Watch a video presentation of this article
Introduction
The coagulopathy of cirrhosis and portal hypertension is complicated and often challenging to predict.1, 2 While patients with cirrhosis are clearly prone to bleed, there is often a concurrent paradoxical propensity toward thrombosis.3 A finely regulated balance between bleeding and thrombosis is maintained in the healthy individual. When vascular breach or injury occurs, the clotting system activates (Fig. 1). In cirrhosis, a more precarious balance exists with heightened sensitivity to exogenous factors.4, 5, 6, 7 This rebalanced system is maintained by compensatory mechanisms, such as elevated levels of procoagulant factors like von Willebrand factor, FVIII, and reduced levels of anticoagulant factors like protein C.4, 5, 7 Additionally, a fibrinolysis pathway (not shown) governs clot dissolution, which may be altered in cirrhosis. Factors like infection, renal dysfunction, and endothelial injury can alter this balance. Tests commonly used to predict bleeding such as the prothrombin time/international normalized ratio do not reliably predict bleeding risk in patients with cirrhosis.8 Other, more global measures are currently under study and available in some centers (e.g., thromboelastography, thrombin generation assay), but there is no single test available today that can accurately predict bleeding or thrombosis tendency in patients with cirrhosis.
Figure 1.
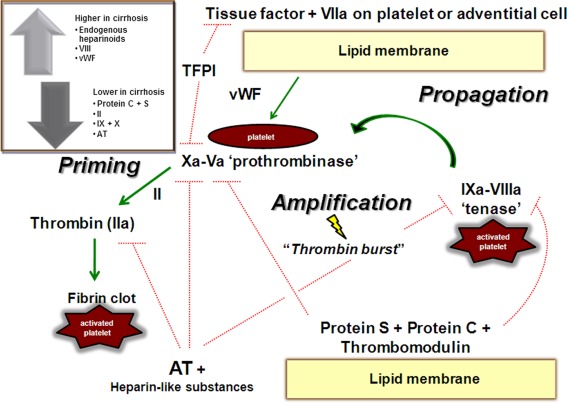
The coagulation cascade in normal conditions and the changes observed in cirrhosis (inset). Abbreviations: AT, antithrombin; TFPI, tissue factor pathway inhibitor; vWF, von Willebrand factor. Green lines indicate the pathway to generate of thrombosis (priming step, amplification, and propagation of thrombosis). Red lines indicate inhibition pathways. Reprinted with permission from Hepatology 2012;55:1634–1637. Copyright 2012, American Association for the Study of Liver Diseases.
Management of the bleeding cirrhosis patient is well studied with established clinical guidelines.9, 10 Treating cirrhosis patients with thrombosis is less defined and clinicians must currently rely on expert recommendation, inference, and extrapolation from data in other patient populations.11 As our understanding of the intricacies of the coagulation system in cirrhosis develops, new tests and treatments for thrombosis and bleeding will likely emerge.
The Bleeding Patient
The ultimate goal in caring for a patient with cirrhosis is to prevent bleeding before it occurs. Current recommendations effectively guide physicians treating portal hypertension–related bleeding.10 Particular attention to the risk of overtransfusion is imperative, and practitioners should recognize the suggested target hemoglobin level (7‐8 g/dL) when transfusing patients with cirrhosis.9, 12
Procedural‐related bleeding is a feared complication for the clinician caring for patients with cirrhosis. Without clear guidelines, clinicians are left to extrapolate approaches to care from other patient populations. There are several blood products and medications available for support of hemostasis (Table 1). Most procedures usually do not require routine prophylaxis (e.g., paracentesis, liver biopsy, most endoscopic procedures). At our institution, we routinely monitor levels of fibrinogen and platelets as a guide prior to major invasive procedures or to support hemostasis during active bleeding (Table 2). We advocate against routine use of fresh frozen plasma to correct the international normalized ratio in patients with cirrhosis, as it is often futile, increases portal pressures, and may cause transfusion‐related injury. Rather, when necessary, we use cryoprecipitate to raise the fibrinogen level (goal: >120 mg/dL) and/or platelets transfused to a range of ∼50‐100 k/μL (depending on the type or invasiveness of the procedure and the clinical situation). Although more evidence is needed to support and guide clinical practice, we feel this is a rational approach based on current evidence and understanding of pathophysiology.
There are other agents available for prophylaxis and treatment of bleeding that have been studied in patients with cirrhosis. Routine use of recombinant factor VIIa is not currently recommended for prophylaxis in patients with cirrhosis but may have a role in rescue therapy.10 The use of aminocaproic acid (an antifibrinolytic) topically after dental extraction is often effective in this patient population and used routinely in our center. Systemic administration for patients exhibiting clinical signs of hyperfibrinolysis (delayed mucosal or puncture site bleeding) should also be considered in select cases. Other therapies—such as desmopressin, vitamin K for malnourished patients, and prothrombin complex concentrates—offer potential adjunct therapies, which can be considered for prophylaxis or treatment of active bleeding.
The Clotting Patient
There is emerging evidence that prevention of venous thromboembolism and portal vein thrombosis is a safe and effective strategy in patients with cirrhosis.13, 14, 15 A recent prospective study in outpatient cirrhosis patients demonstrated that therapy with prophylactic dosing of a low molecular weight heparin decreased formation of portal vein thrombosis, reduced decompensation events, and improved survival.15 Further studies are necessary in larger populations to corroborate these important findings. In an effort to prevent peripheral venous thrombosis during hospitalizations, medical prophylaxis should be considered in cirrhosis patients without contraindications.
Clinical signs of thrombosis are often subtle, hence clinicians who care for patients with cirrhosis should exercise persistent suspicion. Once thrombosis is established, the decision to treat may provoke considerable apprehension. Moreover, there are now numerous available anticoagulants compounding the complexity of treatment decisions (Table 3). There are no current guidelines to provide direction to clinicians, as most clinical trials exclude patients with cirrhosis. Eradication of esophageal varices via band ligation is recommended prior to initiating any long‐term anticoagulation. Furthermore, use of nonselective beta‐blockers is recommended if varices are present to reduce risk of bleeding. Low molecular weight heparin and warfarin are the most widely studied anticoagulants for treatment of portal vein thrombosis in patients with cirrhosis.16, 17, 18, 19, 20 New oral therapies offer great promise, but use is currently limited due to lack of knowledge regarding pharmacodynamics, safety, and efficacy.
Conclusions
Over the last decade, a remarkable paradigm shift in the approach to the coagulopathy of cirrhosis has occurred. It is perplexing to care for patients who profusely hemorrhage from an acute esophageal variceal bleed one day, only to discover an acute venous thrombus soon thereafter. However, this paradox is not so rare as once perceived. Predicting tendencies to bleed or clot and developing strategies to prevent these complications is the ultimate goal of study in this field and will inevitably lead to improved outcomes.
References
- 1. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147‐156. [DOI] [PubMed] [Google Scholar]
- 2. Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology 2006;44:1039‐1046. [DOI] [PubMed] [Google Scholar]
- 3. Northup PG, McMahon MM, Ruhl AP, Altschuler SE, Volk‐Bednarz A, Caldwell SH, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol 2006;101:1524‐1528; quiz 1680. [DOI] [PubMed] [Google Scholar]
- 4. Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, et al. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 2006;44:53‐61. [DOI] [PubMed] [Google Scholar]
- 5. Tripodi A, Primignani M, Chantarangkul V, Dell'Era A, Clerici M, de Franchis R, et al. An imbalance of pro‐ vs anti‐coagulation factors in plasma from patients with cirrhosis. Gastroenterology 2009;137:2105‐2111. [DOI] [PubMed] [Google Scholar]
- 6. Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology 2005;41:553‐558. [DOI] [PubMed] [Google Scholar]
- 7. Tripodi A, Primignani M, Lemma L, Chantarangkul V, Mannucci PM. Evidence that low protein C contributes to the procoagulant imbalance in cirrhosis. J Hepatol 2013;59:265‐270. [DOI] [PubMed] [Google Scholar]
- 8. Ewe K. Bleeding after liver biopsy does not correlate with indices of peripheral coagulation. Dig Dis Sci 1981;26:388‐393. [DOI] [PubMed] [Google Scholar]
- 9. de Franchis R, Baveno VF. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762‐768. [DOI] [PubMed] [Google Scholar]
- 10. Garcia‐Tsao G, Sanyal AJ, Grace ND, Carey W, Practice Guidelines Committee of the American Association for the Study of Liver Disease, Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922‐938. [DOI] [PubMed] [Google Scholar]
- 11. Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol 2013;11:1064‐1074. [DOI] [PubMed] [Google Scholar]
- 12. Zimmon DS, Kessler RE. The portal pressure‐blood volume relationship in cirrhosis. Gut 1974;15:99‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Intagliata NM, Henry ZH, Shah N, Lisman T, Caldwell SH, Northup PG. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int 2014;34:26‐32. [DOI] [PubMed] [Google Scholar]
- 14. Bechmann LP, Sichau M, Wichert M, Gerken G, Kroger K, Hilgard P. Low‐molecular‐weight heparin in patients with advanced cirrhosis. Liver Int 2010;31:75‐82. [DOI] [PubMed] [Google Scholar]
- 15. Villa E, Camma C, Marietta M, Luongo M, Critelli R, Colopi S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253‐1260. [DOI] [PubMed] [Google Scholar]
- 16. Amitrano L, Guardascione MA, Menchise A, Martino R, Scaglione M, Giovine S, et al. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol 2010;44:448‐451. [DOI] [PubMed] [Google Scholar]
- 17. Delgado MG, Seijo S, Yepes I, Achecar L, Catalina MV, Garcia‐Criado A, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol 2012;10:776‐783. [DOI] [PubMed] [Google Scholar]
- 18. Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005;54:691‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia‐Fuster MJ, Abdilla N, Fabia MJ, Fernandez C, Oliver V, Forner MJ. Venous thromboembolism and liver cirrhosis [in Spanish]. Rev Esp Enferm Dig 2008;100:259‐262. [DOI] [PubMed] [Google Scholar]
- 20. Senzolo M, Sartori M T, Rossetto V, Burra P, Cillo U, Boccagni P, et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int 2012;32:919‐927. [DOI] [PubMed] [Google Scholar]
- 21. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD, American Association for the Study of Liver Diseases . Liver biopsy. Hepatology 2009;49:1017‐1044 [DOI] [PubMed] [Google Scholar]


