Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- ARs
adverse reactions
- DILI
drug‐induced liver injury
- DILIN
drug‐induced liver injury network
- FDA
US Food and Drug Administration
- HDS
herbal and dietary supplements
- NAPQI
N‐acetyl‐p‐benzoquinoneimine.
Overview
Herbal and dietary supplements (HDS) are the most common form of complementary and alternative medicine used in the United States.1 American consumers spend about $6 billion on HDS yearly.2 HDS include vitamins, minerals, herbs or other plant materials, and materials extracted from such plants. They are taken by mouth and are intended to supplement the diet and enhance health and well‐being. HDS are consumed by about one‐half of the US population, and their use has been on the rise over the past several years.3 HDS can cause hepatotoxicity. The estimated incidence of HDS hepatotoxicity has risen almost threefold over the past decade (Fig. 1).
Figure 1.
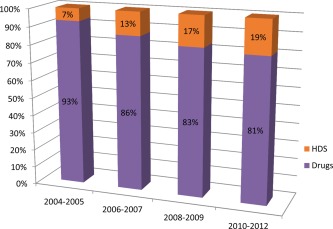
Rising incidence of liver injury due to herbal and dietary supplements (HDS) over the past decade. Data are from the US Drug‐Induced Liver Injury Network database, as reported at the Annual Meeting of AASLD, November 1‐5, 2013, Washington, DC.4
Drug and HDS Metabolism
The human liver and kidneys metabolize and excrete a wide array of drugs and chemicals, which are either introduced from outside (xenobiotics) or generated endogenously (hormones, chemokines, cytokines). The metabolism of xenobiotics or endogenously produced compounds by the liver is a complex, multistep process. Most drugs or HDS are taken orally, absorbed primarily in the proximal small bowel, enter the portal circulation, and reach the liver where they undergo “first‐pass metabolism.” Most drugs or components of HDS undergo an initial hydroxylation/oxidation reaction (phase 1 metabolism) after entering the hepatocytes, which is catalyzed by cytochrome P450 enzymes. They subsequently undergo additional changes (phase 2 metabolism) to increase their water solubility. The metabolites are eventually transported out of the hepatocytes (phase 3 metabolism) and into the bloodstream (to be excreted in urine) or into the bile (to be excreted in feces).
In addition to their potential therapeutic benefit, drugs and HDS can result in several side effects or adverse reactions (ARs) involving one or more organ systems. Drug‐ or HDS‐induced liver injury is one of the major ARs caused by such chemicals. The pathogenesis of liver injury due to most drugs or HDS [DILI] is not completely understood. A complex and variable interplay of features related to the drug or chemical, host, and environmental factors likely occurs in most instances (Fig. 2). Some drugs and chemicals are known, dose‐dependent hepatotoxins. The best known and most important of these is acetaminophen (aka paracetamol). It will cause liver injury in any animal or human who takes a sufficient dose, and its metabolic activation to a highly reactive, potentially toxic quinoneimine (NAPQI) intermediate has been widely studied.6 It is a prototypical “intrinsic” hepatotoxin. However, many more drugs only rarely cause DILI (1/1000 to 1/1,000,000) and without clear dependence on dose or duration of drug use. For many drugs, reactive metabolites are probably involved, but the pathogenesis appears to involve idiosyncratic host immune responses to metabolic particularities. Such drugs are said to cause “idiosyncratic” DILI. Clinicopathologically, DILI can present as hepatocellular, cholestatic or mixed patterns—or as hepatic steatosis. The spectrum of hepatic injury can vary from mild, asymptomatic, liver test elevation to acute liver failure causing death or requiring liver transplantation.
Figure 2.
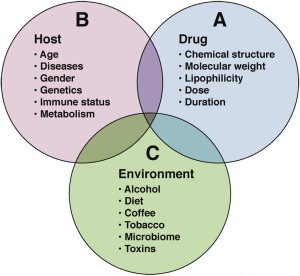
Pathogenesis of idiosyncratic DILI. Diagram representing complex interplay among host, drug, and environmental factors.
From Fontana5 with permission of the author and publisher.
HDS Use and Surveillance Systems
The US Food and Drug Administration (FDA) collects and maintains a large database of ARs voluntarily reported by practitioners and those in pharmaceutical industry (eg, Medwatch). Unfortunately, such mechanisms do not exist for HDS. Patients tend to underreport their HDS use to their physicians. According to Verma et al, about 31% to 40% of patients do not disclose HDS use.2 HDS products are consumed for multiple purposes such as weight loss, bodybuilding, immune support, general well‐being, etc (Fig. 3). Previous studies have indicated that the prevalence of HDS use, even among individuals with chronic liver disease, is about 40%.7 These individuals may be at a higher risk for developing HDS liver toxicity compared to the general population. Certainly, if they do develop toxicity, they are at increased risk of having adverse outcomes.
Figure 3.
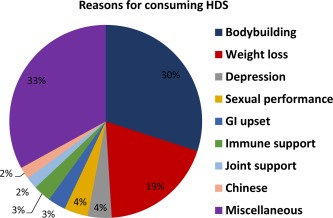
Reasons for consuming herbal and dietary supplements (HDS) in patients enrolled into the US DILIN network.4
HDS products are often marketed as complex mixtures containing several ingredients. The exact biologic role of each individual component in a mixture is difficult to ascertain. The concentrations and biologic activity of the ingredients can also vary from batch to batch within the same product by the same manufacturer. In addition, HDS products are sometimes adulterated with prescription drugs such as steroids, thyroid hormones, phosphodiesterase inhibitors, phentermine, etc.9 In addition, because they are heavily promoted as being “natural,” they are perceived to be “safe” by consumers. Unlike prescription drugs, HDS are not stringently regulated by the FDA. Indeed, under current statutes, the FDA may not take action to limit distribution of HDS until after they have been found to cause ARs.
HDS Hepatotoxicity
The incidence of liver injury due to HDS appears to have risen about threefold in the US during the past decade (Fig. 1). This is probably due in part to the growing consumption of HDS products. Among the patients enrolled into the prospective Drug‐Induced Liver Injury Network (DILIN), HDS as a group are the second most common cause of drug‐induced liver injury.10 Among HDS implicated in hepatotoxicity, the bodybuilding products are the single most common cause of liver injury (Fig. 3).
The spectrum of liver injury due to HDS is similar to DILI and varies from mild elevation in liver tests to acute liver failure. Histologically, liver injury due to HDS in most instances is not distinguishable from other forms of drug and/or toxin‐induced liver damage. Specific clinical‐histologic patterns have been described for some HDS products (Table 1). Patients who develop liver injury due to bodybuilding HDS typically have prolonged jaundice and severe itching.4 In contrast, “other or nonbodybuilding” HDS products typically cause a more hepatocellular‐type of liver injury.4 Patients with liver injury due to bodybuilding HDS have a better prognosis compared to those with liver injury due to other HDS, and they generally improve without the need for liver transplantation.4 In contrast, the all‐cause mortality was 4%, and liver transplantation was required in 13% of patients with liver injury due to non‐bodybuilding HDS products.9 The need for liver transplantation was higher in patients with liver injury due to non‐bodybuilding HDS products compared to DILI due to conventional drugs (13% vs 3%) (Fig. 4).
Table 1.
Herbs and Associated Liver Injury
| HDS | Nature of Liver Injury |
|---|---|
| Atractylis gummifera | Diffuse hepatic necrosis |
| Black cohosh | Elevated liver tests, liver failure |
| Camphor | Elevated liver tests, Reye syndrome |
| Cascara | Bridging fibrosis, bile duct proliferation |
| Chaparral | Cholestasis, zone 3 necrosis |
| Chaso (and onshido) | Elevated liver tests, liver failure |
| Greater celandine | Cholestasis |
| Germander | Zone 3 necrosis, cirrhosis |
| Green tea extracts | Acute hepatitis, hepatocellular injury |
| Impila | Hepatic necrosis |
| Ju bu huan | periportal fibrosis, steatosis |
| Kava | Elevated liver tests, liver failure |
| Ma huang | Elevated liver tests, liver necrosis |
| Mistletoe (skullcap, valerian) | Elevated liver tests, acute hepatitis |
| OxyElite Pro (aegeline) | Acute hepatitis, liver failure |
| Noni juice | Acute hepatitis, liver failure |
| Pennyroyal | Acute hepatitis, liver failure |
| Pyrrolizidine (comfrey, mate, bush tea) | Veno‐occlusive disease |
| Sho‐saiko‐to (dai‐saiko‐to, TJ‐9) | Bridging fibrosis, steatosis |
This table has been adapted and modified from Zakim and Boyer.1
Figure 4.
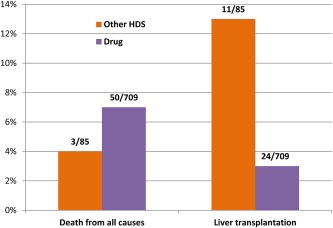
Difference in all‐cause mortality and liver transplantation in liver injury due to “other” HDS and conventional drugs.4
In summary, HDS are widely used and their use is on the rise. They are perceived to be safe, but they can cause severe liver injury. The exact incidence of HDS‐induced liver injury is difficult to ascertain, but it is probably underreported. Liver injury is generally hepatocellular, but bodybuilding HDS may also cause a cholestatic pattern of injury. Attribution of liver injury as being due to HDS (or to drugs) is a diagnosis of exclusion. Causality is difficult to establish because of the complex nature of the marketed HDS products. Improved regulatory mechanisms are required to oversee all aspects of the HDS industry in order to better protect the consumers and prevent HDS‐induced adverse events.
This study was supported by institutional funds from Carolinas HealthCare System and by a cooperative agreement [U01 DK 065201] and a grant [R15 HL 117199] from NIH.
Potential conflict of interest: Nothing to report.
References
- 1. Strader DB, Navarro VJ, Seeff LB. Hepatotoxicity of Herbal Preparations Chapter 26. In: Boyer TD, Manns MP, Sanyal AJ, eds. Zakim and Boyer's Hepatology 6th ed Philadelphia, PA: Saunders‐Elsevier; 2012: 462‐475. [Google Scholar]
- 2. Verma S, Thuluvath PJ. Complementary and alternative medicine in hepatology: review of the evidence of efficacy. Clin Gastroenterol Hepatol 2007;5:408‐416. [DOI] [PubMed] [Google Scholar]
- 3. Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med 2013;173:355‐361. [DOI] [PubMed] [Google Scholar]
- 4. Navarro VJ, Barnhart H, Bonkovsky HL, Davern TJ, Fontana RJ, Grant L, et al. The rising burden of herbal and dietary supplement‐induced hepatotoxicity in the U.S.A. [Abstract #113, presented at Annual Meeting of AASLD, November 1–5, 2013, Washington, DC] Hepatology 58 [4, suppl]: 264A, 2013.
- 5. Fontana RJ. Pathogenesis of idiosyncratic drug‐induced liver injury and clinical perspectives. Gastroenterology 2014;146:914‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonkovsky HL, Jones DP, Russo MW, Shedlofsky SI. Drug‐Induced Liver Injury Chapter 25. In: Boyer TD, Manns MP, Sanyal AJ, editor. Zakim and Boyer's Hepatology 6th ed Philadelphia, PA: Saunders‐Elsevier; 2012: 417‐461. [Google Scholar]
- 7. Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, et al. Herbal product use by persons enrolled in the hepatitis C Antiviral Long‐Term Treatment Against Cirrhosis (HALT‐C) Trial. Hepatology 2008;47:605‐612. [DOI] [PubMed] [Google Scholar]
- 8. Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, Wright EC, et al. Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol 2002;97:2391‐2397. [DOI] [PubMed] [Google Scholar]
- 9. Klinsunthorn N, Petsom A, Nhujak T. Determination of steroids adulterated in liquid herbal medicines using QuEChERS sample preparation and high‐performance liquid chromatography. J Pharm Biomed Anal 2011;55:1175‐1178. [DOI] [PubMed] [Google Scholar]
- 10. Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug‐induced liver injury in the United States. Gastroenterology 2008;135:1924–1934, 1934.e1‐e4. [DOI] [PMC free article] [PubMed] [Google Scholar]


