Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- AaPO2
alveolar‐arterial oxygen tension difference
- GMT
geometric mean of technetium
- HPS
hepatopulmonary syndrome
- IPVD
intrapulmonary vasodilatation
- LTOT
long‐term oxygen therapy
- LTX
liver transplantation
- MELD
model of the end‐stage liver disease
- NOS
nitric oxide synthetase
- PaO2
partial pressure of oxygen
- PFT
pulmonary fuction testing
- SaO2
Oxygen saturation
Hepatopulmonary syndrome (HPS) is a gas exchange abnormality in patients suffering from liver disease as a consequence of intrapulmonary vasodilatation (IPVD) and pulmonary angiogenesis. It is the most frequent complication of the pulmonary vascular bed in patients with liver cirrhosis and is found in up to 30% of patients undergoing evaluation for LTX (liver transplantation). However, the presence of HPS is also reported in other forms of acute and chronic noncirrhotic liver diseases such as acute liver failure, hypoxic hepatitis, and chronic viral hepatitis.1 Mortality is more than twofold increased in patients with cirrhosis compared to patients without HPS.2, 3 Consequently, early detection of HPS and subsequent initiation of adequate therapeutic measures is warranted.
Diagnosis
HPS is defined as a triad of arterial deoxygenation due to IPVD in patients with liver disease.1 Diagnostic criteria of HPS are illustrated in Table 1.4
Table 1.
Diagnostic Criteria for HPS.
| Presence of liver disease | |
| Intrapulmonary vasodilatation | Positive contrast enhanced echocardiography, lung perfusion scanning |
| Gas exchange abnormality | arterial blood gas analysis: AaPO2 > 15 mmHg or > 20 mmHg in patients > 64 years |
Abbreviations: AaPO2: alveolar‐arterial oxygen tension difference
Pulse oximetry is able to detect gas exchange abnormalities nonspecifically in patients with cirrhosis and may facilitate detection of moderate to severe HPS.5 However, arterial blood gas analysis is usually warranted to establish diagnosis and to graduate severity of HPS. Arterial blood gas analysis should be performed in upright position on room air. Alveolar‐arterial oxygen tension difference (AaPO2) is the most sensitive parameter for the detection of gas exchange abnormalities. It increases prior to a decline of partial pressure of oxygen (PaO2) because partial pressure of carbon dioxide (frequently decreased as a consequence of hyperventilation in patients with cirrhosis) is incorporated in its calculation. The severity of HPS is classified by the degree of hypoxemia while breathing room air (Table 2).
Table 2.
Classification of HPS According to Severity of Hypoxia.
| Stage | PaO2 | Clinical consequence |
|---|---|---|
| Mild | ≥ 80 mmHg | clinical follow up |
| Moderate | < 80 mmHg ‐ 60 mmHg | clinical follow up |
| Severe | < 60 mmHg ‐ 50 mmHg | LTOT, evaluation for LTX |
| Very severe | < 50 mmHg | LTOT, evaluation for LTX |
Abbreviations: PaO2 : partial pressure of oxygen; mmHg: milimeter of mercury; LTOT: long term oxygen therapy; LTX means liver transplantation
Contrast‐enhanced echocardiography is the gold standard for the detection of IPVD. Agitated saline usually is used as a contrast agent. Microbubbles can only be detected in the left heart four to six heartbeats after the initial appearance in the right side of the heart as a consequence of IPVD. Intracardiac shunting can easily be delineated where the appearance of microbubbles in the left heart occurs within three cardiac cycles after entering the right heart. Figure 1 illustrates the detection of IPVD by transthoracic contrast‐enhanced echocardiography in a patient suffering from severe HPS. Transesophageal contrast‐enhanced echocardiography usually is reserved for patients when the transthoracic echocardiographic image quality is not satisfactory.
Figure 1.
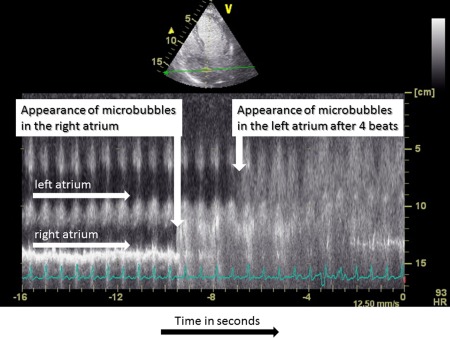
Contrast enhanced echocardiography in a patient with severe HPS. The figure illustrates the time‐dependent appearence of the contrast agent in the right atrium and four heartbeats thereafter in the left atrium using the M‐mode technique. Abbreviations: M‐mode, motion mode.
Lung perfusion scanning is an alternative method for the detection of IPVD. In this test, accumulation of radiolabeled (technetium)‐macroaggregated albumin in the brain is quantified after intravenous injection. The extrapulmonary shunt fraction, assuming that 13% of the cardiac output is delivered to the brain, is calculated as the geometric mean of technetium counts (GMT) according to the following formula: [(GMTbrain)/0.13]/[(GMTbrain)/0.13+(GMTlung). Usually, a shunt fraction of more than 6% is considered positive for intrapulmonary vasodilatation.7 Although lung perfusion scanning has a high sensitivity of detection of severe HPS, the ability to detect mild or moderate stages of HPS is limited. Furthermore, lung perfusion scanning cannot distinguish IPVD from intracardiac shunting.
Pulmonary diseases such as chronic obstructive pulmonary disease, bronchial asthma, and interstitial lung disease may coexist with HPS. Consequently, pulmonary comorbid conditions should be ruled out by chest imaging and pulmonary function testing in patients with respiratory insufficiency and cirrhosis.
A screening algorithm for HPS is proposed in Figure 2.
Figure 2.
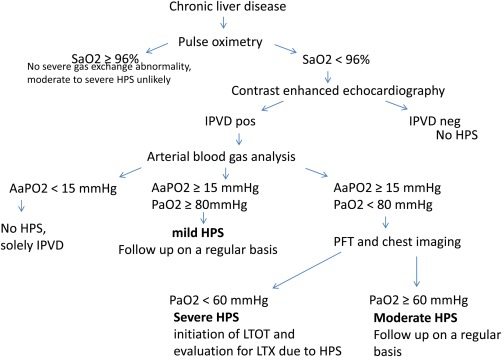
Clinical Algorithm for Screening patients with chronic liver disease for HPS. Abbreviations: AaPO2, alveolar‐arterial oxygen tension difference; HPS: hepatopulmonary syndrome; IPVD: intrapulmonary vasodilatation; SaO2: oxygen saturation; Pao2: partial pressure of oxygen.
Clinical Features
Dyspnea at rest or during exercise is the typical leading symptom in patients with HPS. However, it is not HPS‐specific because other underlying causes such as ascites, hepatic hydrothorax, anemia, and pulmonary comorbid conditions may also lead to respiratory insufficiency. Additionally, patients with mild HPS may be free of respiratory complaints. Spider nevi, digital clubbing, and cyanosis can be observed in patients with advanced stages of HPS. Orthodeoxia (decrease in PaO2 > 4 mm Hg or > 5% on moving from a supine to an upright position) and platypnea (dyspnea in the upright position that ameliorates when assuming supine position) are characteristic clinical features observed in up to 25% of patients with HPS.
Quality of life is significantly reduced in patients with HPS.2 The risk for mortality is increased more than twofold, independently of the severity of cirrhosis and seems to be highest in patients with the most advanced stages of HPS.2, 3 The progression of hypoxia was reported in a cohort of untreated HPS‐positive patients (annual decline in PaO2 of 5 mm Hg/year).9 Therefore, early diagnosis and the subsequent initiation of adequate measures are of central importance. The common causes of death in patients with HPS are linked to nonpulmonary complications of cirrhosis such as infection, gastrointestinal bleeding, and hepatorenal syndrome.3
Medical Therapy
There is no established medical therapy for the treatment of HPS. Liver transplantation is the only successful therapy for HPS. Candidates with HPS and PaO2 > 60 mm Hg are prioritized for LTX in the US and several European countries by the award of ‘exceptional’ Model for End‐Stage Liver Disease (MELD) points. Recently, the impact of the HPS MELD exception policy on outcomes in patients after LTX was assessed using the United Network for Organ Sharing database.8 Because HPS‐positive patients had a significantly reduced risk of dying, this policy is currently being reevaluated to optimize equitable organ allocation. Initiation of long‐term oxygen therapy (LTOT) is recommended in patients with severe and very severe HPS. The therapy should be applied continuously to increase PaO2 levels > 60 mm Hg.
Various therapeutic options have been evaluated in experimental and clinical settings.1, 5, 11 The inhibition of the nitric oxide synthetase (NOS) pathway by different substances has been reported to ameliorate experimental HPS. However, although systemic administration of methylene blue—a potent inhibitor of guanylate cyclase—improved short‐term oxygenation in a case series of patients, inhaled L‐N(G)‐nitroarginine methyl ester (L‐NAME) failed to demonstrate an improvement of gas exchange in human HPS.12, 13 Garlic extracts may also interact with the nitric oxide pathway. Their administration improved gas exchange in patients with HPS in smaller studies.14, 15 To some extent, the phosphodiesterase inhibitor pentoxifylline inhibits tumor necrosis factor alpha and nitric oxide. Its administration resulted in contradictory results in patients with HPS.16, 17 Selective ETB receptor blockade decreased the pulmonary endothelial NOS expression and prevented the onset of HPS in rats.18 However, there is a lack of data concerning ETB receptor blockade in patients. Despite promising experimental findings, the administration of the antibiotic norfloxacin did not improve gas exchange in human HPS.19 Additionally, other substances such as aspirin, somatostatin, indomethacin, and almitrine bismesylate failed to ameliorate HPS. Recent reports indicate that pulmonary angiogenesis seems to be activated in HPS. Case reports indicate the improvement of HPS by substances with antiangiogenic properties such as sorafenib and mycophenolate mofetil. However, larger studies are needed to confirm these findings. Although some case reports described improvement of gas exchange in HPS patients undergoing transjugular intrahepatic portosystemic shunts (TIPS), a case series did not find improved oxygenation after TIPS in patients affected by HPS.20, 21 Therefore, guidelines do not recommend TIPS as a therapeutic option for HPS.6, 8
Conclusion
In summary, HPS is the most common respiratory complication in patients with hepatic disease. It leads to more than twofold increased mortality in patients with cirrhosis. The only established therapy is LTX and the administration of LTOT in patients with severe HPS.
Potential conflict of interest: Nothing to report.
References
- 1. Rodriguez‐Roisin R, Krowka MJ. Hepatopulmonary syndrome—a liver‐induced lung vascular disorder. N Engl J Med 2008;358:2378‐2387. [DOI] [PubMed] [Google Scholar]
- 2. Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology 2008;135:1168‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schenk P, Schoniger‐Hekele M, Fuhrmann V, Madl C, Silberhumer G, Muller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology 2003;125:1042‐1052. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez‐Roisin R, Krowka MJ, Herve P, Fallon MB. Pulmonary‐hepatic vascular disorders (PHD). Eur Respir J 2004;24:861‐880. [DOI] [PubMed] [Google Scholar]
- 5. Machiaco V, Balakrishnan M, Fallon M. Pulmonary complications in chronic liver disease. Hepatology 2014;59:1627‐1637. [DOI] [PubMed] [Google Scholar]
- 6. Abrams G, Jaffe C, Hoffer P, Binder H, Fallon M. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology 1995;109:1283–1289.. [DOI] [PubMed] [Google Scholar]
- 7. Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology 2005;41:1122‐1129. [DOI] [PubMed] [Google Scholar]
- 8. Goldberg D, Krok K, Batra S, Trotter J, Kawut S, Fallon M. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology 2014;146:1256‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horvatits T, Fuhrmann V. Therapeutic options in pulmonary hepatic vascular diseases. Expert Rev Clin Pharmacol 2014;7:31‐42. [DOI] [PubMed] [Google Scholar]
- 10. Schenk P, Madl C, Rezaie‐Majd S, Lehr S, Muller C. Methylene blue improves the hepatopulmonary syndrome. Ann Intern Med 2000;133:701‐706. [DOI] [PubMed] [Google Scholar]
- 11. Gomez FP, Barbera JA, Roca J, Burgos F, Gistau C, Rodriguez‐Roisin R. Effects of nebulized N(G)‐nitro‐L‐arginine methyl ester in patients with hepatopulmonary syndrome. Hepatology 2006;43:1084‐1091. [DOI] [PubMed] [Google Scholar]
- 12. Najafi Sani M, Kianifar HR, Kianee A, Khatami G. Effect of oral garlic on arterial oxygen pressure in children with hepatopulmonary syndrome. World J Gastroenterol 2006;12:2427‐2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De BK, Dutta D, Pal SK, Gangopadhyay S, Das Baksi S, Pani A. The role of garlic in hepatopulmonary syndrome: a randomized controlled trial. Can J Gastroenterol 2010;24:183‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta LB, Kumar A, Jaiswal AK, et al. Pentoxifylline therapy for hepatopulmonary syndrome: a pilot study. Arch Intern Med 2008;168:1820‐1823. [DOI] [PubMed] [Google Scholar]
- 15. Tanikella R, Philips GM, Faulk DK, Kawut SM, Fallon MB. Pilot study of pentoxifylline in hepatopulmonary syndrome. Liver Transpl 2008;14:1199‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Ling Y, Tang L, Luo B, Pollock DM, Fallon MB. Attenuation of experimental hepatopulmonary syndrome in endothelin B receptor‐deficient rats. Am J Physiol Gastrointest Liver Physiol 2009;296:G704‐G708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta S, Faughnan ME, Lilly L, Hutchison S, Fowler R, Bayoumi AM. Norfloxacin therapy for hepatopulmonary syndrome: a pilot randomized controlled trial. Clin Gastroenterol Hepatol 2010;8:1095‐1098. [DOI] [PubMed] [Google Scholar]
- 18. Riegler JL, Lang KA, Johnson SP, Westerman JH. Transjugular intrahepatic portosystemic shunt improves oxygenation in hepatopulmonary syndrome. Gastroenterology 1995;109:978‐983. [DOI] [PubMed] [Google Scholar]
- 19. Martinez‐Palli G, Drake B, Garcia‐Pagan J, Barbera J, Arguedas M, Rodriguez‐Roisin R, Bosch J, Fallon M. Effect of transjugular intrahepatic portosystemic shunt on pulmonary gas exchange in patients with portal hypertension and hepatopulmonary syndrome. World J Gastroenterol 2005;11:6858‐6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyer TD, Haskal ZJ. The Role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010;51:306. [DOI] [PubMed] [Google Scholar]
- 21. Peck‐Radosavljevic M, Angermayr B, Datz C, Ferlitsch A, Ferlitsch M, Fuhrmann V, et al. Austrian consensus on the definition and treatment of portal hypertension and its complications (Billroth II). Wien Klin Wochenschr 2013;125:200‐219. [DOI] [PubMed] [Google Scholar]


