Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CHeCS
Chronic Hepatitis Cohort Study
- CMS
the Centers for Medicare and Medicaid Services
- EMR
electronic medical record
- ER
emergency room
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- NHANES
National Health and Nutrition Examination Survey
- PCR
polymerase chain reaction
- SVR
sustained viral response.
Chronic infection with hepatitis C virus (HCV) is associated with significant morbidity and mortality, especially in those with advanced liver disease. Studies have also shown that viral replication increases not just the risk for liver‐related complications, but also all‐cause mortality (Fig. 1A).1 However, viral eradication or sustained viral response (SVR)—having a negative HCV RNA by polymerase chain reaction (PCR) 6 months after completing HCV therapy—decreases this risk. SVR even decreases all‐cause mortality (Fig. 1B).2 The efficacy of HCV therapy is rapidly improving. Current regimens have nearly 90% SVR rates, and therapies in development are achieving over 95% rates of viral eradication, even in patients traditionally considered difficult to treat.
Figure 1.
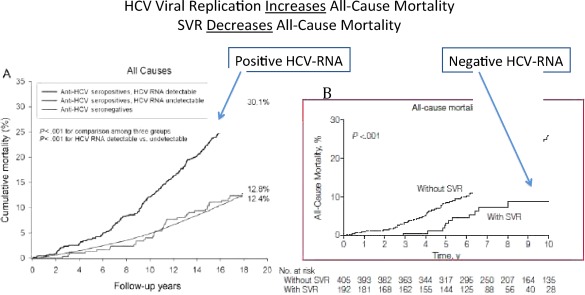
Individuals with active viral replications of hepatitis C have higher rates of all‐cause mortality compared with those with HCV exposure but no replication (anti‐HCV antibody positive, HCV‐RNA negative) and to those with neither antibody nor HCV RNA (A). However, viral eradication (eliminating HCV RNA through antiviral therapy) decreases this risk (B).1, 2
Unfortunately, the limitation to HCV therapy is not treatment efficacy; it is identification of patients and linking them to providers interested in delivering antiviral therapy.
In 1998, the Centers for Disease Control and Prevention (CDC) published risk‐based screening recommendations in an effort to prevent and control HCV. Regrettably, these recommendations met with limited success because 45% to 85% of those infected remain unaware of their HCV status. This includes even those in the highest risk groups; 72% of HCV‐positive injection drug users are unaware of their infection.3
Barriers to risk‐based screening led the CDC to revise the recommendations in 2012 to also include a one‐time screening (irrespective of risk) for all those born between 1945 and 1965. As shown in Fig. 2, the prevalence of anti‐HCV in this birth cohort is five times higher than any other year; thus, it includes 75% of Americans infected with HCV.3
Figure 2.
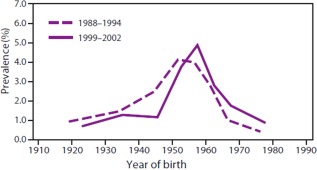
The National Health and Nutrition Examination Survey (NHANES) is a program of studies designed to assess the health and nutritional status of adults and children in the United States. Data collected through this nation survey in both 1988–1994 and 1999–2002 confirmed that those born 1945–1965 had the highest risk of HCV exposure.3
Testing for anti‐HCV is only the first step. The presence of antibody does not diagnose HCV; confirmatory HCV RNA must follow a positive antibody. A positive HCV RNA is essential for diagnosis. Those with active infection require counseling on transmission and disease progression. Lastly, those infected with HCV should be linked to a provider familiar with antiviral therapy.4 This algorithm is clearly visualized in Fig. 3.
Figure 3.
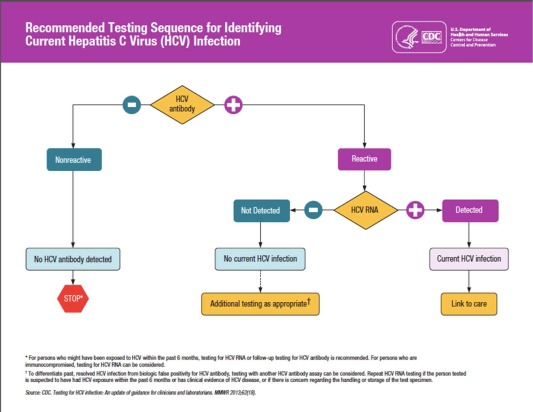
According to the CDC, HCV antibody should be obtained to screen for infection. If this is positive, it must be confirmed with HCV RNA. Those with both a positive antibody and RNA should be linked to care for management of HCV infection.4
Where does the system break down? In our current model, there are deficiencies at every stage. As outlined above, screening is inadequate. Even after an individual is identified as being anti‐HCV positive, there is significant drop‐off at each step from confirmation to linkage to care.
The Chronic Hepatitis Cohort Study (CHeCS) involves more than 13,000 patients with hepatitis C. Researchers from the division of viral hepatitis at the CDC have analyzed this data, as well as information from the National Health and Nutrition Examination Survey (NHANES), and found that unfortunately only 63% of anti‐HCV positive subjects received HCV RNA measurement; only 32% to 38% receive follow‐up hepatitis care; and only 13% to 18% have received antiviral therapy.5, 6 Without effective linkage to care, effective therapy cannot impact morbidity and mortality.
Screening
No obstacle is insurmountable. Recognizing that the old model is ineffective is a valid start and justified the recent revision in the CDC‐screening guidelines to include a one‐time anti‐HCV for the birth cohort.
Still, making a recommendation means nothing without effective implementation. Historically, clinicians have been slow to adopt guidelines into practice. However, using a historical comparison may not be appropriate because several innovations have augmented our ability to identify a specific population.
Electronic medical record (EMR) technology is such a high priority for our health care system that the Centers for Medicare and Medicaid Services (CMS) offers monetary incentives to professionals who adopt the technology. CMS also “implements quality initiatives to assure quality health care for Medicare Beneficiaries through accountability and public disclosure.” In other words, CMS uses specific measures to grade health care providers on the quality of care that they provide. Capturing these values is facilitated by the EMR.7 This push to deliver high‐quality, value‐based medicine makes it easy to imagine that birth cohort screening could be a realistic quality measure.
There are also successful models in which HCV point‐of‐care tests are integrated into high‐risk clinics8 or HCV screening is assimilated into existing human immunodeficiency virus (HIV) screening programs.9
Confirmatory Testing
It would seem that screening should be the hurdle; however, one‐third of the patients who screen positive fail to receive confirmatory testing. Screening performed in high‐risk clinics may lack continuity to communicate results and request additional studies. Patients may fail to follow up due to limited resources, fear, or misunderstanding. Confirmatory test rates are best in continuity clinics, but creative models demonstrate high retention rates even in traditionally difficult populations. This is the appeal of point‐of‐care testing. The results can be discussed, and follow‐up can be arranged before the patient leaves. For example, the University of Alabama performed screening in birth cohort subjects who presented to their emergency room (ER). Participants utilizing the ER included a large number of nonwhite, Medicaid, and uninsured individuals. HCV PCR testing followed rapid HCV‐antibody testing. Six weeks after starting the program, questionnaires were performed in 73% of the birth cohort ER population. Seventy‐five percent of the subjects were unaware of their HCV status. Ninety‐one percent of the subjects accepted testing, and of the subjects who were anti‐HCV positive, confirmatory testing was performed in all but 17%.10
Counseling and Linkage to Care
A diagnosis alone will not impact the consequence of the disease. Alcohol counseling, understanding transmission as well as disease progression, and ultimately therapy are the crux of a positive impact. Yet, linkage to care is the highest hurdle that patients face. Why are not all patients linked to care? This is a complex question without a consistent answer. Superficial barriers are obvious: Specialists may not be readily available; patients may be hesitant to see another doctor; the delay between referral and appointment may dissuade some; and financial barriers exist. Some primary care physicians may determine that a patient is not a treatment candidate or that treatment may not render a cure—and thus a cost/benefit—to their patient. Patients with normal liver enzymes may also be told that their disease is mild and that they are not in need of therapy.
There are several programs that attempt to expand access by having experienced providers mentor health care professionals in areas where there is limited access.12
Still, this is a very superficial approach. In reality, the fix is not as simple as expanding the provider pool. Simplistic, highly effective, accessible therapy is equally vital. This alone will expand the number of willing treaters. Other disciplines outside of gastroenterology and hepatology will become increasingly involved. Providers who are currently managing HIV infection, infectious disease specialists, and primary care providers are familiar with both chronic viral infections, drugs in similar classes to the HCV agents in development, and therapeutic monitoring. These providers also have extensive experience in providing care for a similar population of individuals, as well as the office infrastructure to offer quality care. Once interferon is no longer part of the treatment backbone, these providers are expected to become increasingly interested in managing HCV. Additional health care providers who have traditionally referred HCV‐infected patients to specialists will be more likely to adopt regimens that are easy to apply. For the HCV therapeutic pipeline, all‐oral, pangenotypic, safe, and once‐a‐day agents with few drug interactions are a reality. Providers who care for large groups of high‐risk patients, including those with large numbers of baby boomers (born in the birth cohort 1945–1965), may be motivated to initiate treatment once injection therapy is no longer part of management.
However, accessible therapy also implies that patients in need will also have equal opportunity. Even if linked to a health care professional willing to offer therapy, many individuals with HCV are underinsured and with limited resources. Many companies offer generous indigent care programs, yet drug coverage alone is not adequate. Responsible treatment requires expensive lab assessment and provider appointments. Much of the birth cohort is eligible for Medicare, and additional federal programs could help provide access. HIV‐infected patients benefited significantly with the Ryan White Comprehensive AIDS Resources Emergency Act enacted in 1990, which provides federal funding when no other resources are available.12
Despite extraordinary accomplishments in antiviral therapy for HCV, minimizing HCV‐induced morbidity and mortality remains limited at each intervention point: patient identification, confirmation, counseling, and linkage to care. A coordinated effort, which should include primary care education, creative efforts at screening at‐risk populations, policy, and federal support, could effectively minimize HCV‐related health care burden.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Lee MH, Yang HI, Lu SN, Jen CL, You SL, R.E.V.E.A.L.‐HCV Study Group , et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community‐based long‐term prospective study. J Infect Dis 2012;206(4):469‐477. doi: 10.1093/infdis/jis385. Epub 2012. [DOI] [PubMed] [Google Scholar]
- 2. van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all‐cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584‐2593. [DOI] [PubMed] [Google Scholar]
- 3. Smith BD, Morgan RL, Beckett GA, Falck‐Ytter Y, Holtzman D, Centers for Disease Control and Prevention , et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945‐1965. MMWR Recomm Rep 2012;61(RR-4):1‐32 [PubMed] [Google Scholar]; Erratum in: MMWR Recomm Rep 2012;61(43):886 http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC) . Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep 2013;62(18):362‐365. [PMC free article] [PubMed] [Google Scholar]
- 5. Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the Chronic Hepatitis Cohort Study. Clin Infect Dis 2013;56:40‐50. [DOI] [PubMed] [Google Scholar]
- 6. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med 2013; 368:1859‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services (CMS). Quality Measures. http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/index.html?redirect=/QUALITYMEASURES/. Accessed May 16, 2014.
- 8. Jewett A, Al‐Tayyib AA, Ginnett L, Smith BD. Successful Integration of Hepatitis C Virus Point‐of‐Care Tests into the Denver Metro Health Clinic. AIDS Res Treat 2013;2013:528904. doi: 10.1155/2013/528904. Epub 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cocoros N, Nettle E, Church D, Bourassa L, Sherwin V, Cranston K, et al. Screening for Hepatitis C as a Prevention Enhancement (SHAPE) for HIV: an integration pilot initiative in a Massachusetts County correctional facility. Public Health Rep 2014;129 Suppl 1:5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galbraith JW, Franco R, Rodgers J, Donnelly JP, Morgan J, Overton ET, Saag M, Wang HE. Screening in the Emergency Department Identifies a Large Cohort of Unrecognized Chronic HCV Infection Among Baby Boomers. In Proceedings of the 64th Annual Meeting of the.
- 11. American Association for the Study of Liver Diseases (AASLD) , Washington, DC, 2013.
- 12. Project ECHO, Hepatitis C TeleECHO Clinic . http://echo.unm.edu/clinics/clinic‐hepc‐community.html. Accessed May 15, 2014.
- 13. US Department of Health and Human Services , Health Resources and Services Administration (HRSA). HIV/AIDS Programs. http://hab.hrsa.gov/abouthab/aboutprogram.html. Accessed May 15, 2014.


