Watch a video presentation of this article
Watch the interview with the author
Introduction
As discussed in companion articles in CLD, the clinical course of large duct primary sclerosing cholangitis (PSC) is highly variable and unpredictable.1, 2 Although the median survival from presentation to death or liver transplantation in symptomatic patients is approximately 10‐12 years, 75% of asymptomatic patients will survive 15 years or more. A recent Dutch study has shown an overall median survival of 22 years in PSC patients.3
In the past, a majority of patients have died of hepatic failure following deepening, cholestatic jaundice.2 However, with the advent of successful transplantation, the majority of patients with large duct PSC die of hepatobiliary malignancy (cholangiocarcinoma (CCA), gall bladder cancer, or hepatoma in cirrhosis patients) or colonic adenocarcinoma (Fig. 1).4 One study has suggested an increase in the risk of pancreatic adenocarcinoma in PSC, but this has not been confirmed.4 However, it is apparent that despite an association with nonsmoking, large duct PSC is a premalignant disease. In contrast, small duct PSC has not been associated with an increased risk of either biliary or colonic neoplasia and has a much better prognosis.2
Figure 1.
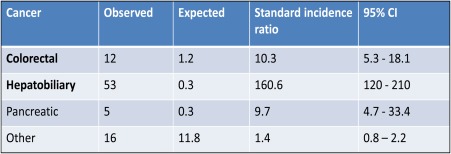
Standard incidence ratio for first cancer after diagnosis in 604 patients with PSC. Reprinted with permission from Journal of Hepatology.4 Copyright 2002, Elsevier.
Carcinogenesis
It is possible that there may be a direct causal genetic contribution of PSC itself to the development of cancer, given its association with the increased risk of cancers in different sites, as discussed previously.1, 2
Various hypotheses have been put forward as an explanation of the increased cancer risk, including an association with abnormal expression of the tumor suppression gene p53, and alternatively, promotion of the expression of sialosyl‐Tn antigen, an antigen that precedes dysplasia formation. It has also been suggested that antinuclear cytoplasmic antibodies, which are prevalent in PSC, may represent an immunological cause of/contribution to carcinogenesis.
It is established that PSC is a premalignant condition that requires long‐term regular surveillance of the hepatobiliary system and colon.5
Hepatobiliary Cancer
Approximately 10%‐30% of patients with large duct PSC die from the development of bile duct carcinoma, which often follows a very aggressive course. Approximately 0.5%‐1.0% of patients with large duct PSC will develop cholangiocarcinoma (CCA) or gall bladder cancer every year (Fig. 2).5 Unfortunately, at present there are no factors that will predict which patients will develop this cancer. Tumor markers such as CEA and CA 19‐9 have been investigated as potential serum markers of the development of bile duct cancer in PSC. Although some centers have found elevations in serum CA 19‐9 a useful predictor, these results have not been confirmed in other investigators.5
Figure 2.
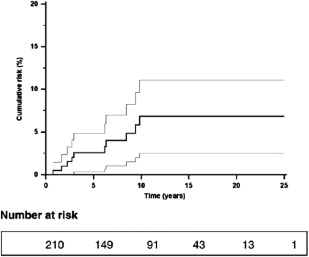
Cumulative risk (95% confidence interval) of CCA for 211 patients with PSC. Reprinted with permission from Journal of Hepatology. Copyright 2009, Elsevier.
The diagnosis of suspected CCA in PSC is often difficult to establish, as brush cytology of biliary strictures performed at endoscopic retrograde cholangiopancreatography (ERCP) is often nonconfirmatory, with unacceptably low levels of sensitivity and specificity obtained in most centers. Fluorescence in situ hybridization (FISH) has been advocated as increasing the sensitivity and sensitivity of cytological analysis, although it is not widely practiced.5
The mean survival after diagnosis of CCA in PSC is only 9 months, although liver transplantation after chemotherapy is possible in selected patients with small tumors (<3.0 cm) and no evidence of metastatic disease. In this highly selected group, survival is comparable to PSC without CCA, as evidenced by a 70%‐80% 5‐year survival posttransplantation. In PSC patients who are found to have unsuspected bile duct cancers at the time of liver transplantation, there is an approximately 30% 5‐year survival.
Annual surveillance with abdominal ultrasound is recommended in both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) PSC guidelines, to exclude the presence of gallbladder polyps which are premalignant.6, 7 Patients with gallbladder polyps should be treated with laporoscopic cholecystectomy. Annual magnetic resonance cholangiopancreatography (MRCP) surveillance for CCA is being investigated, although not yet proven to be of benefit.5 End‐stage patients with cirrhosis are at increased risk of developing hepatocellular carcinoma, although the risk in PSC cirrhosis appears to be small.5
Colorectal Cancer
There is a very close association between PSC and inflammatory bowel disease (IBD), particularly ulcerative colitis (UC).1, 2 Since the early 1990s, evidence has accumulated showing the presence of PSC to be an important additional risk factor for the development of colorectal neoplasia (CRN), dysplasia or cancer in patients with associated IBD (Fig. 3).8 This increased risk has been estimated by a meta‐analysis of 11 studies to be 4‐10 times greater than risk of developing colorectal carcinoma (CRC) in patients with UC without PSC and develops at a much younger age compared with patients with UC alone.9
Figure 3.
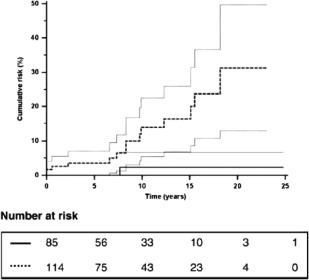
Cumulative risk (95% CI) of colorectal cancer for 211 patients with PSC with ( ) or without (
) or without ( ) concurrent IBD Reprinted with permission from Journal of Hepatology. Copyright 2009, Elsevier.
) concurrent IBD Reprinted with permission from Journal of Hepatology. Copyright 2009, Elsevier.
PSC may be the most important risk factor in the development of CRC in UC. This is becoming increasingly important with the advent of orthotopic liver transplantation for patients with PSC, as the risk of CRC continues and may increase after transplantation, with the need for prolonged immunosuppression. Unlike patients with UC alone, the dysplastic lesions/cancers are present more commonly in the right colon close to the ileocecal valve.8
There are fewer studies with regard to the CRN risk in patients with PSC with associated Crohn's colitis compared with UC, and evidence is conflicting as to whether there may be an increased risk of CRN in PSC patients with Crohn's colitis. However, there are only two retrospective single‐center case‐controlled studies with conflicting results, and further studies are required.
Retrospective studies have provided conflicting results in assessing the efficacy of ursodeoxycholic acid (UDCA) in reducing the risk of CRC in patients with PSC and UC. However, although there have been no prospective, controlled trials investigating the role of UDCA in chemoprevention, recent meta‐analyses have suggested that low‐dose UDCA may have a positive chemoprotective effect.10
Annual screening colonoscopy with multiple colonic biopsies and/or chromoendoscopy as soon as a diagnosis of PSC is established is recommended in all patients with PSC/IBD (UC and Crohn's colitis) both in the AASLD and EASL PSC guidelines.6, 7 In patients without associated IBD, a 5‐yearly colonoscopy is recommended, even in the absence of symptoms of colitis.6, 7
The reason why the incidence of right‐sided CRN is increased in patients with UC and PSC remains unclear.8 It is particularly surprising in light of the generally low levels of colonic inflammation in PSC/UC compared with UC patients alone. It has been suggested that it may be due to a deleterious effect of increased concentration of hydrophobic bile acids such as lithocholic acid in the right colon, which may be carcinogenic in nature.8
Abbreviations
- CCA
cholangiocarcinoma
- CRC
colorectal carcinoma
- CRN
colorectal neoplasia
- IBD
inflammatory bowel disease
- PSC
primary sclerosing cholangitis
- UC
ulcerative colitis
- UDCA
ursodeoxycholic acid
Potential conflict of interest: Nothing to report.
References
- 1. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet 2013;382:1587‐1599. [DOI] [PubMed] [Google Scholar]
- 2. Karlsen TH, Boberg KM. Update on primary sclerosing cholangitis. J Hepatol 2013;59:517‐582. [DOI] [PubMed] [Google Scholar]
- 3. Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, et al. Population‐based epidemiology,malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013;58:2045‐2055. [DOI] [PubMed] [Google Scholar]
- 4. Bergquist A, Broome U. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 2002;36:321‐327. [DOI] [PubMed] [Google Scholar]
- 5. Razumilava N, Gores GJ, Lindor K. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011;54:1842‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapman RW, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis (PSC). Hepatology 2010;51:660‐678. [DOI] [PubMed] [Google Scholar]
- 7. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009;51:237‐267. [DOI] [PubMed] [Google Scholar]
- 8. Torres J, de Chambrun GP, Itzowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther 2011;34:497‐508. [DOI] [PubMed] [Google Scholar]
- 9. Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta‐analysis. Gastrointest Endosc 2002;56:48‐54. [DOI] [PubMed] [Google Scholar]
- 10. Singh S, Khanna S, Pardi DS, Loftus EV, Talwalkar JA. Effect of ursodeoxycholic acid use on the risk of colorectal neplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta‐analysis. Inflamm Bowel Dis 2013;9:1631‐1638. [DOI] [PubMed] [Google Scholar]


