Watch a video presentation of this article
The liver is a complex three‐dimensional structure that consists of epithelial and mesenchymal elements arranged in repetitive microscopic units. Such structural and functional organization allows assessment of diffuse disease processes in small representative biopsy specimens. This review describes the normal microanatomy of the liver with the aim of establishing a basic understanding that will help recognize patterns of injury in liver diseases.
Functional Units: Acinus and Lobule
The microscopic structure is conceptualized in several ways, the two most common being the acinus and the lobule. The acinus is a unit that contains a small portal tract at the center and terminal hepatic venules at the periphery. It is the smallest functional unit and is divided into zones 1, 2, and 3, wherein zone 1 surrounds the portal tract and zone 3 surrounds the hepatic venule. Blood from the portal tract flows though these zones to the venule with a decreasing oxygen and nutrient gradient. Alternatively, the traditional concept of lobule may also be used, in which the central structure is the terminal hepatic venule (“central vein”) and the periphery is delineated by portal tracts. Acinar zones 1, 2, and 3 correspond to the periportal, mid‐, and pericentral zones of the lobule, respectively1, 2 (Fig. 1).
Figure 1.
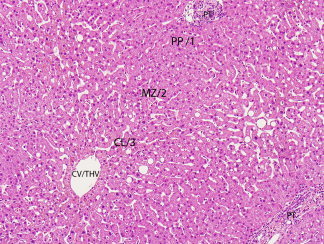
This photomicrograph illustrates the basic microscopic architecture, structural relationships, and terminology used to describe the microanatomy of the liver. The hepatocellular cords are mostly one or two layers thick and are divided into three zones: 1, 2, and 3 of the acinus, and periportal (PP), mid‐ (MZ), and centrilobular (CL) zones of the lobule. Blood flows from the portal tract (PT) to the central vein, also referred to as terminal hepatic venule (CV/THV) (hematoxylin and eosin stain, magnification ×100).
Portal Tracts
Portal tracts are channels that originate at the hilum and course through the liver in a branching pattern. Structures within these tracts include bile duct and ductules, hepatic artery, portal vein, lymphatic vessels, nerve fibers, and a few inflammatory cells (Fig. 2). The normal supporting connective tissue contains mainly type 1 collagen that stains blue with a trichrome stain.
Figure 2.
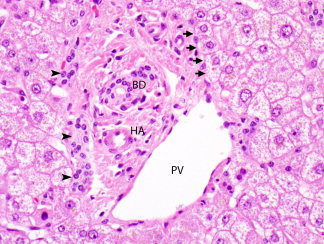
Normal portal tract with a bile duct (BD), hepatic artery (HA), portal vein (PV), and bile ductules located at the periphery (arrowheads). The limiting plate of hepatocytes surrounds the tract (arrows) (hematoxylin and eosin stain, magnification ×400).
Hepatocytes
Hepatocytes are normally arranged in cords that are one or two cells thick separated by sinusoids. Presence of thicker cords suggests regenerative activity, but in the setting of a lesion, may also indicate a neoplasm. Hepatocytes bordering portal tracts form a sheath‐like layer called the limiting plate (Fig. 2). In inflammatory conditions, injury to this layer indicates active hepatitis. The hepatocyte is polygonal, measures about 25 to 40 μm, and has abundant eosinophilic cytoplasm and a central nucleus. The nucleus is round or oval, and may contain glycogen, which is more common in certain conditions, such as young age, diabetes, and Wilson disease. The cell membrane partly faces the sinusoids (basolateral surface), forms the biliary canaliculus with the adjacent hepatocyte (canalicular surface), and has a portion adjacent to the canaliculus that attaches to the neighboring hepatocyte with specialized junctions (lateral surface). The cytoplasm contains abundant endoplasmic reticulum and glycogen, the latter highlighted with a periodic acid‐Schiff stain. Minimal iron may be normally be present, usually identified only with an iron stain. When increased, iron appears as brown refractile granules, similar to copper (Fig. 3). In hemochromatosis, iron deposition initially occurs within zone 1 hepatocytes, progressively extending to zone 3. In secondary iron overload, the distribution is mainly in Kupffer cells. In contrast, lipofuscin, the “wear and tear” pigment, accumulates mostly in zone 3 as a finely granular golden brown pigment (Fig. 4). Bile is normally not seen, but when present in cytoplasm, is green‐yellow and less granular than iron and lipofuscin. In cholestatic conditions, bile is more easily seen in the biliary canaliculi in zone 3 and may also be present in portal ductules and ducts (Figs. 3 and 4). The canaliculi drain into portal ductules through the canals of Hering, which are not seen on routine microscopy.1, 2, 3
Figure 3.
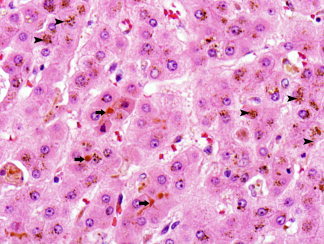
Hepatocyte cords with accumulation of brown coarsely granular iron (arrowheads) and greenish‐brown bile within biliary canaliculi (arrows). The canaliculi are located between hepatocytes (hematoxylin and eosin stain, magnification ×500).
Figure 4.
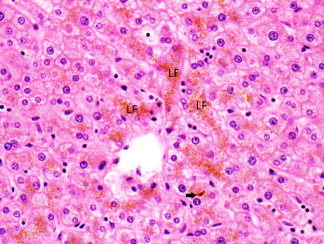
Hepatocyte cords with accumulation of golden brown finely granular lipofuscin pigment (LF). Bile is present in a canaliculus (arrow) (hematoxylin and eosin stain, magnification ×400).
Sinusoidal Lining Cells
Sinusoids are channels through which blood flows from portal tracts to the hepatic venule. They are lined by endothelial cells and Kupffer cells, the latter of which is a specialized population belonging to the macrophage phagocytic system. These cells rest on a layer of reticulin that is easily localized by a reticulin stain. The endothelial lining is plate‐like and fenestrated, allowing easy exchange between blood and hepatocytes through the space of Disse. In contrast, Kupffer cells are irregular, with cytoplasmic extensions that facilitate their phagocytic function (Fig. 5). Also lining the sinusoids are liver‐associated lymphocytes (pit cells), which are in contact with endothelial or Kupffer cells.4, 5 These cells are also present in the space of Disse and have a T lymphocyte or natural killer cell phenotype.
Figure 5.
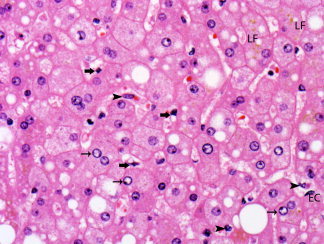
Sinusoids are lined by endothelial cells (EC) and Kupffer cells (arrowheads). Stellate (Ito) cells (thick arrows) are present in the space of Disse. Clear glycogenated nuclei (thin arrows) and lipofuscin pigment (LF) are also present. Hepatocytes show steatosis, with accumulation of small and large droplets of fat (hematoxylin and eosin stain, magnification ×500).
Space of Disse
The space of Disse is a compartment between endothelial cells and hepatocytes that contains the microenvironment for exchange between blood and hepatocytes. The space contains plasma, connective tissue (collagen type 3) forming the reticulin framework, and hepatic stellate cells (Ito cells) (Figs. 5 and 6). The stellate cells belong to the myofibroblast family and are closely related to small nerve endings. They play an important role in the control of sinusoidal blood flow, fibrogenesis, and storage of vitamin A.3, 4, 5
Figure 6.
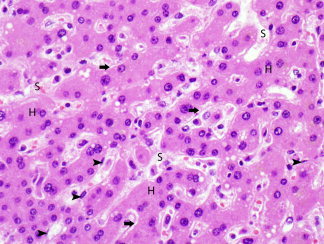
Area of regenerative change, characterized by mostly smaller hepatocytes (H) that are arranged in cords two or more cells thick, separated by sinusoids (S). The space of Disse is expanded (arrows). A few endothelial cells are also seen (arrowheads). (hematoxylin and eosin stain, magnification ×500).
Bile Ducts and Ductules
Bile ducts are present in portal tracts and are lined by cuboidal to columnar epithelium attached to a basement membrane. The smallest portal tract contains one or more bile ducts usually accompanied by an artery and a vein. The diameter of the duct approximates that of the artery. Ductules are located in the periphery of the tracts and transport bile from the canals of Hering to the duct (Fig. 2). They often proliferate in response to liver injury, particularly in conditions in which there is impairment of bile flow. The portal bile ducts anastomose to form larger ducts that exit at the hilum as right and left hepatic ducts. The ducts derive blood supply from a surrounding plexus supplied by the hepatic artery.2, 6
Vasculature
The hepatic arterial supply terminates in the smallest tracts as terminal hepatic arterioles and plexuses around the portal vein and bile duct. The portal vein terminates as terminal portal venules. Both arterial and venous blood from portal tracts flows across the limiting plate and through the sinusoids to drain into the terminal hepatic venule. These venules are lined by endothelium and are surrounded by a thin layer of collagen and elastic fibers. They combine to form larger segmental and lobar hepatic veins that drain into the inferior vena cava. Lymphatic drainage in the liver follows several different pathways. Lymph forms in the space of Disse and drains into the portal lymphatic vessels. These vessels form plexuses accompanying the artery, vein, and bile ducts, exiting at the porta hepatis to drain into the hepatic artery and celiac lymph nodes. Lymphatic drainage also occurs as a plexus around the hepatic veins and within the capsule of the liver. The plexuses are interconnected within the liver and follow multiple drainage routes, making liver the largest source of lymph in the body.
Normal Variations
Fibrous bands and expanded portal tracts are commonly present under the hepatic capsule and may be mistaken for a more diffuse process. Therefore, biopsies obtained for staging of liver disease should represent deeper tissue, preferably obtained with a core biopsy (Fig. 7). The liver is a major site of hematopoiesis during the first two trimesters of intrauterine development, and remnants of the process may be seen during the first few weeks after birth. During childhood the hepatic cords are thicker, nuclear glycogenation is more common, and hepatocyte nuclei are more uniform compared with adulthood.
Figure 7.
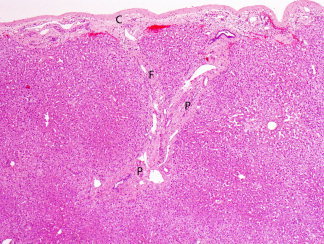
Capsule (C) and underlying subcapsular tissue. Expanded portal tracts (P) and bands of fibrosis (F) present in this region may be mistaken for advanced stage liver disease if these areas are included in biopsy specimens (hematoxylin and eosin stain, magnification ×40).
Potential conflict of interest: Nothing to report.
References
- 1. Suriawinata AA, Thung SN. Liver In: Mills SE, ed. Histology for Pathologists. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007: 685–703. [Google Scholar]
- 2. Crawford AR, Lin X‐Z, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology 1998; 28: 323–331. [DOI] [PubMed] [Google Scholar]
- 3. Roskams T, Desmet VJ, Verslype C. Development, structure and function of the liver In: Burt AD, Portmann BC, Ferrell LD, eds. MacSween's Pathology of the Liver. 5th ed. Edingurgh,UK: Churchill Livingstone/Elsevier; 2007: 1–74. [Google Scholar]
- 4. Saxena R, Theise ND, Crawford JM. Microanatomy of the human liver–exploring the hidden interfaces. Hepatology 1999; 30: 1339–1346. [DOI] [PubMed] [Google Scholar]
- 5. Wisse E, Braet F, Luo D, De Zanger R, Jans D, Crabbé E, et al. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol 1996; 24: 100–111. [DOI] [PubMed] [Google Scholar]
- 6. Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec 2008; 291: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]


