Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- CI
confidence interval
- EHR
electronic health records
- HCV
hepatitis C virus
- PQRS
Physician Quality Reporting System
- QAPI
Quality Assessment and Program Improvement
- QCDR
qualified clinical data registry
- QI
quality indicator
- SBP
spontaneous bacterial peritonitis
Quality Assessment in Chronic Diseases
Quality assessment in chronic disease has become increasingly widespread, with published quality measurement sets in conditions as diverse as diabetes, cardiac diseases, stroke, and cancer.1 The need to measure and improve quality of care is now more urgent given the advent of pay‐for‐performance under the Affordable Care Act. The criteria for selecting conditions for quality assessment include mortality and morbidity burden, variation in care (performance gaps), and the evidence for effective care (process‐outcome link).2
Chronic liver disease is a common condition, with cirrhosis of the liver accounting for nearly 60,000 deaths in the United States each year.3 Chronic liver disease is the 12th leading cause of death in the United States (3rd or 4th leading cause of death among persons aged 45–64 years).3, 4 Among all digestive disease‐related conditions, liver disease contributes the greatest number of years of potential life lost.4 Despite improvements in technology and treatments, liver‐related mortality has remained relatively unchanged over the last 3 decades.
It is well known that appropriate medical care for chronic liver disease can delay complications, improve quality of life, and possibly extend survival.5 Indeed, the link between care processes and desirable patient outcomes is clearly proven for most of the clinical practices in chronic liver disease. However, existing data are sparse on the extent to which these patients receive guideline recommended care, but existing studies indicate significant shortfalls.6, 7, 8 Given the mismatch between published standards of care and clinical practice, quality improvement efforts are needed to narrow this gap.
The first step toward improving the quality of chronic liver disease care is to systematically measure such care. Quality of health care can generally be measured using : a) structural measures (e.g., dedicated physician assistant in clinic, multidisciplinary clinics), b) process measures (e.g., prescribing beta‐blockers for patients with large varices), and c) outcome measures of care (e.g., mortality and health‐related quality of life) (Fig. 1).9 Although each measurement type has strengths and limitations, most quality assessment tools primarily include process measures because they require less risk adjustment than outcome measures and are actionable and more completely under provider or system control than outcome measures.2
Figure 1.
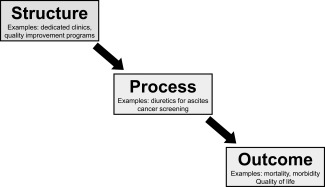
Domains of health care quality.
Quality Assessment in Chronic Liver Disease
Explicit process‐based quality indicators (QIs) exist for patients with chronic hepatitis C virus infection (HCV) and cirrhosis. These QIs have been used to assess the quality of health care delivered to patients with chronic liver disease.
Quality Assessment in HCV
HCV measures were developed by American Medical Association‐convened Physician Consortium for Performance Improvement and are currently included in the Center for Medicare's pay‐for‐ performance program: the Physician Quality Reporting System (PQRS).10 These measures include confirmation of hepatitis C viremia, testing for genotype before treatment, testing for viral load before treatment, testing for viral load between 4 and 12 weeks of therapy, and hepatitis A vaccination. Under PQRS, eligible professionals (defined as professionals who furnish Medicare Professional Fee Schedule‐covered services to Medicare part B beneficiaries) can use the following option(s) for quality reporting based on what fits their practice: claims‐based reporting of individual HCV measures, registry‐based reporting of individual HCV measures, claims‐based reporting of HCV measures group, or registry‐based reporting of HCV measures group. In addition to reporting on individual measures, the PQRS also gives the option to report on a measures group.
Using a nationwide U.S. health‐insurance company research database, we found substantial variation in the proportion of patients who met the HCV QIs.8 For example, 79% of patients received HCV genotype test prior to initiating antiviral treatment, while only 21.5% received at least one vaccination for hepatitis A or had documented immunity to it. Other QIs fell between these extremes (Table 1).8 These measures as well as performance on these measures may change with the availability of the new direct antiviral agents‐based treatments.
Table 1.
Rate of Meeting Hepatitis C Quality Indicators
| Quality Indicators | Score (%) |
|---|---|
| Confirmation of hepatitis C viremia | 72.0 |
| Hepatitis A vaccination | 21.5 |
| HCV genotype testing prior to treatment | 79.0 |
| RNA testing prior to treatment | 62.6 |
| RNA testing at week 12 of treatment | 60.3 |
These data were derived from nationwide US health insurance company research database.
Abbreviation: RNA, ribonucleic acid.
Quality Assessment in Cirrhosis
Cirrhosis QIs were developed using a modified Delphi Panel process that combined existing evidence base with the collective judgment of clinical experts.5 The selected QIs cover six domains of care: 1) ascites (13 QIs), 2) variceal bleeding (18 QIs), 3) hepatic encephalopathy (4 QIs), 4) hepatocellular cancer (1 QI), 5) liver transplantation (2 QIs), and 6) general cirrhosis care (3 QIs). Of these 41 QIs, Table 2 lists the subset of QIs considered as most important (i.e., those with largest beneficial effect) by the expert panel members. These include antibiotic treatment in patients who have an ascitic fluid polymorphonuclear count of > 250 cells/mm3 (i.e., have documented spontaneous bacterial peritonitis), managment with salt restriction and diuretics for clinically apparent ascites, diagnostic paracentesis in patients admitted for ascites or hepatic encephalopathy, use non‐selective beta‐blockers or esophageal variceal ligation (EVL) for patients with large varices that have not bled, upper endoscopy and use of somatostatin analogues in patients presenting with upper GI bleeding, use of EVL or sclerotherapy in patients with variceal hemorrhage, and hepatocellular cancer surveillance.
Table 2.
The Cirrhosis Quality Indicator Subset
| Domain | Quality Indicators | Evidence |
|---|---|---|
| Ascites | If hospitalized patients with ascites have an ascitic fluid polymorphonuclear count of ≥ 250 cells/mm3, they should receive empiric antibiotics within 6 hours of the test result for hospitalized patients and within 24 hours for ambulatory patients. |
Grade 1 Class 1, Level A |
| Ascites | If patients have clinically apparent (i.e., moderate to severe) ascites and normal renal function, they should be managed with both salt restriction and diuretics (including a combination of spirolonactone and loop diuretics). |
Grade I Class I, Level A |
| Ascites | If patients with ascites are admitted to the hospital for evaluation and management of symptoms related to ascites or encephalopathy, they should receive a diagnostic paracentesis during the index hospitalization. |
Grade II‐3 Class I, Level C |
| Variceal bleeding | If patients have cirrhosis, no documented history of previous GI bleeding, and have medium/large varices on endoscopy, they should receive either nonselective beta‐blockers or EVL within 1 month of varices diagnosis. |
Grade I Class I, Level A |
| Variceal bleeding | If patients with cirrhosis present with upper GI bleeding, they should receive upper endoscopy within 24 hours of presentation. |
Grade I Class I, Level A |
| Variceal bleeding | If patients with cirrhosis are admitted with or develop suspected variceal bleeding, they should receive somatostatin or analogues (somatostatin, octreotide, terlipressin) within 12 hours of presentation. |
Grade I Class IIA, Level A |
| Variceal bleeding | If patients with cirrhosis survive an episode of acute variceal hemorrhage, they should receive one of the following therapies to prevent recurrence of variceal hemorrhage: EVL every 1‐2 weeks until obliteration, beta‐blockers, or a combination or EVL and beta‐blockers. |
Grade I Class I, Level A |
| Variceal bleeding | If patients with cirrhosis are found to have bleeding esophageal varices, they should receive EVL or sclerotherapy at the time of index endoscopy. |
Grade I Class I, Level A |
| Liver cancer | If patients have cirrhosis, they should receive surveillance for hepatocellular cancer using imaging with or without alpha fetoprotein every 6‐12 months. |
Grade I, Class IIa, Level A |
Cirrhosis quality indicators that were endorsed as important based on the magnitude of health or health‐related quality of life benefit derived from performing the indicated processes. The last column presents the evidence that supported each indicator.
Abbreviations: EVL, endoscopic variceal ligation; GI, gastrointestinal.
Figure 2 displays the rates of meeting these QIs in a cohort of patients with cirrhosis seen at three Veterans Affairs Medical Centers between 2000 and 2007.6, 7 Although nearly all patients with documented spontaneous bacterial peritonitis (SBP) received antibiotics for treatment of SBP, only 57% of patients underwent the recommended paracentesis during the hospital admission.6 Similarly, 60% (95% confidence interval (CI) = 50.2%–70.7%) of patients with varices received either beta‐blockers or endoscopic variceal ligation for primary prophylaxis of variceal bleeding, and 59.3% (95% CI, 50.1%–68.5%) of patients received somatostatin analogues at the time of variceal bleeding.7
Figure 2.
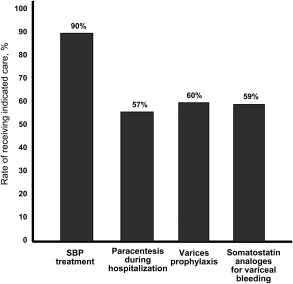
Rate of meeting cirrhosis quality indicators.
Methods of Quality Assessment in Chronic Liver Disease
The QIs described above provide the framework for quality assessment for patients with HCV and cirrhosis and can be collected using either medical record abstraction or electronic health records (EHR), particularly by creating new standard codes that explain exceptions or patient preferences for treatment decisions. As compared to EHR data, medical records contain more complete medical information. However, medical records are costly to obtain and abstract. This burden of data abstraction becomes multiplied in continuous quality improvement initiatives in which repeated measurements may be needed. Indeed, the movement in the quality measurement field is to track QIs that are readily obtainable from EHR and related databases. Although, the assessments using EHR data may be limited by inadequate documentation, inaccurate documentation, or missing data, previous studies have shown that the use of EHR (such as diagnosis and procedures codes) is reasonably valid for several HCV and cirrhosis QIs.6, 7, 11 Availability of EHR may also enhance the feasibility of repeated measurement needed for continuous quality improvement. Qualified clinical data registry (QCDR) is a new reporting mechanism available for the PQRS beginning in 2014. A QCDR will complete the collection and submission of PQRS quality measures data on behalf of professionals. There is still a major need for linking various EHR with qualified data registries to allow automated data pulls and reporting.
Quality Improvement in Chronic Liver Disease
Most of the published health‐care quality research in liver disease has focused on quality assessment. Measuring quality of cirrhosis care, although essential, is insufficient to improve the quality of health care in cirrhosis. Furthermore, the unintended consequences of focusing on available process measures remains a concern; rewarding professionals on the basis of a limited set of measures has the potential to undermine other aspects of care that are important but not captured in the current measurement systems.12 The next steps include expanding the measurement sets to include relevant outcomes as well as opportunities to improve these outcomes. Specifically, there remains a need to identify a set of outcomes13 that are relevant to our patients and that can be reliably tracked over time. These outcomes should cover both near‐term and longer‐term health, and include sufficient measurement of risk factors to allow for risk adjustment.13 Next, systems should be built that will facilitate a feedback loop in order to provide physicians and other health care professionals with the necessary information for continuous quality improvement. One such example is the Quality Assessment and Program Improvement (QAPI) system for solid organ transplant program.14 QAPI allows the transplant programs to track processes and outcomes, identify gaps in performance, take actions that result in improvement, and to ensure that these improvements are sustained.14 Similar programs and emerging data can then serve as a blueprint to guide the next step of continuous quality improvement initiatives for the burgeoning population of patients with chronic liver diseases.
Potential conflict of interest: Nothing to report.
References
- 1. Agency for Healthcare Research and Quality . National quality measures clearinghouse. Available at: http://www.qualitymeasures.ahrq.gov/browse/by‐topic.aspx. Last accessed May 2014. [DOI] [PubMed]
- 2. McGlynn EA, Asch SM. Developing a clinical performance measure. Am J Prev Med 1998;14(suppl 3):14‐21. [DOI] [PubMed] [Google Scholar]
- 3. Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver‐related mortality in the United States. Gastroenterology. 2013;145(2):375‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Everhart JE, editor. The burden of digestive diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office, 2008; NIH Publication No. 09–6443. Available at: http://www.niddk.nih.gov/about‐niddk/strategic‐plans‐reports/Pages/burden‐digestive‐diseases‐in‐united‐states‐report.aspx#CHAPTER25. Last accessed May 2014. [Google Scholar]
- 5. Kanwal F, Kramer J, Asch SM, El‐Serag H, Spiegel BM, Edmundowicz S, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol 2010;8:709‐717. [DOI] [PubMed] [Google Scholar]
- 6. Kanwal F, Kramer JR, Buchanan P, Asch SM, Assioun Y, Bacon BR, Li J, El‐Serag HB. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology 2012;143:70‐77. [DOI] [PubMed] [Google Scholar]
- 7. Buchanan P, Kramer JR, El‐Serag HB, Asch SM, Assioun Y, Bacon BR, Kanwal F. The quality of care provided to patients with cirrhosis and varices in the Department of Veterans Affairs. Am J Gastroenterology 2014;109:934‐940. doi: 10.1038/ajg.2013.487. [DOI] [PubMed] [Google Scholar]
- 8. Kanwal F, Schnitzler MS, Bacon BR, Hoang T, Buchanan PM, Asch SM. Quality of care in patients with chronic hepatitis C virus infection: a cohort study. Ann Intern Med 2010;153:231‐239. [DOI] [PubMed] [Google Scholar]
- 9. Donabedian A. Explorations in Quality Assessment and Monitoring. Volume 1. The Definition of Quality and Approaches to its Assessment. Ann Arbor, MI: Health Administration Press; 1980. [Google Scholar]
- 10. Centers for Medicare and Medicaid Services Physician Quality reporting System . Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/PQRS/MeasuresCodes.html. Last accessed May 2014.
- 11. Kanwal F, Hoang T, Kramer J, Chrusciel T, El‐Serag H, Dominitz JA, Asch SM. The performance of process measures in hepatitis C. Am J Gastroenterol 2012;107:1512‐1521. [DOI] [PubMed] [Google Scholar]
- 12. Berenson RA, Kaye DR. Grading a physician's value—the misapplication of performance measurement. N Engl J Med 2013;369:2079‐2081. [DOI] [PubMed] [Google Scholar]
- 13. Porter ME. What is value in health care? N Engl J Med 2010;363:2477‐2481. [DOI] [PubMed] [Google Scholar]
- 14. Hamilton TE. Improving organ transplantation in the United States—a regulatory perspective. Am J Transplant 2008;8:2503‐2505. [DOI] [PubMed] [Google Scholar]


