correcting the systemic and splanchnic vasodilatation and the resultant decreased effective arterial blood volume (Fig. 2).5
Figure 2.
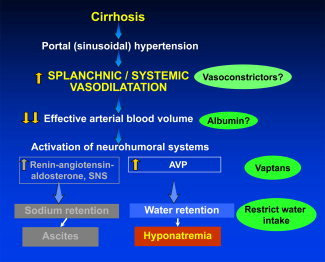
Therapeutic targets to treat hypervolemic hyponatremia in the setting of cirrhosis. As a result of splanchnic and systemic vasodilatation, the renin‐angiotensin‐aldosterone system and SNS are triggered and lead to increased sodium retention and the development of ascites. In addition, there is increased release of AVP, which results in significant water retention by the kidneys and the development of hyponatremia. Water restriction has been the mainstay of therapy, but other targets along this pathway may also provide benefits. Vasoconstrictors and infusions of albumin may improve the effective arterial blood volume (information is limited, and their use is not routinely recommended). Vaptans (V2 AVP receptor antagonists) prevent the binding of AVP to receptors in the kidneys and increase free water excretion.
The restriction of fluids to 1.5 L daily is recommended for severe hyponatremia; however, patient tolerance is very poor, and it leads to decreased quality of life.8 An alternative approach is to increase the renal excretion of free water with V2 receptor antagonists, the so‐called vaptans (Fig. 3). Satavaptan is the most investigated from this drug class within the setting of cirrhosis and has been shown to improve serum sodium levels in placebo‐controlled trials.13 However, the drug's effect lasts only as long as the patient is on the medication. In addition to serum sodium correction, it had been suggested that the long‐term (12‐week) use of satavaptan could be useful also in the control of ascites. However, the combined results of 3 randomized controlled trials using satavaptan and a recent meta‐analysis of 12 trials showed that the vaptans, while improving sodium levels, were not more effective than a placebo in the control of ascites.3, 14 Importantly, there were no observed differences in mortality, and one study actually demonstrated a higher mortality rate in patients treated with satavaptan.14 Satavaptan has been withdrawn from the market.
Figure 3.
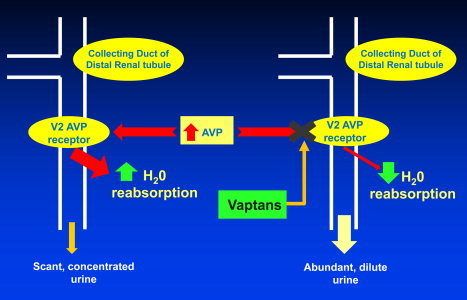
Mechanism of action for vaptans in the treatment of hypervolemic hyponatremia. In the setting of decompensated cirrhosis, the reduction of the effective arterial blood volume results in the increased release of AVP. AVP binds to the V2 AVP receptor located on the basolateral membrane of distal collecting duct cells within the kidneys. This process activates the binding of aquaporin water channels to collecting duct cell membranes and causes increased reabsorption of water. With excessive water retention, the urine becomes solute‐concentrated, and the serum becomes dilute; this leads to hyponatremia. However, if a patient receives a vaptan drug, the vaptan prevents the binding of AVP to the V2 AVP receptor and thus inhibits water reabsorption. In turn, this results in abundant solute‐diluted urine output and maintains serum sodium homeostasis.
A subanalysis of patients with cirrhosis within a large, multicenter, randomized, placebo‐controlled trial using a different V2 receptor antagonist, tolvaptan, to treat hyponatremia revealed that tolvaptan did significantly increase serum sodium levels during the 30‐day treatment period. However, in a subgroup of patients with severe hyponatremia, the drug's effect on serum sodium levels appeared to be transient, and they reverted back to abnormal levels by day 10 of therapy.15 Larger studies with tolvaptan that primarily focus on patients with cirrhosis are necessary in order to clarify these issues. For now, it appears that vaptans are beneficial in increasing serum sodium but only for the short term.
Therapies to correct the decreased effective arterial blood volume in patients with hyponatremia have been confined to the use of intravenous albumin. An infusion of albumin was found to be useful in a very small number of patients in short‐term, nonrandomized studies.16 However, the long‐term benefit of albumin or even vasoconstrictors remains unknown and requires further investigation.
Conclusions
Hyponatremia remains an important marker for severe hepatic dysfunction in cirrhosis, and future investigations are necessary for effective management. Even though the correction of hyponatremia may not improve survival, the presence of hyponatremia limits the use of diuretic therapy, lowers patients' quality of life, and increases the risk of complications before and after liver transplantation. Dietary fluid restriction remains the standard of care but is poorly tolerated by patients. V2 receptor antagonists increase sodium levels in the short term but without an effect on ascites or mortality. Therefore, vaptans could be considered for hospitalized patients with hyponatremia who are high on the liver transplant waiting list. Exploration of the development of new drugs that can effectively resolve the pathophysiology of systemic vasodilation in patients with cirrhosis is an additional avenue for study.
This work was supported by the National Institutes of Health (grant P‐30DK 034989).
Potential conflict of interest: Nothing to report.
References
- 1. Eisenmenger WJ, Blondheim SH, Bongiovanni AM, Kunkel HG. Electrolyte studies on patients with cirrhosis of the liver. J Clin Invest 1950; 29: 1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver‐transplant waiting list. N Engl J Med 2008; 359: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dahl E, Gluud LL, Kimer N, Krag A. Meta‐analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther 2012; 36: 619–626. [DOI] [PubMed] [Google Scholar]
- 4. Angeli P, Wong F, Watson H, Ginès P; for CAPPS Investigators . Hyponatremia in cirrhosis: results of a patient population survey. Hepatology 2006; 44: 1535–1542. [DOI] [PubMed] [Google Scholar]
- 5. Gines P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology 2008; 48: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 6. Gines P, Berl T, Bernardi M, Bichet DG, Hamon G, Jimenez W, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology 1998; 28: 851–864. [DOI] [PubMed] [Google Scholar]
- 7. Planas R, Montoliu S, Balleste B, Rivera M, Miquel M, Masnou H, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 2006; 4: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 8. Sola E, Watson H, Graupera I, Turon F, Barreto R, Rodriguez E, et al. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol 2012; 57: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 9. Guevara M, Baccaro ME, Torre A, Gomez‐Anson B, Rios J, Torres F, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time‐dependent analysis. Am J Gastroenterol 2009; 104: 1382–1389. [DOI] [PubMed] [Google Scholar]
- 10. Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology 1994; 20: 1495–1501. [DOI] [PubMed] [Google Scholar]
- 11. Garcia‐Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008; 48: 2064–2077. [DOI] [PubMed] [Google Scholar]
- 12. Yun BC, Kim WR, Benson JT, Biggins SW, Therneau TM, Kremers WK, et al. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology 2009; 49: 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ginès P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D; for HypoCAT Study Investigators . Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology 2008; 48: 204–213. [DOI] [PubMed] [Google Scholar]
- 14. Wong F, Watson H, Gerbes A, Vilstrup H, Badalamenti S, Bernardi M, et al. Satavaptan for the management of ascites in cirrhosis: efficacy and safety across the spectrum of ascites severity. Gut 2012; 61: 108–116. [DOI] [PubMed] [Google Scholar]
- 15. Cardenas A, Gines P, Marotta P, Czerwiec F, Oyuang J, Guevara M, et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol 2012; 56: 571–578. [DOI] [PubMed] [Google Scholar]
- 16. McCormick PA, Mistry P, Kaye G, Burroughs AK, McIntyre N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut 1990; 31: 204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]


