Watch a video presentation of this article
Watch the interview with the author
Liver transplantation has emerged as a lifesaving operation for patients fortunate enough to receive a donated organ. Today, the 1‐year unadjusted survival rate for a liver transplant recipient is 88.2%.1 For the 5805 adult liver transplant recipients in 2011 alone, this number represents a remarkable outcome achieved through the efforts of transplant and organ procurement teams as well as research advances in transplant immunology and organ preservation. Although the tremendous benefit for recipients cannot be understated, a shortage of organs remains the most significant hurdle in liver transplantation, as demonstrated by the 2456 wait‐list deaths in 2011 and the additional 482 patients who were removed from the wait list because they were too sick to undergo transplantation.1 Furthermore, this disparity between supply and demand varies with a patient's geography, and it has been demonstrated to alter practice patterns by the greater reliance on living donor liver transplantation in those areas most affected by the organ shortage.2, 3 With the continual growth of the liver transplant wait list, additional avenues for expansion of the donor pool have been pursued. This has been manifested in a greater reliance on extended criteria donor (ECD) liver allografts with a subsequent demonstration of reduced wait‐list mortality through their use.4, 5 The use of ECD allografts is a balance between the benefit to the patient in need of liver transplantation (often in a more expedited fashion) and the higher‐than‐normal risk associated with each type of ECD liver. This review is focused on the various types of ECD liver allografts (Table 1) with the understanding that the art of matching an ECD donor liver to the appropriate recipient requires experience, careful planning, and judgment and is a topic beyond the scope of this review.
Table 1.
Risks Associated With ECDs
| Type of Donor | Primary Risk |
|---|---|
| DCD | Biliary complication |
| Advanced age | Delayed graft function |
| Hepatic steatosis | Delayed graft function |
| SLT | Vascular or biliary complication |
| High risk according to CDC | Infectious risk |
| HCV, HBcAb, or HTLV positive | Infectious risk |
| Cancer history | Transfer of malignancy |
Donation After Cardiac Death (DCD)
Livers from donation after brain death (DBD) donors constitute the majority of transplanted organs. DBD allografts undergo continuous organ perfusion until the time of procurement. In contrast, DCD donors (who provided grafts for approximately 5% of all liver transplants in 2011)6 are individuals with severe injuries who do not meet the strict criteria for neurological brain death. DCD donors undergo withdrawal of life support, and procurement begins 5 minutes after asystole. Therefore, organ recovery commences after a period of poor and, subsequently, no organ perfusion. As a result of the variable periods of warm ischemia experienced by DCD allografts, there remains a risk for poor function in the immediate posttransplant period. In addition, the extended period of warm ischemia can lead to biliary complications in 29% and ischemic cholangiopathy in as many as 16% of recipients of DCD livers, whereas the rates are only 17% and 3% for recipients of DBD livers.7 Patients with ischemic cholangiopathy present with jaundice, pruritus, and recurrent cholangitis, and this ultimately leads to early graft loss and the need for retransplantation. Although retrospective analyses have demonstrated that allograft survival and patient survival after DCD transplantation are inferior to those after DBD transplantation,8, 9, 10 there remains a survival benefit for select populations, especially when one considers the risk of death on the wait list.11 The 1‐ and 3‐year survival rates have been shown to be 84.3% and 74.5%, respectively, for adult patients receiving DCD liver allografts and 86.1% and 77.8%, respectively, for patients receiving DBD organs.9 The careful selection of DCD donors is paramount to success and includes the utilization of younger donors (<50 years old), the use of livers with an asystole‐to‐cross‐clamp duration < 30 minutes, and the minimization of the cold ischemia time (CIT) after procurement.
Donors of Advanced Age
Liver allografts from older donors (>65 years old) pose a higher risk of poor function in the immediate postoperative period, and this may negatively affect overall patient survival (Fig. 1A). However, success has been obtained with the use of older liver allografts. Singhal et al.12 retrospectively analyzed United Network for Organ Sharing/Organ Procurement and Transplantation Network data and found a 1‐year patient survival rate of 83.8% with allografts from donors who were 60 to 79 years old, and a 1‐year patient survival rate of 81.0% with allografts from octogenarian donors. Surgeons attempting to use allografts from donors of an advanced age must ensure careful selection of the recipient and work toward minimization of CIT. It should be noted that the risk associated with older liver allografts is heightened in the hepatitis C virus (HCV)–positive recipient, and thus the latter combination should be avoided.13
Figure 1.
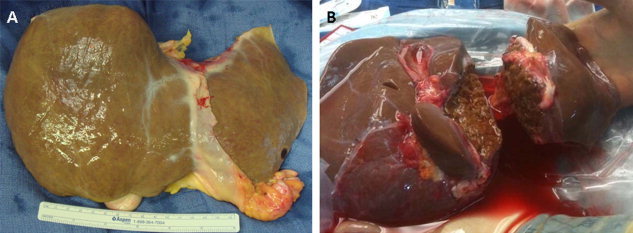
(A) Allograft from a donor of advanced age versus (B) an ideal liver that has undergone ex vivo splitting for pediatric and adult recipients. Although the young donor liver is brown with a smooth surface, the allograft from the donor of advanced age is not as smooth and has yellowish discoloration suggesting a component of steatosis.
Split Liver Transplantation (SLT)
SLT provides another mechanism for increasing the number of organs available for transplantation, and it currently accounts for 4% of liver transplants annually. Typically, one allograft is split, with the left lateral segment going to a pediatric recipient and the right trisegment being transplanted into an adult (Fig. 1B). Less frequently, a liver can be split into right and left lobes for two adult recipients. Liver splitting can be performed before liver removal (in situ), much like a living donor operation, or can be performed after the entire liver has been removed (ex vivo). The initial application of SLT included a higher risk of primary nonfunction for these allografts, and this prompted the current practice of splitting only the highest quality donor livers and transplanting them into recipients who are capable of tolerating a reduced functional hepatic mass. Centers with technical expertise in liver splitting have reported excellent outcomes with SLT that are equivalent to those of whole organ recipients.14, 15 However, much like living donor liver transplantation, SLT carries with it a higher risk of morbidity associated with biliary and vascular complications.
Donors With Hepatic Steatosis
The epidemic of obesity and metabolic syndrome has generated a considerable number of potential donors with hepatic steatosis from fatty liver disease. Much like livers from donors of an advanced age, fatty livers (allografts with >30% macrovesicular steatosis) pose the additional risk of delayed graft function and thus may adversely affect early and late posttransplant outcomes. A large US registry found that allografts with more than 30% macrovesicular steatosis were independently associated with lower 1‐year graft survival.16 The decision to use allografts with more than 30% macrovesicular steatosis remains controversial but can be considered with an otherwise young donor and minimal CIT. The assessment of the steatosis percentage of a donor organ is essential for a procurement team and often requires biopsy, which can be performed either before or at the time of procurement. A biopsy sample can be subject to variability in interpretation because of frozen section artifact17 (Fig. 2). Undoubtedly, fatty livers will continue to be an important source of ECD allografts, and their safe use will be the topic of further investigations.
Figure 2.
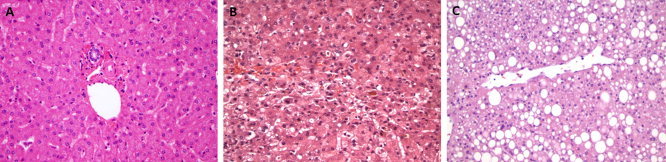
Histopathology of (A) a normal liver, (B) preservation injury after procurement showing zone 3 degeneration with hepatocyte loss and parenchymal collapse, and (C) a nonalcoholic fatty liver showing zone 3 macrovesicular steatosis (grade 2) without hepatocyte ballooning or lobular inflammation (all the panels show hematoxylin and eosin stains and are courtesy of Joseph Misdraji, M.D., Department of Pathology, Massachusetts General Hospital, Boston, MA).
Donors With an Increased Infectious Risk
Allografts from donors with infectious risk factors can often be appropriate for liver transplant recipients, and they include liver allografts from donors with HCV, hepatitis B core antibody (HBcAb), or human T‐lymphotropic virus (HTLV) and from donors who are high‐risk according to the Centers for Disease Control and Prevention (CDC).
HCV‐Positive Donors
HCV‐positive donors have been reserved for HCV genotype 1–positive recipients who have not achieved a sustained virological response to anti‐HCV therapy before transplantation. Studies show that HCV patients who receive HCV‐positive allografts have the same 5‐year survival as HCV patients who receive HCV‐negative liver allografts.18, 19 In addition to normal liver function tests, the selection of HCV‐positive allografts is best suited for HCV‐positive donors who are less than 50 years old and whose liver biopsy samples lack fibrosis or significant inflammation. The use of older HCV‐positive donors for HCV‐positive recipients may lead to more rapid progression of fibrosis in the postoperative period.20
HBcAb‐Positive Donors
Hepatitis B vaccination before transplantation, in addition to extremely effective single‐agent antiviral prophylaxis after transplantation, has allowed excellent outcomes with the use of HBcAb‐positive allografts in recipients without prior exposure to hepatitis B.21 However, the use of hepatitis B surface antigen–positive donors is a rare occurrence, with only 92 liver transplants using allografts from these donors having been reported in the United States from 1990 to 2009.22 The majority of these allografts (74%) were used in recipients with hepatitis B virus–related disease.
HTLV
HTLV I and HTLV II are human retroviruses with up to a 30% prevalence in endemic areas of the developing world as well as the Caribbean. HTLV I can cause myelopathy and adult T cell leukemia, whereas HTLV II is not known to cause any human disease. Transplanting an organ from a donor raised in an area endemic for HTLV I or HTLV II should be considered on an individual basis with vigilant monitoring in the posttransplant setting.
Donors Who Are High‐Risk According to the CDC
In 1994, the CDC issued classifications of high‐risk organ donors based on previous behavior or circumstances. These criteria have been maintained to date despite advances in testing for bloodborne pathogens and currently include men who have sex with men, injection drug users, hemophiliacs, commercial sex workers, people who have high‐risk sex (i.e., sex with people in any of the foregoing groups), people who have been exposed to human immunodeficiency virus (HIV) through blood, and people who are incarcerated. Donors at high infectious risk have been reported to account for up to 9% of the donor population.23 Advances in testing (specifically nucleic acid testing for HIV and HCV), although more costly than traditional serological testing, have mitigated much of the posed infectious risk by significantly shortening the window period of exposure. Because of differences in the infectious risk between the various categories of high‐risk donors, the estimated risk of an undetected window‐period HIV infection ranges from 0.035 to 4.9 per 10,000 donors, whereas the risk of an undetected window‐period HCV infection ranges from 0.027 to 32.4 per 10,000 donors.23 It should be noted that although nucleic acid testing has been shown to be more sensitive for detection, it comes at the cost of a higher false‐positive rate.
Donors With a Cancer Risk
Donors with a previous history of malignancy require a thorough investigation before procurement with respect to the cancer type, the stage or grade, and any treatment or response. Although the compilation of these data in the immediate preprocurement time period can be difficult, it is beneficial to thoroughly carry out this effort in order to prevent not only a posttransplant malignancy of donor origin but also the alternative of passing on a potential organ when minimal risk is present. Thus, the decision to use a donor allograft with a previous malignancy should be individualized on the basis of the recipient's need and the potential risk of a donor‐derived malignancy in the posttransplant period.
Conclusion
In summary, despite technical advances and excellent outcomes, there remains a great disparity between supply and demand in the field of liver transplantation. This has prompted the transplant community to investigate avenues that have allowed the expansion of the donor population. These advances have included not only technical innovations leading to living donor liver transplantation and SLT but also the use of ECD liver allografts. Because of the increased potential risks associated with the various types of ECD allografts, a thorough discussion of the risks and benefits should be undertaken between the transplant team and the patient in advance of organ acceptance. With the careful selection of ECD liver donors and appropriate recipient matching, excellent survival results can be obtained with a demonstrated reduction of wait‐list mortality.
Potential conflict of interest: Nothing to report.
References
- 1. Kim WR, Stock PG, Smith JM, Heimbach JK, Skeans MA, Edwards EB, et al. OPTN/SRTR 2011 annual data report: liver. Am J Transplant 2013; 13(suppl 1): 73‐102. [DOI] [PubMed] [Google Scholar]
- 2. Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation 2011; 91: 479‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vagefi PA, Ascher NL, Freise CE, Dodge JL, Roberts JP. Use of living donor liver transplantation varies with the availability of deceased donor liver transplantation. Liver Transpl 2012; 18: 160‐165. [DOI] [PubMed] [Google Scholar]
- 4. Renz JF, Kin C, Kinkhabwala M, Jan D, Varadarajan R, Goldstein M, et al. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Ann Surg 2005; 242: 556‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barshes NR, Horwitz IB, Franzini L, Vierling JM, Goss JA. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant 2007; 7: 1265‐1270. [DOI] [PubMed] [Google Scholar]
- 6. Monbaliu D, Pirenne J, Talbot D. Liver transplantation using donation after cardiac death donors. J Hepatol 2012; 56: 474‐485. [DOI] [PubMed] [Google Scholar]
- 7. Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta‐analysis. Ann Surg 2011; 253: 259‐264. [DOI] [PubMed] [Google Scholar]
- 8. Selck FW, Grossman EB, Ratner LE, Renz JF. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased‐donor pool. Ann Surg 2008; 248: 599‐607. [DOI] [PubMed] [Google Scholar]
- 9. Harring TR, Nguyen NT, Cotton RT, Guiteau JJ, Salas de Armas IA, Liu H, et al. Liver transplantation with donation after cardiac death donors: a comprehensive update. J Surg Res 2012; 178: 502‐511. [DOI] [PubMed] [Google Scholar]
- 10. Jay C, Ladner D, Wang E, Lyuksemburg V, Kang R, Chang Y, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant—an analysis of the national registry. J Hepatol 2011; 55: 808‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dageforde LA, Feurer ID, Pinson CW, Moore DE. Is liver transplantation using organs donated after cardiac death cost‐effective or does it decrease waitlist death by increasing recipient death? HPB (Oxford) 2013; 15: 182‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singhal A, Sezginsoy B, Ghuloom AE, Hutchinson IV, Cho YW, Jabbour N. Orthotopic liver transplant using allografts from geriatric population in the United States: is there any age limit? Exp Clin Transplant 2010; 8: 196‐201. [PubMed] [Google Scholar]
- 13. Condron SL, Heneghan MA, Patel K, Dev A, McHutchison JG, Muir AJ. Effect of donor age on survival of liver transplant recipients with hepatitis C virus infection. Transplantation 2005; 80: 145‐148. [DOI] [PubMed] [Google Scholar]
- 14. Yersiz H, Renz JF, Farmer DG, Hisatake GM, McDiarmid SV, Busuttil RW. One hundred in situ split‐liver transplantations: a single‐center experience. Ann Surg 2003; 238: 496‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vagefi PA, Parekh J, Ascher NL, Roberts JP, Freise CE. Outcomes with split liver transplantation in 106 recipients: the University of California, San Francisco, experience from 1993 to 2010. Arch Surg 2011; 146: 1052‐1059. [DOI] [PubMed] [Google Scholar]
- 16. Spitzer AL, Lao OB, Dick AA, Bakthavatsalam R, Halldorson JB, Yeh MM, et al. The biopsied donor liver: incorporating macrosteatosis into high‐risk donor assessment. Liver Transpl 2010; 16: 874‐884. [DOI] [PubMed] [Google Scholar]
- 17. McCormack L, Dutkowski P, El‐Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol 2011; 54: 1055‐1062. [DOI] [PubMed] [Google Scholar]
- 18. Velidedeoglu E, Desai NM, Campos L, Olthoff KM, Shaked A, Nunes F, et al. The outcome of liver grafts procured from hepatitis C‐positive donors. Transplantation 2002; 73: 582‐587. [DOI] [PubMed] [Google Scholar]
- 19. Northup PG, Argo CK, Nguyen DT, McBride MA, Kumer SC, Schmitt TM, et al. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transpl Int 2010; 23: 1038‐1044. [DOI] [PubMed] [Google Scholar]
- 20. Lai JC, O'Leary JG, Trotter JF, Verna EC, Brown RS Jr, Stravitz RT, et al.; for Consortium to Study Health Outcomes in HCV Liver Transplant Recipients (CRUSH‐C). Risk of advanced fibrosis with grafts from hepatitis C antibody‐positive donors: a multicenter cohort study. Liver Transpl 2012; 18: 532‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti‐hepatitis B core positive donors: a systematic review. J Hepatol 2010; 52: 272‐279. [DOI] [PubMed] [Google Scholar]
- 22. Saidi RF, Jabbour N, Shah SA, Li YF, Bozorgzadeh A. Liver transplantation from hepatitis B surface antigen‐positive donors. Transplant Proc 2013; 45: 279‐280. [DOI] [PubMed] [Google Scholar]
- 23. Kucirka LM, Singer AL, Segev DL. High infectious risk donors: what are the risks and when are they too high? Curr Opin Organ Transplant 2011; 16: 256‐261. [DOI] [PubMed] [Google Scholar]


