Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- GCCR
glucocorticoid receptor gene
- HELLP
hemolysis, elevated liver function tests, low platelet counts
- LCHAD
long chain 3‐hydroxyacyl‐CoA dehydrogenase
- LDH
lactate dehydrogenase
- PET
plasma exchange therapy
- TLR4
toll‐like receptor 4 gene
Preeclampsia‐induced Liver Dysfunction and HELLP Syndrome
Definition and Epidemiology
Preeclampsia‐induced liver disease is a disorder unique to pregnancy and is frequently seen in the third trimester. Severe preeclampsia is defined by extreme elevation in systemic blood pressure and evidence of organ compromise. HELLP syndrome is a unique liver‐related disorder of pregnancy that was first described by Weinstein in 1982 as a constellation of clinical and laboratory abnormalities in pregnant women in their third trimester.1 This disorder was termed HELLP syndrome, with (H) for hemolysis, (EL) for elevated liver functions tests, and (LP) for low platelet counts.
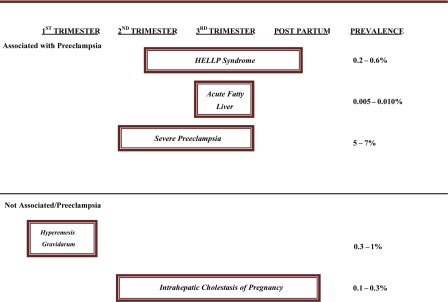
The incidence of HELLP syndrome is approximately 0.6% of all pregnancies and is considered a variant of severe preeclampsia. Seventy percent of cases occur between weeks 27 and 37, with 20% occurring within 48 hours of delivery. Features of preeclampsia occur in the majority of patients presenting with HELLP syndrome. Ten percent to 20% of patients with severe preeclampsia will develop HELLP. Liver involvement in preeclampsia is not common, however, if present signifies severe disease. Table 1 lists the prevalence of liver disorders unique to pregnancy throughout the gestation period and their association with preeclampsia.2
Table 1.
Liver Diseases Unique to Pregnancy
|
Pathogenesis
Vascular remodeling, uteroplacental ischemia, defects in the formation of the placenta, and immune‐mediated mechanisms have been proposed. Several gene variants such as glucocorticoid receptor gene (GCCR), toll‐like receptor 4 gene (TLR4), vascular endothelial growth factor gene, FAS gene, cluster of differentiation 95 (CD95), and the coagulation factor V Leiden mutation are associated with increased risk of HELLP compared to healthy women. Inadequate vascular placental invasion has been the leading hypothesis in the etiology of preeclampsia‐eclampsia and HELLP syndrome. Thrombotic microangiopathy leads to microangiopathic hemolytic anemia and liver damage in patients with HELLP. HELLP syndrome has also been reported in a few cases to be associated with defects in β‐oxidation of fatty acids.
Clinical Presentation
Affected pregnant women typically present with nonspecific symptoms of weight gain, right upper quadrant pain (90%), nausea or vomiting (50%), or nonspecific viral‐like illness. Jaundice is seen in 40% of patients. Concomitant signs and symptoms of preeclampsia may or may not be present. Severe right upper‐abdominal pain may herald impending hepatic rupture. Other causes of right upper‐quadrant pain must be sought such as acute cholecystitis, acute pancreatitis, peptic ulcer disease, and acute fatty liver disease of pregnancy. Pregnant women with preeclampsia/HELLP may present with involvement of other organs, resulting in cerebral or visual symptoms, pulmonary edema, and renal insufficiency.
Diagnosis
Table 2 shows both the Tennessee and Mississippi classifications for the diagnosis of HELLP. Liver enzyme abnormalities are seen in up to 10% of pregnant women with severe preeclampsia. These include two‐ to three‐fold elevation in alanine and aspartate aminotransferases. The frequency and severity of elevation of liver aminotransferases are much higher in HELLP syndrome than in severe preeclampsia.3 Thus, elevation in liver transaminases, lactate dehydrogenase (LDH), and uric acid in the setting of severe preeclampsia might indicate a progression to HELLP syndrome. Hemolysis is the hallmark of the triad of HELLP syndrome. The presence of an abnormal peripheral smear (microangioplastic anemia with schistocytosis); thrombocytopenia; and elevated levels of aspartate aminotransferase, alanine aminotransferase, bilirubin, and LDH are diagnostic.4 Liver transaminases are typically > 500 U/L. The Mississippi classification has been proposed for assessment of the severity of the pathologic process of HELLP. Class 1 denotes worse prognosis and longer hospital stay. The trends in platelet count and serum LDH levels are predictive of speed of recovery.5 Other causes that simulate HELLP syndrome are acute viral hepatitis, hemolytic uremic syndrome (HUS), thrombotic thrombocytopenic purpura (TTP), antiphospholipid syndrome, and AFLP. The classic hepatic lesion associated with the HELLP syndrome is periportal or focal parenchymal necrosis (Fig. 1A, 1B).6
Table 2.
Diagnostic Criteria for HELLP Syndrome
| Tennessee Classification | Mississippi Classification |
|---|---|
| Complete syndrome: | Class 1: platelets ≤ 50 × 109/L |
| Platelets ≤ 100 × 109/L | 1. AST or ALT ≥ 70 units/L |
| AST ≥ 70 units/L | 2. LDH ≥ 600 units/L |
| LDH ≥ 600 units/L | |
| Incomplete syndrome: | |
| Any one or two of the above | |
| Class 2: platelets ≤ 100 × 109/L | |
| 1. ≥ 50 × 109/L | |
| 2. AST or ALT ≥ 70 units/L | |
| 3. LDH ≥ 600 units/L | |
| Class 3: platelets ≤ 150 × 109/L | |
| 1. ≥ 100 × 109/L | |
| 2. AST or ALT ≥ 40 units/L | |
| 3. LDH ≥ 600 units/L |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Figure 1.
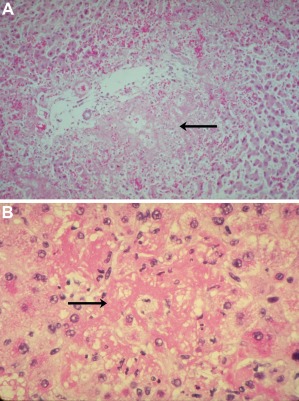
Liver in HELLP syndrome. (A) Periportal patchy hemorrhage and necrosis (arrow). (B) Sinusoidal deposition of fibrin (arrow); hematoxylin and eosin stain. Reproduced with permission from Hammoud.13
Management
Bed rest and control of hypertension is mandatory for all women with HELLP syndrome. All women diagnosed with preeclampsia and HELLP syndrome should receive IV‐infused magnesium sulfate for the prevention of eclamptic seizures and cerebral changes. Prompt delivery is indicated if the syndrome develops after 34 weeks of gestation, or earlier if there is multiorgan dysfunction, disseminated intravascular coagulation (DIC), liver infarction or hemorrhage, renal failure, suspected abruption placentae, or nonreassuring fetal status.
Corticosteroids improve laboratory abnormalities but do not alter the natural progression of liver disease.7, 8 Plasma exchange therapy (PET) has been used in the management of severe cases with HELLP syndrome and organ failure refractory to conservative management.9 PET is generally started on the day of delivery or postpartum until improvement in laboratory parameters.10
Hepatic rupture, although rare, is perhaps the most catastrophic complication of HELLP syndrome. Surgical and interventional radiologic procedures such as hepatic artery ligation, hepatic packing or lobectomy, arterial embolization, and liver transplantation have been described. HELLP syndrome can recur in subsequent pregnancies and is associated with a high risk of complications such as fetal prematurity and intrauterine growth retardation.
Acute Fatty Liver of Pregnancy
Definition and Epidemiology
Acute fatty liver of pregnancy (AFLP) is a serious and rare maternal liver disease unique to pregnancy that occurs in the third trimester. It was first described as a specific clinical entity in 1900.11 It carries a significant perinatal and maternal mortality. AFLP has an incidence of one per 7,270 to 13,000 deliveries. AFLP is more frequent in primaparous than multiparous women but can occur after multiple uneventful pregnancies.
Pathogenesis and Clinical Implications
There is a strong association between AFLP and mitochondrial long chain 3‐hydroxyacyl‐CoA dehydrogenase (LCHAD) deficiency in the fetus.12 LCHAD is part of a complex mitochondrial enzyme alpha subunit, the mitochondrial trifunctional protein, and its deficiency is known to be secondary to a common G1528C mutation. LCHAD deficiency in the fetus likely causes accumulation of long chain 3‐hydroxy‐fatty acyl metabolites particularly toxic to the liver. The interaction between the affected fetus and the mother may result from passage of these metabolites from the fetus to the maternal circulation. In addition, metabolic and environmental stresses in the third trimester can lead to further accumulation of hepatotoxic LCHAD‐metabolites in the maternal circulation (Fig. 2).13
Figure 2.
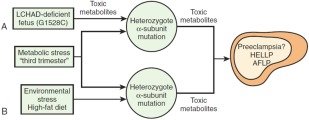
Hypothesis illustrating the possible role of fetal and maternal mitochondrial trifunctional protein mutations in developing AFLP. (A) Carrying an LCHAD‐deficient fetus is the major determining factor in the development of maternal illness. Hepatotoxic metabolites produced by the fetus and/or placenta may cause liver disease in the obligate heterozygous mother when combined with the metabolic stress of the third trimester. (B) Environmental stress may lead to the further accumulation of toxic metabolites in the genetically susceptible mother, causing maternal liver disease. Reproduced with permission from Hammoud.13
This association between AFLP and the common LCHAD G1528C mutation in the fetus is significant.
Approximately 20% of women who develop AFLP carry LCHAD‐deficient fetuses.14 Thus, screening the offspring of women who develop AFLP at birth for this mutation can be lifesaving.14, 15 Most newborns who are LCHAD‐deficient present with a metabolic crisis in the first year of life and can suffer a sudden unexpected death at a few months of age. Thus, genetic counseling and screening newborns for LCHAD deficiency in pregnancies complicated by AFLP should be sought as early as possible after delivery.14, 15 LCHAD‐deficient newborns can be treated by dietary modifications, with dramatic reduction in morbidity and mortality.
Clinical Presentation
The initial manifestations are nonspecific and include headache, fatigue, nausea and vomiting (70%), right upper quadrant or epigastric pain (50%‐80%), and jaundice (70%). Maternal morbidity includes hypoglycemia, renal failure, and coagulopathy, which can be seen in up to 90% of cases. Hepatic encephalopathy occurs later in the disease and should immediately alert the physician to the possibility of AFLP. Hypertension, edema, and ascites may be present. Half of the patients may have associated preeclampsia.16 Upper gastrointestinal hemorrhage due to coagulation abnormalities may be seen in some cases. In severe untreated cases, progression is rapid over a few hours or days to hepatic failure with coma, hypoglycemia, hyperammonemia, renal failure, and severe coagulopathy, with hemorrhage leading to death of the mother and fetus.
Diagnosis
Peripheral blood smears may demonstrate thrombocytopenia. Disseminated intravascular coagulopathy is common. Prothrombin time, partial thromboplastin time, and fibrinogen maybe abnormal. Urine analysis may reveal ketones, protein, or bilirubin. Blood urea nitrogen and creatinine may be elevated, and uric acid is increased. Histologically, AFLP is characterized microscopically by microvesicular hepatic steatosis (Fig. 3). The centrally located nuclei are dense, widespread inflammation or necrosis is absent, and mitochondrial dense bodies are present.
Figure 3.
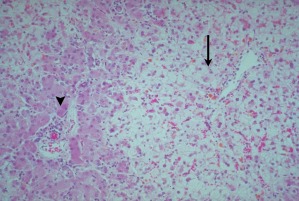
Acute fatty liver of pregnancy. Fat accumulation is greater in pericentral hepatocytes (arrow) compared with periportal hepatocytes (arrowhead); hematoxylin and eosin stain. Reproduced with permission from Hammoud.13
Distinguishing HELLP From AFLP
Table 3 lists the overlap distinguishing and clinical, laboratory, histological, and imaging features between HELLP syndrome and AFLP.
Table 3.
Distinguishing Features of HELLP and AFLP
| Features | HELLP | AFLP |
|---|---|---|
| Incidence in pregnancy | 0.2%‐0.6% | 0.005%‐0.01% |
| Onset during pregnancy | Third trimester or postpartum | Third trimester or postpartum |
| Presence of preeclampsia | Yes | > 50% |
| Abdominal pain | Common | Common |
| Clinical features | Hemolysis, thrombocytopenia, hyperuricemia | Liver failure with coagulopathy, encephalopathy, hypoglycemia, hyperuricemia, and DIC |
| Aminotransferases | Typically > 500 units/L | Typically 300‐500 units/L but could be > 1000 unites/L |
| Alkaline phosphatase | Exceeds those normally observed in pregnancy | 3‐10 fold upper limit |
| Bilirubin | < 5 mg/dL | < 5 mg/dL |
| LDH | Markedly elevated | Elevated |
| Peripheral smear | Schistocystes, spherocytes, reticulocytes | Thrombocytopenia, normoblasts |
| Renal impairment | Mild increase in BUN/creatinine | Mild to moderate increase in BUN/creatinine |
| Imaging | May show hepatic rupture or infarction | May show fatty infiltrate |
| Histology | Periportal hemorrhage, extensive necrosis, and hemorrhage | Microvesicular steatosis in zone 3 |
| Immunohistologic | Cytokine and immune‐mediated | Reactive oxygen species‐mediated mitochondrial apoptosis |
| Perinatal/fetal outcomes | Perinatal mortality, prematurity | Fetal mortality 9‐23% |
Abbreviation: BUN, blood urea nitrogen.
Management
Timely delivery after initial maternal stabilization is a cure. Prothrombin time improvement is the first sign of hepatic recovery. Liver transplantation is an option if the patient did not improve after delivery due to liver failure.
Conclusion
HELLP syndrome and AFLP are both rare and serious disorders unique to pregnancy. These disorders are seen in the third trimester of pregnancy and carry significant perinatal and maternal morbidity and mortality. Preeclampsia is associated with both disorders. Expedited delivery when fetal maturity is optimal is the main stay of therapy in both conditions.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol 1982;142:159‐167. [DOI] [PubMed] [Google Scholar]
- 2. Bearelly D, Hammoud GM, Koontz G, Merrill DC, Ibdah J. Preeclampsia‐induced liver disease and HELLP syndrome In: Ibdah J. A., ed. Maternal Liver Disease. 1st ed Austin, TX: Landes Bioscience; 2012: 73‐92. [Google Scholar]
- 3. Martin JN, Jr. , Rinehart BK, May WL, Magann EF, Terrone DA, Blake PG. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol 1999;180:1373‐1384. [DOI] [PubMed] [Google Scholar]
- 4. Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol 2004;103:981‐991. [DOI] [PubMed] [Google Scholar]
- 5. Sibai BM, Taslimi MM, el‐Nazer A, Amon E, Mabie BC, Ryan GM. Maternal‐perinatal outcome associated with the syndrome of hemolysis, elevated liver enzymes, and low platelets in severe preeclampsia‐eclampsia. Am J Obstet Gynecol 1986;155:501‐509. [DOI] [PubMed] [Google Scholar]
- 6. Arias F, Mancilla‐Jimenez R. Hepatic fibrinogen deposits in pre‐eclampsia. Immunofluorescent evidence. N Engl J Med 1976;295:578‐582. [DOI] [PubMed] [Google Scholar]
- 7. Woudstra DM, Chandra S, Hofmeyr GJ, Dowswell T. Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy. Cochrane Database Syst Rev 2010;9:CD008148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katz L, Amorim M, Souza JP, Haddad SM, Cecatti JG, Group CS. COHELLP: collaborative randomized controlled trial on corticosteroids in HELLP syndrome. Reprod Health 2013;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eser B, Guven M, Unal A, et al. The role of plasma exchange in HELLP syndrome. Clin Appl Thromb Hemost 2005;11:211‐217. [DOI] [PubMed] [Google Scholar]
- 10. Eckford SD, Macnab JL, Turner ML, Plews D, Liston WA. Plasmapheresis in the management of HELLP syndrome. J Obstet Gynaecol 1998;18:377‐379. [DOI] [PubMed] [Google Scholar]
- 11. Findlay JW. Remarks on the pathology of acute yellow atrophy of the liver. Br Med J 1900;1:1330‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ibdah JA, Bennett MJ, Rinaldo P, et al. A fetal fatty‐acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med 1999;340:1723‐1731. [DOI] [PubMed] [Google Scholar]
- 13. Hammoud GM, Ibdah JA. The liver in pregnancy In: Boyer T. D., Manns M. P., Sanyal A. J., eds. Zakim and Boyer's Hepatology: A Textbook of Liver Disease. Vol 52 6th ed Philadelphia, PA: Elsevier Saunders; 2012: 919‐940. [Google Scholar]
- 14. Yang Z, Yamada J, Zhao Y, Strauss AW, Ibdah JA. Prospective screening for pediatric mitochondrial trifunctional protein defects in pregnancies complicated by liver disease. JAMA 2002;288:2163‐2166. [DOI] [PubMed] [Google Scholar]
- 15. Ibdah JA. Role of genetic screening in identifying susceptibility to acute fatty liver of pregnancy. Nat Clin Pract Gastroenterol Hepatol 2005;2:494‐495. [DOI] [PubMed] [Google Scholar]
- 16. Buytaert IM, Elewaut GP, Van Kets HE. Early occurrence of acute fatty liver in pregnancy. Am J Gastroenterol 1996;91:603‐604. [PubMed] [Google Scholar]


