Watch a video presentation of this article
Watch the interview with the author
Perihilar or hilar cholangiocarcinoma is an adenocarcinoma of the bile ducts arising from the main right or left hepatic ducts or their biliary confluence.1 Perihilar cholangiocarcinoma is associated with a poor prognosis and a 5‐year overall survival rate less than 10%.2 Surgical resection is the only potentially curative treatment for perihilar cholangiocarcinoma.1 Eighty to ninety percent of patients, however, are not surgical candidates and should be considered for palliative therapies, including biliary drainage, most commonly with endoscopic stents.2, 3, 4 Percutaneous biliary drainage can be considered as well but is less comfortable for the patient, often requires an external drainage bag, and usually requires more maintenance than endoscopic stents. Palliative endoscopic biliary drainage leads to improved quality of life by relieving jaundice and its associated symptoms, such as diarrhea, sleep pattern disturbances, anorexia, and pruritus.2, 5, 6 This review is focused on the utility of metal stents versus plastic stents (PSs) and unilateral stents versus bilateral stents in these patients.
Metal Stents Versus PSs
For more than 30 years, PSs have been used to provide endoscopic biliary drainage.7, 8, 9 PSs are easy to insert, are removable, and are less expensive than self‐expanding metal stents (SEMSs), although PSs are much narrower.9 PSs are still widely used for perihilar cholangiocarcinoma, especially in pretransplant patients (Fig. 1). Unfortunately, PSs have a shorter period of patency than SEMSs, and clogging can progress to jaundice and/or cholangitis.10 PS clogging is thought to be multifactorial and associated with bacterial contamination of undrained bile ducts.9, 11, 12 PSs may be a viable option for patients with a short life expectancy (usually defined as less than 3 months). The prophylactic administration of antibiotics, the administration of ursodeoxycholic acid, and the utilization of wider PSs have been evaluated as ways of reducing PS clogging, but the benefits have been limited.9, 13, 14
Figure 1.
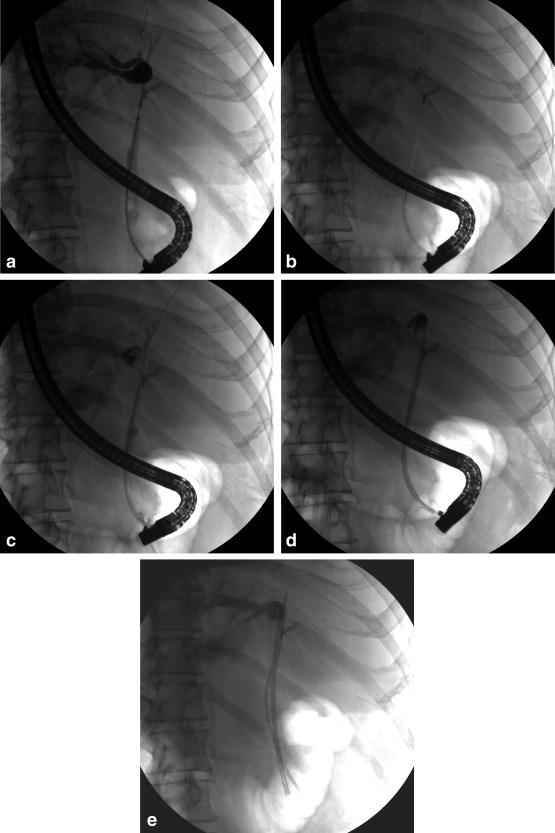
Bilateral PS placement for perihilar cholangiocarcinoma in a pretransplant patient. (A) An occlusion cholangiogram shows a tight hilar stricture. (B) With two separate guidewires, bilateral biliary access is obtained. (C) Contrast injection confirms good bilateral wire placement. (D) A long 8.5‐Fr PS is placed into the left biliary system. (E) A long 7‐Fr PS is placed into the right biliary system. The patient now has bilateral biliary drainage.
SEMSs were developed to overcome the limitations of PSs.9, 15 In patients with hilar obstructions, uncovered SEMSs are generally used to prevent obstruction of the contralateral biliary system.9 SEMSs have been found to have higher insertion and patency rates than PSs. SEMSs are typically placed in patients with unresectable disease (Fig. 2). Raju et al.16 found that the median patency times were 1.86 months with PSs and 5.56 months with SEMSs (P < 0.0001) in patients with cholangiocarcinoma. On average, 4.60 and 1.52 reinterventions were performed for the PS and SEMS groups, respectively. In a study comparing biliary drainage in stents, SEMS patients had a higher rate of successful drainage than patients with PSs (70.4% versus 46.3%, P = 0.011).17 In another study comparing SEMSs to PSs in cholangiocarcinoma, long‐term stent failure (>30 days) occurred in 50% of the PS patients and in 18.2% of the SEMS patients, although the results did not reach statistical significance.18
Figure 2.
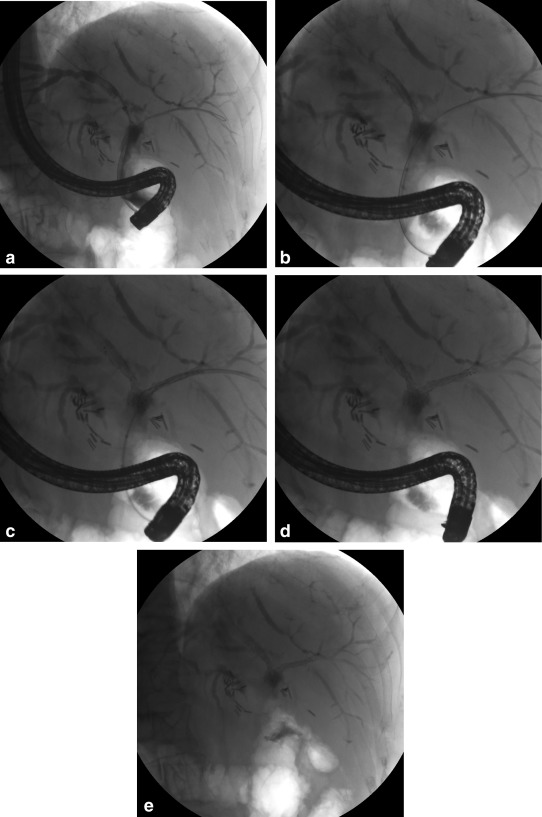
Bilateral SEMS placement in a patient with unresectable perihilar cholangiocarcinoma. (A) A cholangiogram reveals a malignant obstruction of the left hepatic duct, right hepatic duct, and bifurcation. Bilateral guidewire access is obtained. (B) An SEMS is deployed across the left hepatic duct. (C) An SEMS is deployed across the right hepatic duct. (D) The appearance of bilateral stents immediately after their deployment is shown. (E) The final appearance of the stents after endoscope withdrawal is shown (note the lower magnification).
SEMSs are associated with fewer adverse events than PSs. Adverse outcomes (stent occlusion, migration, cholangitis, perforation, and a need for unplanned endoscopic retrograde cholangiopancreatography/percutaneous transhepatic cholangiography) were measured in a study by Perdue et al.10 Adverse outcomes occurred in 39.3% of the PS patients and in 11.8% of the SEMS patients (P = 0.017).
Unilateral Stents Versus Bilateral Stents
The need for bilateral stents versus unilateral stents in all patients with cholangiocarcinoma is debatable. Unilateral stent placement is sufficient in many cases because only 25% to 30% of the liver needs to be drained to relieve jaundice.19 However, many centers today prefer bilateral stents because they are more effective in terms of biliary drainage and cumulative stent patency.
A retrospective study of 106 patients by Vienne et al.20 examined the volume of liver drainage with respect to outcomes. That study found that draining more than 50% of the liver volume, which frequently required bilateral stent placement, was associated with longer median survival for patients with hilar cholangiocarcinoma (119 versus 59 days, P = 0.005). The study also found that patients were less likely to develop cholangitis when the drained liver volume was more than 50% (55% versus 30%, P = 0.03).
In a study of 46 patients comparing bilateral and unilateral SEMSs, the cumulative stent patency period was significantly longer for the bilateral stent group (488 versus 210 days).21 Superior cumulative stent patency rates with bilateral SEMS or PS placement were also seen in a retrospective study by Liberato and Canena22 when they compared them to unilateral SEMSs or PSs. If bilateral stents are used, preprocedural imaging (computed tomography and/or magnetic resonance imaging with magnetic resonance cholangiopancreatography) is recommended in order to identify the dominant biliary system in the event that only one side can be drained.
Complications
Despite the advantages of SEMSs, these devices can become clogged by stones or become obstructed via tumor ingrowth or overgrowth.10, 13, 22, 23, 24 A recent retrospective study by Siddiqui et al.25 found that metal stent occlusion in cholangiocarcinoma was directly related to the stage of disease, with more advanced tumors resulting in faster SEMS occlusion. Age, sex, stricture length, and SEMS diameter and length were not associated with SEMS patency. Other complications of biliary stenting have included stent migration, pancreatitis, cholecystitis, cholangitis, perforation, fistula formation, and bleeding.10, 22, 26, 27
Conclusions
Perihilar cholangiocarcinoma is associated with a poor prognosis. The majority of patients are not surgical candidates and require palliation. Biliary stenting is commonly used for the palliation of perihilar cholangiocarcinoma and is associated with improved quality of life. Currently, uncovered SEMSs and PSs are used for the endoscopic drainage of perihilar cholangiocarcinoma. In comparison with PSs, SEMSs are associated with fewer complications. Our general preference is to use metal stents in these patients because of their superior patency rates, although we use PSs in some patients in accordance with the overall clinical situation. Although unilateral stent placement provides sufficient drainage in many cases, bilateral stents are recommended when they are possible in order to maximize biliary drainage.
Abbreviations
- PS
plastic stent
- SEMS
self‐expanding metal stent.
Potential conflict of interest: Nothing to report.
References
- 1. Lau SH, Lau WY. Current therapy of hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2012;11:12‐17. [DOI] [PubMed] [Google Scholar]
- 2. Cheng JL, Bruno MJ, Bergman JJ, Rauws EA, Tytgat GN, Huibregtse K. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self‐expandable metallic Wallstents. Gastrointest Endosc 2002;56:33‐39. [DOI] [PubMed] [Google Scholar]
- 3. Stain SC, Baer HU, Dennison AR, Blumgart LH. Current management of hilar cholangiocarcinoma. Surg Gynecol Obstet 1992;175:579‐588. [PubMed] [Google Scholar]
- 4. Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg 1988;12:39‐47. [DOI] [PubMed] [Google Scholar]
- 5. Luman W, Cull A, Palmer KR. Quality of life in patients stented for malignant biliary obstructions. Eur J Gastroenterol Hepatol 1997;9:481‐484. [DOI] [PubMed] [Google Scholar]
- 6. Ballinger AB, McHugh M, Catnach SM, Alstead EM, Clark ML. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut 1994;35:467‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soehendra N, Reynders‐Frederix V. Palliative bile duct drainage—a new endoscopic method of introducing a transpapillary drain. Endoscopy 1980;12:8‐11. [DOI] [PubMed] [Google Scholar]
- 8. Donelli G, Guaglianone E, Di Rosa R, Fiocca F, Basoli A. Plastic biliary stent occlusion: factors involved and possible preventive approaches. Clin Med Res 2007;5:53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park YJ, Kang DH. Endoscopic drainage in patients with inoperable hilar cholangiocarcinoma. Korean J Intern Med 2013;28:8‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perdue DG, Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, et al. Plastic versus self‐expanding metallic stents for malignant hilar biliary obstruction: a prospective multicenter observational cohort study. J Clin Gastroenterol 2008;42:1040‐1046. [DOI] [PubMed] [Google Scholar]
- 11. Freeman ML, Sielaff TD. A modern approach to malignant hilar biliary obstruction. Rev Gastroenterol Disord 2003;3:187‐201. [PubMed] [Google Scholar]
- 12. Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9:43‐57. [DOI] [PubMed] [Google Scholar]
- 13. Libby ED, Leung JW. Prevention of biliary stent clogging: a clinical review. Am J Gastroenterol 1996;91:1301‐1308. [PubMed] [Google Scholar]
- 14. Galandi D, Schwarzer G, Bassler D, Allgaier HP. Ursodeoxycholic acid and/or antibiotics for prevention of biliary stent occlusion. Cochrane Database Syst Rev 2002:CD003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neuhaus H, Hagenmüller F, Classen M. Self‐expanding biliary stents: preliminary clinical experience. Endoscopy 1989;21:225‐228. [DOI] [PubMed] [Google Scholar]
- 16. Raju RP, Jaganmohan SR, Ross WA, Davila ML, Javle M, Raju GS, et al. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self‐expanding metal stents. Dig Dis Sci 2011;56:1557‐1564. [DOI] [PubMed] [Google Scholar]
- 17. Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc 2012;76:93‐99. [DOI] [PubMed] [Google Scholar]
- 18. Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy 1993;25:213‐218. [DOI] [PubMed] [Google Scholar]
- 19. Dowsett JF, Vaira D, Hatfield AR, Cairns SR, Polydorou A, Frost R, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology 1989;96:1180‐1186. [DOI] [PubMed] [Google Scholar]
- 20. Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc 2010;72:728‐735. [DOI] [PubMed] [Google Scholar]
- 21. Naitoh I, Ohara H, Nakazawa T, Ando T, Hayashi K, Okumura F, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol 2009;24:552‐557. [DOI] [PubMed] [Google Scholar]
- 22. Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol 2012;12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loew BJ, Howell DA, Sanders MK, Desilets DJ, Kortan PP, May GR, et al. Comparative performance of uncoated, self‐expanding metal biliary stents of different designs in 2 diameters: final results of an international multicenter, randomized, controlled trial. Gastrointest Endosc 2009;70:445‐453. [DOI] [PubMed] [Google Scholar]
- 24. Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self‐expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet 1992;340:1488‐1492. [DOI] [PubMed] [Google Scholar]
- 25. Siddiqui A, Shahid H, Sarkar A, Cox K, Kowalski TE, Loren DE, et al. Stage of hilar cholangiocarcinoma predicts recurrence of biliary obstruction in patients with metal stents. Clin Gastroenterol Hepatol 2013;11:1169‐1173. [DOI] [PubMed] [Google Scholar]
- 26. Yoon WJ, Ryu JK, Yang KY, Paik WH, Lee JK, Woo SM, et al. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost‐effectiveness in a country with a low ERCP cost. Gastrointest Endosc 2009;70:284‐289. [DOI] [PubMed] [Google Scholar]
- 27. Moon SK, Cheung DY, Kim JH, Im EJ, Ha JH, Kim JI, et al. A case of choledochoduodenal fistula as a delayed complication after biliary metallic stent placement in distal cholangiocarcinoma [in Korean]. Korean J Gastroenterol 2008;51:314‐318. [PubMed] [Google Scholar]


