Watch a video presentation of this article
Watch the interview with the author
Proof‐of‐Concept Studies of Interferon‐free Regimens
With more than 30 direct‐acting antiviral agents (DAAs) in clinical trials, the hepatitis C community (scientists, physicians, patients) expect that the right combinations of DAAs will emerge, permitting treatment of hepatitis C virus (HCV) genotype 1 with interferon (IFN)‐free regimens. In 2012, the first report of sustained virologic response (SVR) with an IFN‐free regimen in patients with genotype 1 was published. In this study, 4 of 11 (36%; 2 of 9 with genotype 1a, 2 of 2 with genotype 1b) noncirrhotic null responders to pegylated IFN (PEG‐IFN) and ribavirin (RBV) achieved SVR after a 24‐week course of asunaprevir, a protease inhibitor, and daclatasvir, a NS5A inhibitor.1 Whereas the number of patients studied was small and the SVR rate was low in patients with genotype 1a, these data provided proof of concept that SVR can be achieved without IFN or RBV in patients with HCV genotype 1. These data have encouraged the evaluation of many other IFN‐free regimens.
IFN‐free Regimens Expected to Be Approved in 2014/2015
In late 2013, two new DAAs for genotype 1 were approved by the US Food and Drug Administration (FDA): simeprevir, a protease inhibitor, and sofosbuvir, a nucleotide polymerase inhibitor. Both drugs are to be used with PEG‐IFN and RBV. Although sofosbuvir was also approved to be used with RBV (without PEG‐IFN) for IFN‐ineligible patients, the SVR rate of the 2‐drug regimen is substantially lower than the 3‐drug regimen: 68% to 76% versus 89%.2, 3, 4 Many experts have advocated the off‐label combination of simeprevir and sofosbuvir based on data from a phase 2 study, and this approach is endorsed by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America HCV guidelines.5 However, only 167 patients were included in that study, and the cost of this off‐label combination is substantially higher than the approved regimens.
Two IFN‐free regimens for HCV genotype 1 are expected to be approved by the FDA in late 2014. Phase 3 trials of both regimens have been completed and results published.
The ION‐1, 2, and 3 trials compared sofosbuvir plus ledipasvir, an NS5A inhibitor coformulated as a single pill, with or without RBV, for 8 to 24 weeks (Table 1) (Fig. 1). In the ION‐1 trial in treatment‐naïve patients (16% with cirrhosis), SVR rates after 12 weeks of therapy were 97% and 98% in the groups with or without RBV, respectively7; results in the 24‐week treatment groups were 99% and 98% with and without ribavirin respectively.7 In the ION‐3 trial, the possibility of shortening the duration of treatment to 8 weeks was tested in treatment‐naïve patients with no to moderate fibrosis (Metavir F0‐F2); SVR rates after 8 weeks of therapy were 93% and 94% in the groups with or without RBV, respectively, and 95% after 12 weeks of treatment without RBV.6 In the ION‐2 trial in treatment‐experienced patients (20% with cirrhosis), SVR rates after 12 weeks of therapy were 96% and 94% in the groups with or without RBV, respectively, and 99% in patients who received 24 weeks of treatment, with or without RBV.8 Anemia was reported in 0.5% of patients in the RBV‐free groups compared to 9.2% in the RBV‐containing groups. Fewer than 1% of patients in the ION studies discontinued treatment due to treatment‐related adverse events.
Table 1.
SVR Rates in Phase 3 Trials of Sofosbuvir‐based Regimens in Patients with HCV Genotype 1 (see references 6, 7, 8)
| Study | Population | Treatment | Duration | SVR12 Rates |
|---|---|---|---|---|
| ION‐1 | GT 1 treatment‐naïve, including 15.7 percent (136/865) with cirrhosis | SOF/LDV | 12 weeks | 97.7% (209/214) |
| SOF/LDV + RBV | 12 weeks | 97.2% (211/217) | ||
| SOF/LDV | 24 weeks | NA (n = 217) | ||
| SOF/LDV + RBV | 24 weeks | NA (n = 217) | ||
| ION‐2 | GT 1 treatment‐experienced, including 20.0 percent (88/440) with cirrhosis | SOF/LDV | 12 weeks | 93.6% (102/109) |
| SOF/LDV + RBV | 12 weeks | 96.4% (107/111) | ||
| SOF/LDV | 24 weeks | 99.1% (108/109) | ||
| SOF/LDV + RBV | 24 weeks | 99.1% (110/111) | ||
| ION‐3 | GT 1 treatment‐naïve | SOF/LDV | 8 weeks | 94.0% (202/215) |
| SOF/LDV + RBV | 8 weeks | 93.1% (201/216) | ||
| SOF/LDV | 12 weeks | 95.4% (206/216) |
SOF 400 mg once daily and LDV 90 mg once daily coformulated as a single pill; RBV weight‐based dosing twice daily.
Abbreviations: GT, genotype; LDV, ledipasvir; SOF, sofosbuvir.
Figure 1.
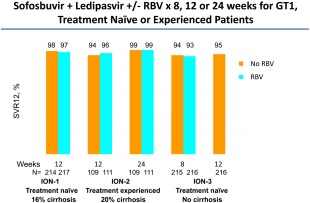
SVR12 rates in ION‐I, ION‐II, and ION‐III trials of sofosbuvir and ledipasvir with or without ribavirin in treatment‐naïve and treatment‐experienced patients with HCV genotype 1.
The AbbVie regimen consisted of fixed‐dose combination of ABT‐450 (a protease inhibitor) with ritonavir boost coformulated with ombitasvir (ABT‐267, an NS5A inhibitor) plus dasabuvir (ABT‐333, a non‐nucleoside polymerase inhibitor) with or without RBV for 12 or 24 weeks (Table 2).9, 10, 11, 12 SAPPHIRE‐I, PEARL‐III, and PEARL‐IV included treatment naïve, noncirrhotic patients; and SAPPHIRE‐II and PEARL‐II included treatment‐experienced, noncirrhotic patients, whereas TURQUOISE‐II included both treatment‐naïve and treatment‐experienced patients with compensated cirrhosis. In SAPPHIRE‐I, all patients received 12 weeks treatment with RBV, and SVR rates were 95% for subtype 1a and 98% for subtype 1b (Fig. 2a).11 In PEARL‐III and PEARL‐IV, all patients received 12 weeks treatment with or without RBV; SVR rates for PEARL‐III (subtype 1b) were 99% in both groups; and for PEARL‐IV (subtype 1a) rates were 97% and 90% in the groups with and without RBV, respectively (Fig. 2a). In SAPPHIRE‐II, 49% of the patients were null responders to PEG‐IFN and RBV. All patients received 12 weeks treatment with RBV, and SVR rates were 96% for subtype 1a and 97% for subtype 1b (Fig. 2b).12 In PEARL‐II, all patients had subtype 1b, and SVR rates were 97% and 100% in the groups with and without RBV, respectively (Fig. 2b). In TURQUOISE‐II, all patients had cirrhosis and all received RBV. SVR rates were 92% and 96% for the groups that received 12 and 24 weeks treatment, respectively (Fig. 2c).9 Only 2% of the patients in either treatment group in TURQUOISE‐II discontinued treatment because of adverse events.
Table 2.
SVR Rates to Phase 3 Trials of IFN‐free AbbVie Regimens in Patients with HCV Genotype 1(see references 9, 10, 11, 12)
| Study (duration) | Patients | Treatment Regimen | SVR12 |
|---|---|---|---|
| PEARL‐II (12 weeks) | GT1b treatment‐experienced | AbbVie regimen + RBV (n = 88) | 97% |
| AbbVie regimen only (n = 91) | 100% | ||
| PEARL‐III (12 weeks) | GT1b treatment‐ naïve | AbbVie regimen + RBV (n = 210) | 99% |
| AbbVie regimen only (n = 209) | 99% | ||
| PEARL‐IV (12 weeks) | GT1a treatment‐ naïve | AbbVie regimen + RBV (n = 100) | 97% |
| AbbVie regimen only (n = 205) | 90% | ||
| TURQUOISE‐II (12 and 24 weeks) | GT1 treatment‐ naïve and treatment‐experienced with compensated cirrhosis | AbbVie regimen + RBV, 12 weeks (n = 208) | 92% |
| AbbVie regimen + RBV, 24 weeks (n = 172) | 96% | ||
| SAPPHIRE‐I (12 weeks) | GT1 treatment‐ naïve | AbbVie regimen + RBV (n = 473) | 96% |
| SAPPHIRE‐II (12 weeks) | GT1 treatment‐experienced | AbbVie regimen + RBV (n = 297) | 96% |
AbbVie regimen consists of ABT‐450/ritonavir (150 mg/100 mg) coformulated with Ombitasvir (ABT‐267) (25 mg), dosed once daily, and Dasabuvir (ABT‐333) (250 mg), dosed twice daily. RBV weight‐based dosing, twice daily.
Abbreviation: GT, genotype.
Figure 2.
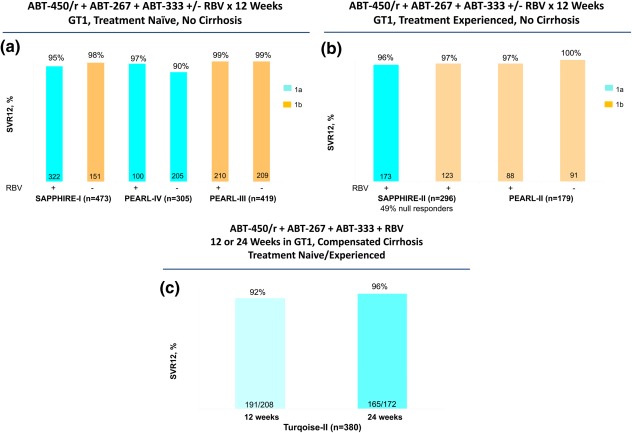
SVR12 rates in phase 3 trials of AbbVie regimens of ABT‐450/ritonavir, Ombitasvir (ABT‐267) and Dasabuvir (ABT‐333) with or without ribavirin in patients with HCV genotype 1.9, 10, 11, 12 (A) Treatment‐naïve noncirrhotic patients: SAPPHIRE‐I (subtype 1a and 1b), PEARL‐IV (subtype 1a), and PEARL‐III (subtype 1a). (B) Treatment‐experienced, noncirrhotic patients: SAPPHIRE‐II (subtype 1a and 1b) and PEARL‐II (subtype 1b). (C) Treatment‐naïve and treatment‐experienced patients with cirrhosis (subtype 1a and 1b).
Other IFN‐free Regimens in the Pipeline
There are several other IFN‐free regimens in the pipeline. The furthest along involves a combination of asunaprevir, daclatasvir, and BMS‐791325—a nonnucleoside polymerase inhibitor. In a phase 2b trial of 166 treatment‐naïve patients including 9% with cirrhosis, SVR rates were 92% and 92% in the groups that received low‐dose or high‐dose BMS‐791325.13 Phase 3 trial of this regimen is ongoing. Combination of daclatasvir and asunaprevir without BMS‐791325 is also pursued in Japan, where nearly all patients with HCV genotype 1 have subtype 1b. As discussed earlier, this combination has low efficacy for subtype 1a. Another regimen involves a combination of a second‐generation protease inhibitor MK‐5172 and a second‐generation NS5A inhibitor MK‐8742.14 Preliminary data showed SVR rates of 96% to 100% with or without RBV.
Are We Ready to Say Good‐bye to Interferon for HCV Genotype 1?
Data from the studies presented above show that a 12‐week course of IFN‐free, all‐oral combination of 2 or 3 DAAs can result in > 90% SVR rates in most patients with genotype 1 infection, including those with subtype 1a, treatment‐experienced patients, and patients with cirrhosis. Some of these regimens would also be RBV‐free. Phase 3 trials of two of the IFN‐free regimens have been completed and qualify for expedited review by the FDA; thus, it is expected that they will be available in late 2014 or early 2015. This is tremendous news for patients and HCV providers because the wait is finally over for most, if not all, patients with genotype 1 infection.
Abbreviations
- DAA
direct‐acting antiviral agent
- HCV
hepatitis C virus
- IFN
interferon
- PEG‐IFN
pegylated interferon
- RBV
ribavirin
- SVR
sustained virologic response
- FDA
United States Food and Drug Administration.
Potential conflict of interest: a.s.f.l. has received research grants from AbbVie, Bristol‐Myers Squibb, Gilead, Idenix, and Merck, and has served on advisory panels of Gilead and Janssen.
References
- 1. Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012;366:216‐224. [DOI] [PubMed] [Google Scholar]
- 2. Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA 2013;310:804‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sulkowski M, Rodriguez‐Torres M, Lalezari JP, Fessel WJ, Mounzer K, Shuhart MC, et al. All‐oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotype 1, 2, and 3 infection in patients co‐infected with HIV (PHOTON‐1). Hepatology 2013;58(suppl 1):313A. [Google Scholar]
- 4. Lawitz E, Mangia A, Wyles D, Rodriguez‐Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878‐1887. [DOI] [PubMed] [Google Scholar]
- 5. AASLD, IDSA . Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org/. Last accessed May 10, 2014.
- 6. KV Kowdley, SC Gordon, KR Reddy, L Rossaro, DE Bernstein, E Lawitz, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879–1888. [DOI] [PubMed] [Google Scholar]
- 7. N Afdhal, S Zeuzem, P Kwo, M Chojkier, N Gitlin, M Puoti, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889–1898. [DOI] [PubMed] [Google Scholar]
- 8. N Afdhal, KR Reddy, DR Nelson, E Lawitz, SC Gordon, E Schiff, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483–1493. [DOI] [PubMed] [Google Scholar]
- 9. F Poordad, C Hezode, R Trinh, KV Kowdley, S Zeuzem, K Agarwal, et al. ABT-0000/r-Ombitasvir and Dasabuvir with Ribavirin for Hepatitis C with Cirrhosis. N Engl J Med 2014;370:1973–1982. [DOI] [PubMed] [Google Scholar]
- 10. P Ferenci, D Bernstein, J Lalezari, D Cohen, Y Luo, C Cooper, et al. ABT-0000/r-Ombitasvir and Dasabuvir with or without Ribavirin for HCV. N Engl J Med 2014;370:1983–1992. [DOI] [PubMed] [Google Scholar]
- 11. JJ Feld, KV Kowdley, E Coakley, S Sigal, DR Nelson, D Crawford, et al. Treatment of HCV with ABT-0000/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1594–1603. [DOI] [PubMed] [Google Scholar]
- 12. S Zeuzem, IM Jacobson, T Baykal, RT Marinho, F Poordad, M Bourlière, et al. Retreatment of HCV with ABT-0000/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1604–1614. [DOI] [PubMed] [Google Scholar]
- 13. Everson GT, Sims KD, Thuluvath PJ, Lawitz E, Hassanein T, Rodriguez‐Torres M, et al. Phase 2b study of the interferon‐free and ribavirin‐free combination of daclatasvir, asunaprevir, and BMS‐791325 for 12 weeks in treatment‐naïve patients with chronic HCV genotype 1 infection. Hepatology 2013;58(suppl):1377A. [Google Scholar]
- 14. Lawitz E, Vierling JM, Murillo A, Kugelmas M, Gerstoft J, Winkle P, et al. High efficacy and safety of the all‐oral combination regimen, MK‐5172/MK‐8742 ± RBV for 12 weeks in HCV genotype 1 infected patients: the C‐WORTHY study. Hepatology 2013;58(suppl 1):244A. [Google Scholar]


