Watch a video presentation of this article
Abbreviations
- ESLD
end stage liver disease
- HPS
hepatopulmonary syndrome
- ICU
intensive care unit
- IPVD
intrapulmonary vascular dilatations
- LOS
length of stay
- LT
liver transplantation
- MELD
model for end stage liver disease
- PEEP
pulmonary end-expiratory pressure
- POPH
portopulmonary hypertension
- RV
right ventrical
- RVSP
right ventricular systolic pressure
- UNOS
United Network for Organ Sharing.
Introduction
We often consider Hepatopulmonary syndrome (HPS) and portopulmonary hypertension (POPH) together as pulmonary vascular complications of end‐stage liver disease (ESLD). Although this is true, the clinical presentation, pathophysiology and management of HPS and POPH are really quite distinct from each other (Table 1). These issues have been discussed extensively in other sections of this series. In this brief review, we will focus on important aspects related to liver transplantation (LT) for HPS.
Table 1.
A Comparison Between HPS and POPH
| POPH | HPS | |
|---|---|---|
| Primary Pathophysiology | PAH | Intrapulmonary shunting |
| Pathology | PAH due to plexiform lesions, thrombosis, obliterative pulmonary arteriopathy | Intrapulmonary vascular dilatations causing intrapulmonary shunting and consequent hypoxemia |
| Severity of hypoxemia | + (typically mild) | +++ (mild to very severe, depending on degree of shunting) |
| Right ventricular function | Significantly elevated RVSP with RV dilatation/systolic dysfunction and low cardiac output | Normal or mildly elevated RVSP (due to high flow state) with normal RV size and function |
| Clinical findings | Loud 2nd heart sound, systolic murmur, RV heave, lower extremity edema along with features of portal hypertension (varices, splenomegaly, ascites etc.) | Clubbing, cyanosis, systolic flow murmur, platypnea, orthodeoxia along with signs of end‐stage liver disease. |
| Treatment | PAH therapy (e.g., ambrisentan, sildenafil, epoprostenol etc.) | Supportive care and management of underlying liver disease until LT (which is curative for HPS) |
| Is LT recommended/feasible? | Only in patients where PH is adequately controlled prior to LT | Recommended/feasible in all patients (even in severe hypoxemia) |
| MELD exception points available? | Yes | Yes |
Abbreviation: LT, liver transplantation; PAH, pulmonary arterial hypertension; RV, right ventricle; RVSP, right ventricular systolic pressure.
Brief Overview of HPS
HPS is characterized by the presence of hypoxemia due to the presence of intrapulmonary vascular dilatations (IPVDs) in the setting of portal hypertension (with/without cirrhosis). The prevalence of HPS varies widely between studies (5%‐32% of patients) and likely reflects diverse patient populations and varying definitions of hypoxemia. HPS is associated with varying severities of hypoxemia and a room air upright PaO2 ≤ 50mmHg is considered to be very severe hypoxemia.1 HPS‐related vascular pathology includes dilatations of pre‐ and postcapillary vessels (IPVDs), neoangiogenesis, and the development of arteriovenous malformations (both pleural and pulmonary). A careful initial evaluation to exclude other potentially treatable causes of hypoxemia is warranted (Table 2).
Table 2.
Initial Workup of ESLD Patient With Hypoxemia
| Test | Rationale |
|---|---|
| Transthoracic echocardiography with “bubble” study | Confirms HPS (presence of intrapulmonary shunt) And excludes other entities (POPH, intracardiac shunting, valvular disease, left ventricular systolic/diastolic dysfunction) |
| Pulmonary function testing with diffusing capacity | Identifies obstructive (e.g., COPD) and restrictive (e.g., interstitial lung disease) pulmonary disease and establishes degree of impairment |
| Room air sitting and standing PaO2 | Establishes degree of hypoxemia |
| 100% oxygen shunt study (sitting and standing) | Establishes degree of hypoxemia and shunting |
| Chest x‐ray and/or chest CT scan | Identifies other pulmonary pathologies (e.g. COPD, interstitial lung disease, chest wall and pleural disease) |
| Overnight oximetry | Establishes need for nocturnal O2 supplementation. Also useful in identifying sleep apnea if clinically suspected. |
| Oxygen titration study | Establishes supplemental O2 needs at rest and during exercise. |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Impact of HPS on Morbidity and Mortality in Patients with ESLD
HPS is an independent risk factor for poor outcomes in patients with ESLD. Mortality for patients with HPS undergoing evaluation for LT is roughly doubled as compared to non‐HPS patients.2 HPS is also associated with a significantly worsened quality of life with increased dyspnea, reduced exercise capacity, hypoxemia, and need for oxygen therapy. To date, no pharmacological therapies have been consistently shown to lead to resolution of HPS or result in sustained clinical improvement. Anecdotal case reports have documented improved oxygenation with norfloxacin, mycophenolate mofetil, garlic, and inhibitors of nitric oxide synthase such as L‐NMMA and methylene blue. One randomized trial showed a substantial beneficial effect of garlic in improving hypoxemia, but the results have not been replicated so far.3 LT remains the only proven and clinically validated treatment for HPS. Even patients with severe hypoxemia (PaO2 < 50 mmHg) have been shown to have complete resolution of hypoxemia post‐LT.4
Hypoxemia was initially considered to be contraindication to LT. However, over the past decade or so, the presence of HPS‐related hypoxemia has become a criterion to expedite LT with patients having a room air PaO2 ≤ 60 mmHg receiving Model for End Stage Liver Disease (MELD) exception points.5 Goldberg et al. recently published on their work utilizing the United Network for Organ Sharing (UNOS) database and suggested that only a fraction of eligible HPS patients actually received MELD exception points. This likely reflects a systematic under‐recognition of HPS and its impact on morbidity and mortality in patients with ESLD.6
Hypoxemia Severity and Mortality Post‐LT
Severe hypoxemia was once considered to be a contraindication for LT. Older studies with small case series did document higher post‐LT mortality in patients with severe hypoxemia. However, our group and Gupta et al. have recently published on the relationship between hypoxemia severity and post‐LT outcomes.4, 7 Both of these series had a large number of patients with severe HPS and reported favorable post‐LT outcomes, even in patients with severe hypoxemia (Fig. 1 and 2). Of note, Goldberg et al.'s recent study using the UNOS database again showed a higher mortality in patients with very severe hypoxemia (PaO2 ≤ 44 mmHg). However, their study also showed a higher mortality rate in patients with less severe hypoxemia (PaO2 ≥ 61 mmHg), raising questions about misclassification bias and other errors stemming from use of a large national database.6 The impact of hypoxemia on post‐LT outcomes is clearly an unresolved area that requires a systematic multi‐center effort to collect and report high quality short‐ and long‐term outcomes data.
Figure 1.
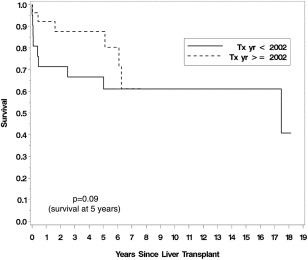
Survival following LT in the MELD exception era.4
Figure 2.
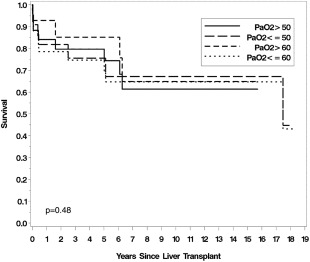
Lack of association between severity of hypoxemia and survival post‐LT.4
Special Considerations for Post‐LT Management in HPS Patients
Management of HPS patients' post‐LT can pose additional challenges for the transplant and intensive care unit (ICU) teams. Hypoxemia significantly improves in many patients by hospital (or even ICU) discharge. Thus it is imperative to provide expert ICU and respiratory support to these patients in the immediate post‐LT period. The most severely hypoxemic patients (PaO2 ≤ 50 mmHg) routinely require additional monitoring and advanced respiratory support in the post‐LT period. We reported a median hospital length of stay (LOS) of 14 days (range 5–65) and a median ICU LOS of 2 days (range 1–15) in 32 HPS patients for whom detailed post‐LT information was available.4 Half of these patients (13/28) had severe hypoxemia with a baseline PaO2 < 50 mmHg. Median intubation and mechanical ventilation period post‐LT was 10 hours (range 1–230 hours), and no patient required a tracheostomy. A suggested approach for post‐LT respiratory care is outlined in Fig. 3. Similar outcomes were also reported by Gupta et al.7 Thus, it appears that even severely hypoxemic patients can be safely transplanted with excellent short‐ and long‐term outcomes using modern ICU care protocols and noninvasive ventilatory support techniques.
Figure 3.
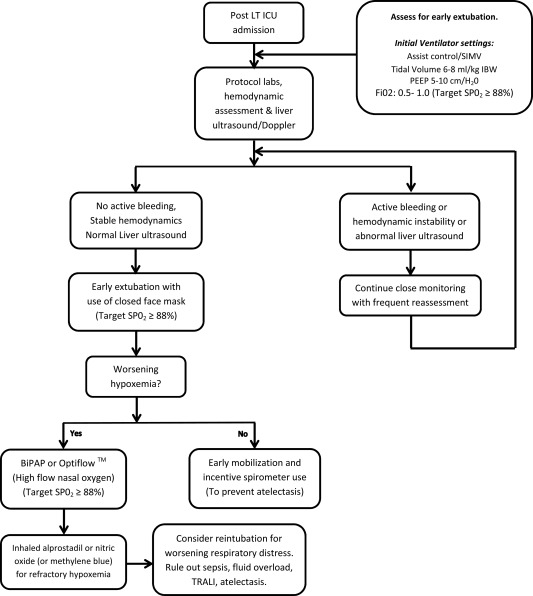
ICU management of HPS patients post‐LT.4 Adapted from Iyer, et al.8 Abbreviations: PEEP, positive‐end expiratory pressure. Abbreviations: IBW, ideal body weight; SIMV, synchronized intermittent mandatory ventilation; TRALI, transfusion related acute lung injury.
HPS Resolution Post‐LT
LT results in near uniform resolution of HPS features including hypoxemia and dyspnea. However, in some patients complete resolution can be delayed by many months, but does eventually occur.8 HPS can also re‐occur post‐LT if there is significant dysfunction in the transplanted liver.9
Development of POPH in Patients with HPS Post‐LT
Patients with HPS very rarely develop POPH post‐LT. It has been shown that HPS patients undergoing LT evaluation have features of right atrial and ventricular enlargement, along with RV hypertrophy.2 The role of activated alveolar macrophages in the pathogenesis of HPS has raised some intriguing possibilities for a common pathophysiological mechanism for both POPH and HPS in ESLD.10 An elegant study by Thenappan et al. provides evidence for the presence of a proliferative arteriopathy and vascular occlusion in a common bile duct ligated rat model of HPS.10 One possibility is that the nitric oxide mediated vasodilatation is reversed post‐LT, leading to unopposed vasoconstriction and the development of pulmonary arterial hypertension due to the underlying proliferative arteriopathy and vascular occlusions. The clinician needs to be alert to the development of dyspnea or other symptoms of right heart failure post‐LT for the early detection of POPH post‐LT. Due to the infrequency of this phenomenon, no recommendations can be made regarding follow‐up echocardiograms and so forth.
Conclusion
HPS is a common complication in ESLD patients receiving evaluation for LT. A screening program along with a comprehensive workup to exclude other treatable pulmonary pathologies is warranted. HPS is one of the conditions eligible for MELD exception. Post‐LT outcomes are favorable even in patients with severe hypoxemia, and LT typically results in complete HPS symptom resolution.
Potential conflict of interest: Nothing to report.
References
- 1. Rodriguez‐Roisin R, Krowka MJ. Hepatopulmonary syndrome—a liver‐induced lung vascular disorder. N Engl J Med 2008;358:2378‐2387. [DOI] [PubMed] [Google Scholar]
- 2. Fallon MB, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology 2008;135:1168‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De BK, et al. The role of garlic in hepatopulmonary syndrome: a randomized controlled trial. Can J Gastroenterol 2010;24:183‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iyer VN, et al. Hepatopulmonary syndrome: favorable outcomes in the MELD exception era. Hepatology 2013;57:2427‐2435. [DOI] [PubMed] [Google Scholar]
- 5. Fallon MB, et al. Model for end‐stage liver disease (MELD) exception for hepatopulmonary syndrome. Liver Transpl 2006;12(suppl 3):S105‐S107. [DOI] [PubMed] [Google Scholar]
- 6. Goldberg DS, et al. Impact of the Hepatopulmonary Syndrome MELD Exception Policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology 2014;146(5):1256‐1265 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta S, et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant 2010;10:354‐363. [DOI] [PubMed] [Google Scholar]
- 8. Iyer VN, Swanson KL, Krowka MJ. Survival benefits of liver transplant in severe hepatopulmonary syndrome. Am J Respir Crit Care Med, 2013;188:514. [DOI] [PubMed] [Google Scholar]
- 9. Krowka MJ, et al. Late recurrence and rapid evolution of severe hepatopulmonary syndrome after liver transplantation. Liver Transpl Surg 1999;5:451‐453. [DOI] [PubMed] [Google Scholar]
- 10. Thenappan T, et al. A central role for CD68(+) macrophages in hepatopulmonary syndrome: reversal by macrophage depletion. Am J Respir Crit Care Med 2011;183:1080‐1091. doi: 10.1164/rccm.201008-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


