Watch a video presentation of this article
Abbreviations
- AFP
alpha‐fetoprotein
- HCC
hepatocellular carcinoma
Hepatocellular carcinoma (HCC), while rare in childhood, is the second most common pediatric liver malignancy and commonly presents in school age and adolescent children.1 As in adults, HCC may occur in the setting of chronic hepatocyte injury caused by cholestatic liver, metabolic and viral (hepatitis B and C) diseases, or de novo without an antecedent history of liver disease. The pathogenesis of HCC is complex and involves genetic and environmental factors. Pathology demonstrates solitary or multifocal disease with a trabecular growth pattern, intervening sinusoid‐like vascular channels, and a reduced reticular network.1 A histologic variant is fibrolamellar HCC, which occurs in young adults and individuals without cirrhosis, yet has a poor prognosis similar to that of typical HCC.2 A novel group of HCC is the transitional liver cell tumors, which often present as large masses in older children and adolescents and histologically appear to be a transitional position between hepatoblastoma and HCC.3
In this review, we present an overview of the approach to a child with HCC, including recognition of patients with increased risk for the development of this malignancy, common presentation, appropriate workup, and treatment strategies. Although the mainstay of treatment is surgical resection, the role of neoadjuvant and adjuvant chemotherapy, embolization techniques, alternative medical therapy, and liver transplantation are also important considerations and may be used in the management of pediatric HCC.
Risk Factors in HCC
HCC can develop in children with underlying liver disease, including cirrhosis, viral hepatitis, and inborn metabolic disorders (Table 2). The most common cause of cirrhosis in children is biliary atresia, an obstructive cholangiopathy of the newborn that, if untreated, leads to end‐stage liver disease. With early recognition, successful drainage may be achieved with a Kasai portoenterostomy, but even with a successful Kasai, most develop cirrhosis. Patients treated for biliary atresia in childhood should be monitored for the development of HCC and cholangiocarcinoma as they transition into adulthood, even though the incidence of either is rare.4 Serum alpha‐fetoprotein (AFP) levels and ultrasound screening performed every 6–12 months should be used to monitor patients for malignant change.
Table 2.
Key Teaching Points
| Risk Factors | Presentation | Laboratory Evaluation | Imaging Evaluation | Management | Alternative Treatments |
|---|---|---|---|---|---|
| Cirrhosis, viral hepatitis, inborn metabolic diseases | Mass, abdominal pain | AFP, ferritin, CEA | Ultrasound, CT, or MRI; chest x‐ray or CT | Chemotherapy, surgical resection, liver transplantation | Sorafenib, RFA/TACE |
Abbreviations: CEA, carcinoembryonic antigen; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
Children afflicted with hereditary tyrosinemia, a disorder caused by a deficiency in the enzyme fumarylacetoacetase, present with neonatal liver disease and early development of HCC.5 Historically, hereditary tyrosinemia was managed by dietary modifications and liver transplantation. Currently, the cornerstone of treatment is nitisinone, a potent inhibitor of 4‐hydroxyphenylpyruvate dioxygenase, decreasing tyrosine degredation and subsequent production of the toxic metabolite succinylacetone. This drug, in combination with dietary restrictions, may delay but not prevent the development of HCC.6 Another metabolic disorder that predisposes for HCC in children is type I glycogen storage disease. A glucose‐6‐phosphatase deficiency leads to glycogen accumulation in the liver and the development of hepatocellular adenomas, which tend to appear during or after puberty and have the potential for malignant transformation.7 Children afflicted with type 2 progressive familial intrahepatic cholestasis, which is caused by mutations in the gene ABCB11, present with pruritus and jaundice due to a bile salt export pump deficiency and is another risk factor for the development of HCC in young children.8 The risk of developing HCC by 2 years of age is 5%‐10% in bile salt export pump deficiency patients, thus these children should be monitored closely.9, 10 Children who are diagnosed with these metabolic disorders or who have cirrhosis induced by chronic liver disease require frequent screening for HCC, with current guidelines recommending liver ultrasound and serum AFP levels every 6 months.10 A rising AFP value should prompt cross‐sectional imaging.
The majority of HCC cases arise de novo in children.11 This finding exemplifies the difference between pediatric and adult HCC, in which the majority occur in the setting of underlying cirrhosis. Furthermore, a reported 50% response rate to neoadjuvant chemotherapy and >50% survival rate after complete resection further support the difference between pediatric and adult HCC, in which no systematic therapy has been identified to improve survival and a high rate of recurrence after resection occurs.12, 13, 14 On a molecular level, pediatric HCC differs from adult tumors by a lower level of cyclin D1 and a higher loss of heterozygosity of chromosome 13q.15 The pathologic basis of pediatric HCC is arguably different than adult HCC; therefore, alternative therapies are warranted.
Presentation and Diagnosis
Children with HCC may present with an asymptomatic abdominal mass or, in more advanced stages, with abdominal, shoulder, and back pain with concomitant anorexia or emesis (Table 1). Laboratory evaluation includes detection of elevated levels of AFP, ferritin, and carcinoembryonic antigen. Imaging for workup may consist of ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI); chest x‐ray or CT is important to evaluate children with advanced disease, as pulmonary metastases are common. Differential diagnosis for a liver mass in the pediatric population encompasses benign lesions, including hemangiomas, mesenchymal hemartomas, focal nodular hyperplasia, and adenomas, as well as malignant tumors, most commonly hepatoblastoma or metastases from distant primaries. Biopsy of the mass is used to confirm diagnosis and can be done percutaneously through normal adjacent parenchyma to avoid tumor spread prior to initiating treatment for HCC.16
Management
Factors influencing prognosis in pediatric HCC are outlined in Table 1.11 One such factor is the pretreatment extent of disease (PRETEXT), a staging and risk stratification system for liver tumors designed by the International Childhood Liver Tumor Strategy Group (SIOPEL) to describe the radiographic appearance of tumors prior to any treatment.17 Treatment of HCC is multimodal and includes chemotherapy and surgical resection, though complete tumor excision is required for cure. Management is stage dependent. In contrast to adult HCC, in which chemotherapy has limited efficacy, neoadjuvant chemotherapy is employed in pediatric HCC, as some studies have shown it may increase the likelihood of tumor resectability with reported response rates to a combination of cisplantinum and doxorubicin approaching 50%.16 In a representative case (Fig. 1), a 6‐year‐old male diagnosed with extensive multifocal HCC was treated with neoadjuvant chemotherapy and had significant decrease in tumor burden and size of the largest mass. The response to therapy demonstrates the chemosensitivity seen in pediatric HCC. Adjuvant chemotherapy has also been given to stage I patients after complete resection; although the number of patients is small, excellent survival has been demonstrated.18 Side effects of this therapy are significant and include neutropenia, anemia, and thrombocytopenia. The prognosis of metastatic disease is dismal; chemotherapy may be employed as palliation, but there is no evidence that it prolongs life.2 An alternative and less toxic use of chemotherapy is hepatic artery chemoembolization, which allows for direct arterial delivery to the tumor resulting in ischemic necrosis. This widely employed technique in adult patients is feasible in children in select circumstances.
Table 1.
Significant Factors Affecting Overall and Event‐Free Survival11
| Parameter | χ2 | df | P | 5‐Year Overall Survival | |
|---|---|---|---|---|---|
| % | 95% CI | ||||
| Factors significant for overall survival | |||||
| PRETEXT | |||||
| I‐II | 6.6 | 2 | 0.09 | 44 | 16–72 |
| III | 22 | 0–49 | |||
| IV | 8 | 0–28 | |||
| Metastases | |||||
| Yes | 15 | 1 | 0.0001 | 9 | 0–26 |
| No | 37 | 19–57 | |||
| Multifocality | 4.52 | 1 | 0.03 | 11 | 0–26 |
| Unifocality | 45 | 21–70 | |||
| Factors significant for event‐free survival | |||||
| Metastases | |||||
| Yes | 17.4 | 1 | 0.0001 | 0 | 0–0 |
| No | 25 | 8–41 | |||
| Vascular Invasion | |||||
| Yes | 3.06 | 1 | 0.08 | 0 | 0‐0 |
| No | 27 | 4–49 | |||
PRETEXT refers to PREtreatment EXTent of disease, a staging and risk stratification system for liver tumors designed by the International Childhood Liver Tumor Strategy Group (SIOPEL) to describe the radiographic appearance of tumors prior to any treatment; X2 refers to Chi‐squared; df: discriminant function.
Figure 1.
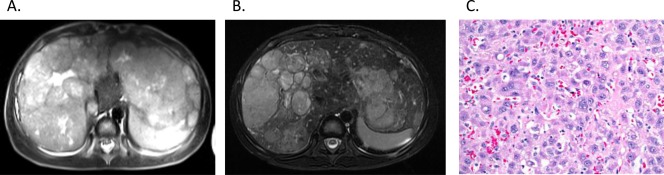
(A) A 6‐year‐old male diagnosed with extensive multifocal HCC and a large left lobe mass as demonstrated on T2‐weighted MRI underwent cisplatinum and doxorubicin therapy. (B) The tumor was chemosensitive, with follow‐up MRI demonstrating decreased tumor burden and size of the largest mass. The patient underwent successful liver transplantation. (C) Pathology demonstrates polygonal tumor cells with a vesicular nuclear chromatic pattern and prominent nucleoli with areas of individual cell apoptosis, consistent with therapeutic response.
For children with HCC localized to the liver, choices in operative management include complete surgical resection of the tumor by conventional surgery or hepatectomy and liver transplantation. Several studies have demonstrated favorable outcomes and equivalent survival rates after transplantation for unresectable HCC without extrahepatic spread.19 Furthermore, Romano et al.9 demonstrated favorable outcomes in children who underwent transplantation for HCC treatment with underlying cirrhosis. Transplantation may also be indicated in patients with tumor response to neoadjuvant chemotherapy that remain unresectable.19 The use of transplantation criteria based on Milan criteria is the accepted approach, but of late, several studies have reported pediatric patients successfully treated by transplantation that were well outside of University of California, San Francisco and Milan criteria. This includes an increased tumor size and angio‐invasion. Additional studies are needed to define pediatric criteria for transplantation.
Recurrence rates of HCC after resection and transplantation are not well delineated due to the rare occurrence of the disease and the likelihood for presentation in an advanced stage. In a study by Pham et al.,20 the recurrence rate for patients who underwent complete resection with negative margins was 45%. Kosola et al.19 examined the survival rates after transplantation and report a recurrence rate of 17% in their small, single site study. Overall survival after liver transplantation for HCC was examined through review of the United Network for Organ Sharing database and was found to be 86%, 63%, and 58% for 1‐, 5‐, and 10‐year patient survival, respectively.21
Children afflicted with advanced staged HCC require alternative strategies. Sorafenib, an oral inhibitor of the vascular endothelial growth factor receptor, platelet‐derived growth factor receptor, and of the Raf pathway, is the only systemic therapy in use for HCC shown to prolong survival. Its use has been approved by the US Food and Drug Administration for use in the treatment of adults with HCC and is being investigated in combination with transcatheter arterial chemoembolization. In a phase 1 trial, Widemann et al.22 investigated the pharmacokinetics of sorafenib in children. Studies are underway to examine the safety and efficacy of sorafenib in children, including a phase 2 trial currently being conducted on patients with relapsed or refractory HCC (ClinicalTrials.gov identifier NCT01502410).
Potential conflict of interest: Nothing to report.
References
- 1. Coran AG, Adzick NS. Pediatric Surgery. 7th ed. Philadelphia, PA: Elsevier Mosby; 2012. [Google Scholar]
- 2. Gupta AA, Gerstle JT, Ng V, Wong A, Fecteau A, Malogolowkin MH, et al. Critical review of controversial issues in the management of advanced pediatric liver tumors. Pediatr Blood Cancer 2011;56:1013–1018. [DOI] [PubMed] [Google Scholar]
- 3. Prokurat A, Kluge P, Kosciesza A, Perek D, Kappeler A, Zimmermann A. Transitional liver cell tumors (TLCT) in older children and adolescents: a novel group of aggressive hepatic tumors expressing beta‐catenin. Med Pediatr Oncol 2002;39:510–518. [DOI] [PubMed] [Google Scholar]
- 4. Fukuda A, Sakamoto S, Kanazawa H, Shigeta T, Karaki C, Hamano I, et al. Incidentally detected cholangiocarcinoma in an explanted liver with biliary atresia after Kasai operation. Pediatr Transplant 2013;17:E62–E66. [DOI] [PubMed] [Google Scholar]
- 5. van Spronsen FJ, Bijleveld CM, van Maldegem BT, Wijburg FA. Hepatocellular carcinoma in hereditary tyrosinemia type I despite 2‐(2 nitro‐4‐3 trifluoro‐ methylbenzoyl)‐1, 3‐cyclohexanedione treatment. J Pediatr Gastroenterol Nutr 2005;40:90–93. [DOI] [PubMed] [Google Scholar]
- 6. Carr BI. Hepatocellular carcinoma: diagnosis and treatment. 2nd ed. Totowa, NJ: Humana Press; 2010. [Google Scholar]
- 7. Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis 2005;28:153–162. [DOI] [PubMed] [Google Scholar]
- 8. Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 2006;44:478–486. [DOI] [PubMed] [Google Scholar]
- 9. Romano F, Stroppa P, Bravi M, Casotti V, Lucianetti A, Guizzetti M, et al. Favorable outcome of primary liver transplantation in children with cirrhosis and hepatocellular carcinoma. Pediatr Transplant 2011;15:573–579. [DOI] [PubMed] [Google Scholar]
- 10. Hadzic N, Finegold MJ. Liver neoplasia in children. Clin Liver Dise 2011;15:443–462. [DOI] [PubMed] [Google Scholar]
- 11. Czauderna P, Mackinlay G, Perilongo G, Brown J, Shafford E, Aronson D, et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol 2002;20:2798–2804. [DOI] [PubMed] [Google Scholar]
- 12. Czauderna P. Adult type vs. childhood hepatocellular carcinoma—are they the same or different lesions? Biology, natural history, prognosis, and treatment. Med Pediatr Oncol 2002;39:519–523. [DOI] [PubMed] [Google Scholar]
- 13. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 14. Lang H, Sotiropoulos GC, Brokalaki EI, Schmitz KJ, Bertona C, Meyer G, et al. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg 2007;205:27–36. [DOI] [PubMed] [Google Scholar]
- 15. Kim H, Lee MJ, Kim MR, Chung IP, Kim YM, Lee JY, et al. Expression of cyclin D1, cyclin E, cdk4 and loss of heterozygosity of 8p, 13q, 17p in hepatocellular carcinoma: comparison study of childhood and adult hepatocellular carcinoma. Liver 2000;20:173–178. [DOI] [PubMed] [Google Scholar]
- 16. Otte JB. Progress in the surgical treatment of malignant liver tumors in children. Cancer Treat Rev 2010;36:360–371. [DOI] [PubMed] [Google Scholar]
- 17. Roebuck DJ, Aronson D, Clapuyt P, Czauderna P, de Ville de Goyet J, Gauthier F, et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol 2007;37:123–132; quiz 249–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Liu‐Mares W, Douglass EC, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children's Cancer Group intergroup study. J Clin Oncol 2002;20:2789–2797. [DOI] [PubMed] [Google Scholar]
- 19. Kosola S, Lauronen J, Sairanen H, Heikinheimo M, Jalanko H, Pakarinen M. High survival rates after liver transplantation for hepatoblastoma and hepatocellular carcinoma. Pediatr Transplant 2010;14:646–650. [DOI] [PubMed] [Google Scholar]
- 20. Pham TH, Iqbal CW, Grams JM, Zarroug AE, Wall JC, Ishitani MB, et al. Outcomes of primary liver cancer in children: an appraisal of experience. J Pediatr Surg 2007;42:834–839. [DOI] [PubMed] [Google Scholar]
- 21. Austin MT, Leys CM, Feurer ID, Lovvorn HN 3rd, O'Neill JA Jr, Pinson CW, et al. Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 2006;41:182–186. [DOI] [PubMed] [Google Scholar]
- 22. Widemann BC, Kim A, Fox E, Baruchel S, Adamson PC, Ingle AM, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children's Oncology Group Phase I Consortium report. Clin Cancer Res 2012;18:6011–6022. [DOI] [PMC free article] [PubMed] [Google Scholar]


