Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- CA19‐9
carbohydrate antigen 19‐9
- CCA
cholangiocarcinoma
- CI
confidence interval
- CK
cytokeratin
- eCCA
extrahepatic cholangiocarcinoma
- FISH
fluoresence in situ hybridization
- HR
hazard ratio
- iCCA
intrahepatic cholangiocarcinoma
- PSC
primary sclerosing cholangitis.
Cholangiocarcinoma (CCA) is defined anatomically as intrahepatic CCA (iCCA), perihilar CCA, and distal CCA. The prognosis of patients with CCA is poor given that the majority of patients are diagnosed at advanced stages, when treatment is less effective. The diagnosis of CCA is complex and is made with a combination of appropriate clinical suspicion, imaging techniques, endoscopic techniques, and cytopathological examination. However, the late‐stage detection and poor prognosis of this tumor has led to an urgent need for biomarkers for the early diagnosis of CCA. We herein discuss the most promising markers for this tumor.
Serum Markers
A list of currently available serum markers and those that are promising is provided in Table 1. Carbohydrate antigen 19‐9 (CA19‐9), the most commonly used serum marker in clinical practice, is a sialylated Lewis blood group antigen targeted by the monoclonal antibody described in 1979 as a tumor‐associated antigen in a colorectal cancer cell line. CA19‐9 has a wide variation in sensitivity (50%‐90%) and specificity (54%‐98%)1 and is often falsely elevated in benign biliary disease and/or cholangitis, with levels falling after relief of biliary obstruction and sepsis. However, very high levels of CA19‐9 (≥1000 U/mL) have been associated with metastatic iCCA, so this assay might be used in disease staging rather than diagnosis. In patients with primary sclerosing cholangitis (PSC), CA19‐9 has been shown to have a sensitivity of 75% and a specificity of 80% in diagnosing extrahepatic CCA (eCCA). For the diagnosis of iCCA, the sensitivity and specificity of CA19‐9 is 62% and 63%, respectively.2 However, CA19‐9 should not be used alone for the diagnosis of iCCA or eCCA. In a recent study of patients with PSC, the combination of cytopathological examination with fluoresence in situ hybridization (FISH) and an elevated CA19‐9 (> 126 U/mL) level was found to be predictive of cancer.3
Cytokeratins (CKs) are intermediate filaments that are an essential part of the cytoskeleton of epithelial cells. Twenty different CK polypeptides have been identified, and CK‐19 has been shown to be associated with CCA. It appears that CK‐19 is more specific for iCCA than for other tumor types, but it also lacks sensitivity for the diagnosis of this tumor.4 In some studies, CK‐19 is a useful marker not only for detecting iCCA early but also for distinguishing iCCA from hepatocellular carcinoma. In fact, hepatocytes contain CK‐8 and ‐18, while bile duct cells contain CK‐7, ‐8, ‐18, and ‐19; therefore, this information can be used for distinguishing these primary tumors in the liver.5 However, CKs need further validation in order to be used clinically, as do other serum markers currently under investigation.
Cytology
The distinction between benign biliary strictures and eCCA is difficult due to the desmoplastic reaction around them and the confounding nature of having concomitant cholangitis. Up to 80% of biliary malignancies exhibit chromosomal abnormalities in the number of chromosomes within the cancer cell, and FISH uses fluorescently labeled DNA probes to detect aneusomy of individual cells. FISH from biliary brushings and/or fine needle aspiration targets the centromeric regions of chromosomes 3 (CEP3), 7 (CEP7), and 17 (CEP17), and band 9p21 (P16/CDKN2A gene). Polysomy of chromosomes 3 and 7 has been shown to have a sensitivity of 46% and a specificity of 88% in patients with biliary stricture and PSC.6 A recent meta‐analysis of the role of FISH for the diagnosis of eCCA in patients with PSC showed that FISH polysomy has a sensitivity of 51% (95% confidence interval [CI], 43%‐59%) and a specificity of 93% (95% CI, 91%‐95%) as shown in Fig. 1.7 This test is now recommended in the evaluation of patients who have biliary strictures, especially if they have PSC.
Figure 1.
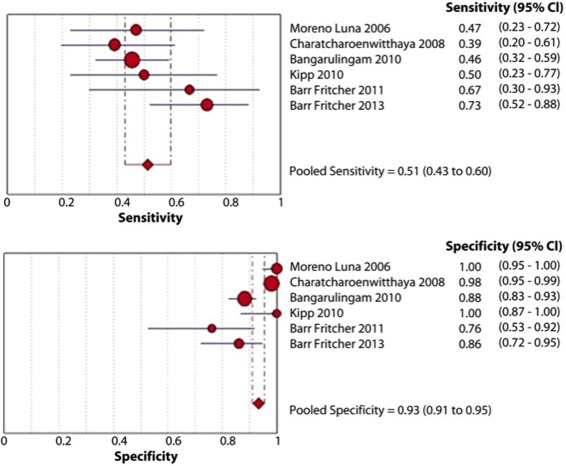
Studies reporting the diagnostic role of any FISH polysomy. Reprinted with permission from Gastrointestinal Endoscopy.7 Copyright 2013, Mosby Yearbook.
Tissue
Tissue‐based biomarkers are not available for early detection but may provide information about diagnosis as well as prognosis. A recent meta‐analysis of tissue markers for the prognosis of CCA, as measured by immuno‐histochemistry, evaluated the existing literature.8 Fourteen studies were graded with a low risk of bias and were deemed high quality; the biomarkers prognostic of overall survival in a pooled analysis included fascin (hazard ratio [HR], 2.58; 95% CI, 1.19‐5.58), epidermal growth factor (HR, 1.79; 95% CI, 1.14‐2.8), MUC1 (HR, 2.52; 95% CI, 1.49‐4.26), MUC4 (HR, 2.45; 95% CI, 1.56‐3.86), and p27 (HR, 0.29; 95%, CI 0.14‐0.6). Other markers showed promising results in single pilot studies, including HSP27, Akt, HDGF, MUC6, p16, p4EBP1, S100A4, a‐SMA, keratin 903, and TROP2. These are only potential biomarkers, however, and need further validation for clinical use.
Bile
Bile has been studied for identifying CCA‐specific biomarkers. Because bile is more proximal to the tumor and is in intimate contact with the tumor, it may provide better biomarkers for the diagnosis of this deadly tumor. CA19‐9, insulin growth factor, and pancreatic amylase/elastase ratio are markers identified in bile that appear to be promising.9 The collection of bile samples can only be performed by using invasive techniques; consequently, their use for screening purposes or for the surveillance of a population at risk is limited, but the information may prove useful as an adjunct to cytopathology and/or endoscopic procedures. Further validation studies are needed.
Table 1.
Serum Markers in CCA
| Markers | Sensitivity | Specificity |
|---|---|---|
| CA19‐9 | ↑↑↑ | ↑↑↑ |
| Carcino‐embryonic antigen | ↑ | ↑ |
| Total sialic acid | ↑ | ↑ |
| C reactive protein | ↑ | ↑ |
| Cytokeratin fragment 21‐1 | ↑↑ | ↑↑ |
| Transforming growth factor β | ↑ | ↑ |
| Chromogranin A | ↑ | ↑ |
| Mucins | ↑↑ | ↑↑ |
| Metalloproteinase‐7 | ↑↑ | ↑↑ |
| Serotonin | ↑ | ↑ |
Potential conflict of interest: Nothing to report.
References
- 1. Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19‐9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol 2000;95:204‐207. [DOI] [PubMed] [Google Scholar]
- 2. Tao LY, Cai L, He XD, Liu W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Am Surg 2010;76:1210‐1213. [PubMed] [Google Scholar]
- 3. Barr Fritcher EG, Voss JS, Jenkins SM, Lingineni RK, Clayton AC, Roberts LR, et al. Primary sclerosing cholangitis with equivocal cytology: fluorescence in situ hybridization and serum CA 19‐9 predict risk of malignancy. Cancer Cytopathol 2013;121:708‐717. [DOI] [PubMed] [Google Scholar]
- 4. Uenishi T, Kubo S, Hirohashi K, Tanaka H, Shuto T, Yamamoto T, et al. Cytokeratin‐19 fragments in serum (CYFRA 21‐1) as a marker in primary liver cancer. Br J Cancer 2003;88:1894‐1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tao LY, Cai L, He XD, Lui W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Br J Cancer 2010;76:1210‐1213. [PubMed] [Google Scholar]
- 6. Bangarulingam SY, Bjornsson E, Enders F, Barr Fritcher EG, Gores G, Halling KC, et al. Long‐term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology 2010;51:174‐180. [DOI] [PubMed] [Google Scholar]
- 7. Navaneethan U, Njei B, Venkatesh PG, Vargo JJ, Parsi MA. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta‐analysis. Gastrointest Endosc 2013. doi: 10.1016/j.gie.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 8. Ruys AT, Groot Koerkamp B, Wiggers JK, Klümpen HJ, Ten Kate FJ, van Gulik TM. Prognostic biomarkers in patients with resected cholangiocarcinoma: a systematic review and meta‐analysis. Ann Surg Oncol 2013. PMID: 24081803. [DOI] [PubMed] [Google Scholar]
- 9. Alvaro D. Serum and bile markers for cholangiocarcinoma. Curr Opin Gastroenterol 2009;25:279‐284. [DOI] [PubMed] [Google Scholar]


