Abstract
Background:
The blood monocyte-to-lymphocyte ratio (MLR) is associated with active tuberculosis (TB) in adults, but has not been evaluated as a TB diagnostic biomarker in HIV-infected children in whom respiratory sampling is difficult.
Setting:
In a cohort of HIV-infected hospitalized Kenyan children initiating antiretroviral therapy, absolute monocyte and lymphocyte counts were determined at enrollment and 4, 12, and 24 weeks thereafter.
Methods:
Children were classified as confirmed, unconfirmed, or unlikely pulmonary TB. ROC curves of MLR cutoff values were generated to distinguish children with confirmed TB from those with unconfirmed and unlikely TB. General estimating equations were used to estimate change in MLR over time by TB status.
Results:
Of 160 children with median age 23 months, 13 (8.1%) had confirmed TB and 67 (41.9%) had unconfirmed TB. Median MLR among children with confirmed TB [0.407 (interquartile range (IQR) 0.378 – 0.675)] was higher than MLR in children with unconfirmed [0.207 (IQR 0.148 – 0.348), p < 0.01] or unlikely [0.212 (IQR 0.138 – 0.391), p = 0.01] TB. MLR above 0.378 identified children with confirmed TB with 77% sensitivity, 78% specificity, 24% positive predictive value, and 97% negative predictive value. After TB treatment, median MLR declined in children with confirmed TB and levels were similar to children with unlikely TB after 12 weeks.
Conclusion:
Blood MLR distinguished HIV-infected children with confirmed TB from those with unlikely TB and declined with TB treatment. MLR may be a useful diagnostic tool for TB in settings where respiratory-based microbiologic confirmation is inaccessible.
Keywords: tuberculosis, HIV, children, monocytes, lymphocytes, biomarkers
INTRODUCTION
Mycobacterium tuberculosis disease (TB) is a leading cause of mortality in HIV-infected children.1 In 2016 there were over 1 million incident cases of TB and 253,000 TB-related deaths in children under 15 years of age.2 Microbiologic diagnosis of TB in children is difficult given paucibacillary disease and the operational challenges of obtaining respiratory specimens in young children who are unable to produce sputum.3 Host biomarkers for TB disease may provide an alternative to pathogen-based biomarkers for TB diagnosis.4 T cell activation markers and transcriptional profiling5–9 have shown utility for diagnosis in children, but are costly and require specialized equipment and training.
The blood monocyte-to-lymphocyte ratio (MLR), derived from blood counts that are routinely collected in resource-limited settings for the management of acute illness, has been shown to predict progression to TB disease in children and adults.10–12 In a cohort of HIV-infected and HIV-exposed uninfected South African and Botswanan children, elevated MLRs at 3–4 months of age predicted onset of TB disease by 2 years of age.10 Among HIV-infected African women, elevated MLR during pregnancy was associated with increased risk for incident TB disease over 18 months of postpartum follow-up, even when controlling for CD4+ count, antiretroviral treatment (ART), and World Health Organization HIV stage.11 Less is known about the diagnostic performance of MLR, but an Italian study of adults without HIV found that an MLR cutoff >0.285 had high sensitivity and specificity (91% and 94%, respectively) to identify patients with culture-confirmed TB.13 Furthermore, MLR may be useful as an indicator of treatment response, as demonstrated in a cohort of Chinese adults with TB in which MLRs normalized to ranges similar to those of healthy controls after a 6-month treatment course.14
To our knowledge, the performance of MLR as a diagnostic biomarker for TB has not been evaluated in children with or without HIV disease. In addition, prior studies performed have not accounted for important co-factors that may alter MLR, such as immunosuppression12, nutritional status15 and malaria co-infection12,15–17. In a cohort of hospitalized Kenyan HIV-infected children starting antiretroviral therapy with well-classified TB disease status, we investigated the association between MLR and active TB disease determined at enrollment, and evaluated MLR changes over a 6-month time period as a potential indicator of treatment response. We evaluated CD4+ count, nutritional status, and malaria infection as potential confounders of the association between MLR and TB.
METHODS
Study Population
We conducted a longitudinal cohort study nested within the Pediatric Urgent Start of HAART (PUSH) randomized clinical trial (NCT02063880).18 Study subjects in the parent trial were HIV-infected, antiretroviral therapy-naïve children age 12 years old or younger, hospitalized in Kenya. Participants were randomized in the parent trial to urgent (less than 48 hours) or early (7–14 days) antiretroviral therapy and were excluded if they had a central nervous system infection at enrollment. Children were excluded from this sub-analysis if they initiated treatment for TB more than 14 days prior to or after enrollment or if they did not complete at least one test for microbiologic confirmation of TB diagnosis.
The parent study was reviewed and approved by the Institutional Review Board (IRB) at the University of Washington, the University of Nairobi/Kenyatta National Hospital Ethical Review Committee (UoN/KNH ERC), and the Pharmacy and Poisons Board in Kenya, and is in accordance with the Helsinki Declaration of 1975. Written informed consent was obtained from all participants’ caregivers in their preferred language (English, Kiswahili, or Dholuo).
Study Procedures
All children were evaluated at enrollment for pulmonary tuberculosis by symptom screening, physical examination, tuberculin skin test (TST), chest radiograph, two sputum or gastric aspirate samples for direct Ziehl-Neelsen (ZN) smear microscopy and liquid culture using BACTEC Mycobacteria Growth Indicator Tube (MGIT)™ 960 system (Becton Dickinson, Sparks, MD, USA), one sputum or gastric aspirate specimen for PCR using Xpert MTB/RIF® (Cepheid, Sunnyvale, CA, USA), and one stool specimen for PCR using Xpert MTB/RIF®. For TST, 5 units (0.1 mL) of purified protein derivative (RT23 solution; Sanofi Pasteur, Lyon, France) were injected intradermally and a study nurse measured induration 48 to 72 hours later. Children were also evaluated at enrollment for symptoms and signs of extrapulmonary TB.
Blood specimens were obtained from each participant for full blood count and differential (including monocyte and lymphocytes) at enrollment and 4, 12, and 24-week follow-up visits. CD4+ percentage was determined at enrollment, 4 and 24-week follow-up visits. Full blood counts were performed on an automated MS4 Haematology analyzer (Melet Schloesing Laboratoires, Osny, France) and AC•TTM 5diff Coulter® counter (Beckman Coulter, Inc., Brea, United States). MLR was calculated after conclusion of the parent trial and was not considered in the diagnostic evaluation of children. A study nurse evaluated growth parameters (height, weight, middle upper arm and head circumference) at every encounter.
Participants were treated with combination antiretroviral therapy (cART) (abacavir and lamivudine with either nevirapine, efavirenz, or lopinavir/ritonavir) according to Kenyan Ministry of Health guidelines.19 Children with suspected TB as assessed clinically by hospital medical officers were treated with a six-month regimen of rifampin, isoniazid, pyrazinamide, and ethambutol per Kenyan National TB Program guidelines.20 ART regimens were adjusted as needed for children receiving concurrent TB treatment to avoid medication interactions.[20]
Definitions
The monocyte-to-lymphocyte ratio (MLR) was determined by dividing absolute monocyte counts by absolute lymphocyte counts at each study time point. Weight-for-age Z-scores (WAZ) and weight-for-height Z-scores (WHZ) were calculated based on WHO growth curves using WHO ANTHRO software (version 3.2.2 World Health Organization, Geneva, Switzerland).21
Children were classified as having microbiologically-confirmed TB, unconfirmed TB (clinical presentation suggestive of TB with at least two of the following: TB symptoms, abnormal CXR, positive tuberculin skin test or TB exposure history, or response to anti-TB treatment), or unlikely TB at enrollment based on international consensus clinical case definitions for pulmonary TB.22 For the purposes of this analysis, failure to thrive was defined by underweight (WAZ ≤−2) or wasting (WHZ ≤−2 if under 5 years of age or MUAC <12.5 for ages 5 to 12 years). Response to anti-TB treatment was defined as increase in weight and resolution of enrollment TB symptoms over 24 weeks of follow-up.
Statistical Analysis
Children were stratified by confirmed, unconfirmed, or unlikely pulmonary TB classification. Descriptive measures of frequency (counts and percentages for categorical variables, medians and interquartile ranges [IQRs] for continuous variables) were calculated for all covariates. Fisher’s exact tests were used to compare the distributions of categorical variables between confirmed versus unlikely TB groups and unconfirmed versus unlikely TB groups. Wilcoxon two-sample tests were used to compare the distributions of continuous variables between confirmed versus unlikely TB groups and unconfirmed versus unlikely TB groups. Study power was calculated using OpenEpi (Version 3.01, updated 04/06/2013).23
ROC curves of MLR cutoff values were used to distinguish children with confirmed TB, unconfirmed and unlikely TB. The optimal MLR diagnostic cutoff was determined based on the maximum value of Youden’s index, J, where J = sensitivity + specificity – 1.24
General estimating equations (GEE) were used to estimate the association between TB status and changes in repeated MLR measures over the study period. We also estimated this association for absolute monocyte count and absolute lymphocyte counts individually. We evaluated baseline and time-varying CD4+ percentage, WAZ and WHZ, and malaria infection as potential confounders of the association between MLR and TB. Time-varying CD4+ percentage was considered a priori as a confounder of interest since CD4+ percentage is associated with active TB and MLR, as was time since enrollment because it is a proxy for TB treatment over time. We assessed for multicollinearity and selected the most parsimonious model. Due to the known association of age with TB disease,25,26 a sensitivity analysis was performed with age included as an additional cofactor in our final adjusted model. Sensitivity analyses restricting participants to children who had completed the study and children who were treated for TB were also conducted.
Two-sided p-values < 0.05 were considered statistically significant. All analyses were conducted using SAS software (version 9.4, SAS Institute Inc, Cary, NC).
RESULTS
Cohort Characteristics
Of 183 randomized children from April 2013 to May 2015 in the parent trial we included 160 children in this secondary analysis. Twenty-three children were excluded for the following reasons: 1 for HIV-negative status, 1 for concurrent CNS infection, 13 for TB treatment 14 days prior to or after enrollment and 8 for failing to receive microbiologic confirmation testing. Three patients had signs of extrapulmonary TB infection in conjunction with pulmonary TB (one child with lymphadenitis, a second with miliary infiltrate on chest radiography and a third with miliary infiltrate and vertebral spondylitis on chest radiography). There were two patients that had extrapulmonary TB (miliary infiltrate on chest radiography) without evidence of pulmonary TB. Thirteen children met criteria for confirmed TB (8.1%), 67 (41.9%) had unconfirmed TB, and 80 (50.0%) were unlikely to have TB (Figure, Supplemental Digital Content 1, patient inclusion flowchart). Overall, the median age at enrollment was 22.8 months (IQR 10.0–62.7), 87 children (54.4%) were male, 30 (18.8%) children died during the study period, and 13 (8.1%) were lost-to-follow-up.
The median enrollment CD4+ percentages for children in the confirmed and unconfirmed groups were similar to the CD4+ percentage in the unlikely TB group (p = 0.10 and p = 0.21, respectively); however, over all study intervals, the median CD4+ percentage was significantly lower for participants in the confirmed and unconfirmed groups as compared to the unlikely TB group (p = 0.01 and p = 0.01, respectively). Over all study intervals, the median WAZ and WHZ were lower for the confirmed and unconfirmed TB children as compared to those in the unlikely TB group (WAZ: p < 0.01 and p < 0.01, respectively; WHZ: p < 0.01 and p < 0.01, respectively). Overall, 25 children were diagnosed with malaria during the study period, with 15 confirmed by positive blood smear and the remainder diagnosed clinically. Of 25 children with malaria, none had confirmed TB (p = 0.23 compared to unlikely TB), 5 had unconfirmed TB (p = 0.03 compared to unlikely TB) and 20 were unlikely to have TB.
MLR Diagnostic Utility
At enrollment, the median MLR for children with confirmed TB [0.407 (IQR 0.378 – 0.675)] was higher compared to children with unconfirmed [0.207 (IQR 0.148 – 0.348), p < 0.01] or unlikely [0.212 (IQR 0.138 – 0.391), p = 0.01] TB. Children with unconfirmed TB had similar MLRs compared to children with unlikely TB (p = 0.87) (Table 2). The study power calculated to detect the difference in MLR between the confirmed TB (n = 13, median MLR = 0.407) and unlikely TB (n = 79, median MLR = 0.212) groups was 70.0% (assuming α = 0.05 with two-tailed t-test).
Table 2:
Median Blood MLR by Visit Week and TB Classification
| Time from Enrollment (wk) |
Overall Median (IQR*), n |
TB-Confirmed Median (IQR), n |
TB-Unconfirmed Median (IQR), n |
TB-Unlikely Median (IQR), n |
TB- Confirmed, P† |
TB- Unconfirmed, P† |
|---|---|---|---|---|---|---|
| 0 | 0.214 (0.148–0.397), 158 | 0.407 (0.378–0.675), 13 | 0.207 (0.148–0.348), 66 | 0.212 (0.138–0.391), 79 | 0.01 | 0.87 |
| 4 | 0.111 (0.079–0.212), 124 | 0.340 (0.212–0.581), 9 | 0.135 (0.092–0.217), 54 | 0.093 (0.064–0.145), 61 | < 0.01 | < 0.01 |
| 12 | 0.095 (0.065–0.145), 118 | 0.119 (0.115–0.532), 5 | 0.094 (0.065–0.143), 52 | 0.091 (0.052–0.145), 61 | 0.21 | 0.60 |
| 24 | 0.078 (0.055–0.121), 116 | 0.109 (0.048–0.348), 6 | 0.077 (0.054–0.119), 50 | 0.078 (0.057–0.119), 60 | 0.51 | 0.79 |
Interquartile range.
Wilcoxon 2-sample test t approximation reported. The reference group is TB Unlikely.
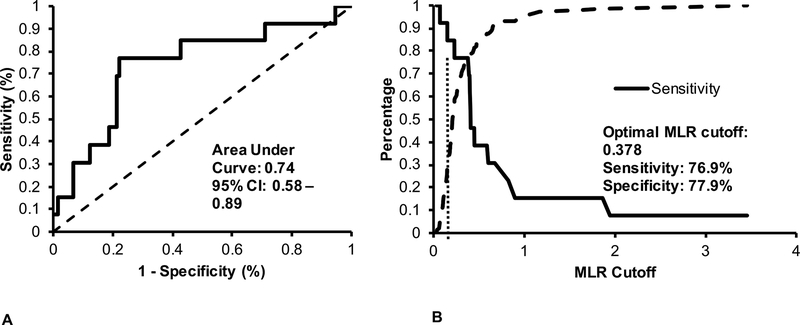
The optimal MLR cutoff value of 0.378 identified 10 of 13 confirmed TB patients as having TB disease with sensitivity 77% (95% CI 50 – 92%), specificity 78% (95% CI 71 – 84%), positive predictive value (PPV) 24% (95% CI 14 – 39%), negative predictive value (NPV) 97% (95% CI 93 – 99%), positive likelihood ratio (LR) 3.5 (95% CI 3.1 – 3.9), and negative LR 0.3 (95% CI 0.2 – 0.6). The corresponding area under the ROC curve was 0.74 (95% CI 0.58 – 0.89) (Figure 2 and Table, Supplemental Digital Content 2, MLR cutoff values). In sensitivity analyses comparing the confirmed TB group with the unlikely TB group yielded similar diagnostic testing results.
Figure 2.
Receiver operating characteristic curve (A) and sensitivity and specificity curve (B) for MLR cutoffs identifying TB confirmed patients. The optimal MLR cutoff above 0.378 had sensitivity 77%, specificity 78%, positive predictive value 24%, and negative predictive value 97%.
Longitudinal changes in MLR
The association between TB status and MLR over all study intervals was significant for the confirmed TB group compared to the unlikely TB group [β = 0.32, standard error (SE) 0.13, p = 0.01] on univariate analysis. There was no significant difference between MLR among children with unconfirmed TB compared to children unlikely to have TB on univariate analysis (Table 3). In our multivariable GEE model adjusting for time-varying CD4+ percentage and visit week since enrollment, the association between confirmed TB diagnosis and MLR remained significant (β = 0.27, SE 0.12, p = 0.02; reference unlikely TB group), and the association between unconfirmed TB and MLR remained non-significant (β = 0, SE 0.03, p = 0.99; reference unlikely TB group). No other potential confounders were added to this model due to lack of association with MLR (malaria status), TB status (age), or collinearity with time-varying CD4 percentage (time-varying WAZ and WHZ). Sensitivity analyses with age included as a covariate did not significantly affect the model. Excluding the two patients with only extrapulmonary TB did not significantly change our findings. In addition, MLRs were not significantly different between the two treatment arms of the parent trial (urgent or early antiretroviral therapy).
Table 3:
Association of TB-Confirmed and TB-Unconfirmed Groups With Absolute Monocyte Count, Absolute Lymphocyte Count, and MLR Over All Study Intervals
| Absolute Monocyte Count (103 Cells/μL) | Absolute Lymphocyte Count (103 Cells/μL) | Monocyte–Lymphocyte Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β* | SE† | Model P | β | SE | Model P | β | SE | Model P | |
| TB-confirmed | |||||||||
| Unadjusted model | 0.65 | 0.38 | 0.09 | −1.82 | 1.01 | 0.07 | 0.32 | 0.13 | 0.01 |
| Adjusted model‡ | 0.27 | 0.12 | 0.02 | ||||||
| TB-unconfirmcd | |||||||||
| Unadjusted model | 0.12 | 0.14 | 0.39 | −0.12 | 0.59 | 0.83 | 0.01 | 0.03 | 0.66 |
| Adjusted model‡ | 0 | 0.03 | 0.99 | ||||||
| TB unlikely | ref | ref | ref | ||||||
Parameter estimate.
Standard error.
Model adjusted for time-varying CD4 percentage and time from enrollment.
The two components of the MLR, absolute blood monocyte count and absolute blood lymphocyte count, were analyzed individually in unadjusted analyses for their association with TB status using GEE modeling (Table, Supplemental Digital Content 3, median monocyte and lymphocyte counts by visit week and TB group). Over all study intervals, there were no statistically significant associations between the absolute monocyte or lymphocyte count and the TB groups (Table 3).
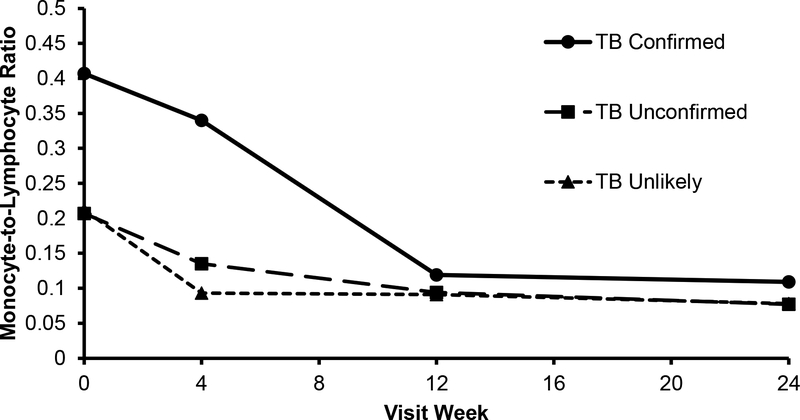
Over 24 weeks of anti-TB treatment, median MLR declined by 0.298 among children with confirmed TB (p = 0.01) and was similar to MLR levels of children unlikely to have TB by week 12 of TB treatment (p = 0.21) (Figure 1, Table 2). Among unconfirmed patients who received TB treatment, however, there was no difference in median MLR when compared to the unlikely TB group at all study intervals.
Figure 1.
Median blood monocyte-to-lymphocyte ratio over visit weeks from enrollment by TB classification (TB confirmed, unconfirmed or unlikely). The median MLR in the TB confirmed group declined to levels similar to the TB unconfirmed and TB unlikely groups by 12 weeks of anti-TB treatment.
In sensitivity analyses restricted to children with complete 6-month follow-up (excluding those who died or who were lost to follow-up), children with confirmed TB had a trend of higher median MLR over all study intervals as compared to children with unconfirmed or unlikely TB, but this did not reach statistical significance (p = 0.09 and p = 0.08, respectively). The trend of MLR among the three TB groups over the study period was similar to the longitudinal findings with all participants included (Table and Figure, Supplemental Digital Content 4, median MLR by visit week and TB group among those patients who completed the study).
When restricting participants to only those treated for TB, children who died during the study period or had treatment failure had a trend of higher enrollment median MLRs [(0.346 (IQR 0.271, 0.632)] compared to those who had response to TB treatment [0.209 (IQR 0.147, 0.375), p = 0.06] (Table, Supplemental Digital Content 5.1, comparing median MLR among those who had TB treatment response versus failure). Among children with TB treatment response, the pattern of longitudinal changes in MLR for the confirmed and unconfirmed TB groups was similar to the curves when all participants were included (Table and Figure, Supplemental Digital Content 5.2 and 5.3, median MLR by visit week and TB group among those patients who had TB treatment response). Longitudinally, children with confirmed TB who did not respond to TB treatment or who died before completing the study showed a trend toward higher MLRs compared to children with unconfirmed or unlikely TB, although sample size was limited for this sensitivity analysis (Table and Figure, Supplemental Digital Content 5.4, 5.5 and 5.6).
DISCUSSION
Among HIV-infected children, blood MLR distinguished children with microbiologically-confirmed pulmonary TB disease from those with unconfirmed or unlikely TB. An MLR value above 0.378 was associated with moderate sensitivity (77%) and specificity (78%) to identify confirmed TB cases at enrollment. By 12 weeks of anti-tuberculosis treatment the median MLR of the confirmed TB group declined to levels similar to the unconfirmed and unlikely TB groups. Furthermore, children across all diagnostic groups who were treated for TB and died or did not respond to treatment had a trend of higher median enrollment MLRs compared to those who had treatment success.
The MLR cutoff value above 0.378 demonstrated good overall TB diagnostic performance in our cohort of hospitalized HIV-infected Kenyan children. Our cutoff was higher and had lower sensitivity and specificity compared to a study of Italian adults in which an MLR cutoff of 0.285 had sensitivity 91% and specificity 94% for identifying active TB in HIV negative adults compared to healthy controls.13 We hypothesize that the optimal cutoff value from our analysis was higher because we compared confirmed TB patients with pooled unconfirmed and unlikely TB patients who were all HIV-infected and hospitalized rather than healthy controls. When La Manna and colleagues compared participants with TB to those with latent TB infection, the optimal MLR cutoff increased to 0.305 with decreased sensitivity and specificity.13
The sensitivity of MLR to detect confirmed TB is comparable to other rapid diagnostic methods for microbiologic confirmation that utilize non-sputum based sample collection in children. Studies evaluating stool Xpert MTB/RIF® reported test sensitivities between 32% and 81% and specificities between 99% and 100% as compared to the gold standard of culture-positive respiratory specimens.27–31. Sensitivity of stool Xpert MTB/RIF® improved when data were restricted to HIV-infected children (63% to 80%).27,29,31 Nasopharyngeal aspirate Xpert MTB/RIF® assays had similar diagnostic performance to stool samples in children (sensitivity: 39% to 65% and specificity: 98% to 99%),32,33 while urinary lipoarabinomannan (LAM) assays performed less well (sensitivity from 0% to 70% and specificity from 60% to 97% depending on HIV infection status and type of assay).31,34–36 A meta-analysis of HIV-negative and positive adults found the sensitivity of serum C-reactive protein (CRP) > 1.0 mg/dL for TB diagnosis ranged between 56% to 96% and specificity between 0% to 67%; elevated CRP levels have been observed in children with active TB but diagnostic performance in children is not known.37,38 While the sensitivities of these rapid diagnostic tests in children are similar to the sensitivity of MLR in our cohort, the specificity of MLR was lower which may be an important limitation for clinicians to consider.
In settings with limited capacity for microbiologic testing, MLR may be an inexpensive and rapid tool to inform clinical TB diagnosis. Despite the scale-up of Xpert MTB/RIF, it is underutilized in many populations and children may have lower odds of getting Xpert MTB/RIF testing.39 In a 2012 study of 47 sites, the test was used for only 4% of patients, possibly due to limitations in electrical power, transportation and cartridge availability.40 Additionally, lack of clinical staff training and program guideline knowledge may hinder Xpert MTB/RIF implementation.41 MLR, therefore, may be a more accessible test in some settings.
We hypothesize that elevation in MLR among children with confirmed TB disease could reflect higher mycobacterial burden. Monocytes proliferate in the presence of mycobacterial growth before migrating and differentiating into macrophages, while CD4+ T-lymphocytes are the primary effectors of adaptive immune response to Mycobacterium tuberculosis infection.42,43 Naranbhai et al. have shown in vitro that higher MLR was associated with mycobacterial growth and that an elevated ratio of gene expression transcripts of monocytes to lymphocytes was associated with TB disease in vivo.44 In our cohort, higher MLR among children with treatment failure or death and decline in MLR with anti-TB treatment (consistent with previous studies in adults)13,14 support our hypothesis that MLR may be a useful biomarker for mycobacterial burden and increased risk of mortality. Future studies to evaluate MLR change over time in the first few weeks after TB treatment initiation will be useful to assess its role as a marker of early treatment response and mortality risk in this population. In our study MLR did not distinguish children with microbiologically unconfirmed TB from children who were unlikely to have TB, possibly because of the lower mycobacterial burden in those with unconfirmed TB. Additional studies to explore the utility of MLR among children with unconfirmed TB may help inform clinical TB treatment decisions if microbiological confirmation cannot be obtained.
This study contributes to a growing body of research on MLR as a biomarker for TB diagnosis in children, which is vital as it is difficult to obtain respiratory samples for microbiologic diagnosis in this population. All children in our study were comprehensively evaluated for pulmonary tuberculosis with two samples for smear microscopy and culture, Xpert MTB/RIF® and chest X-ray. Classifying children who had negative microbiologic testing but clinical signs of TB allowed us to analyze the utility of MLR for patients with unconfirmed TB. We also evaluated MLR longitudinally, allowing us to explore MLR changes over the TB treatment period. Moreover, we assessed for potential confounding by nutritional status and immunosuppression that may affect MLR. Our results may not be generalizable to all pediatric populations, as our cohort was limited to hospitalized, HIV-infected children with TB excluding those with CNS infections. The study was also limited by a relatively small sample size and had a low number of confirmed TB cases among younger children. However, our power to detect differences in MLR between the confirmed and unlikely TB groups was robust.
Conclusion
In summary, the blood monocyte-to-lymphocyte ratio distinguished Kenyan hospitalized HIV-infected children with microbiologically-confirmed pulmonary TB disease from children with unlikely TB. Blood MLR could be a useful diagnostic tool for TB disease in settings where bacteriological confirmation is difficult to obtain. MLR could also be evaluated as a component of future clinical diagnostic algorithms and/or biomarker for TB treatment response.
Supplementary Material
Table 1:
Baseline and Time-varying Characteristics of Study Participants in TB-Confirmed Versus TB Unlikely, and TB-Unconfirmed Versus TB Unlikely Diagnostic Classification Groups
| Baseline Characteristics | Total | TB-Confirmed | TB-Unconfirmed | TB Unlikely | TB- Confirmed |
TB- Unconfirmed |
|---|---|---|---|---|---|---|
| N = 160 | N = 13¶ (8.1%) | N = 67# (41.9%) | N = 80** (50.0%) | P* | P* | |
| Median age at enrollment in months (IQR†) | 22.8 (10.0–62.7) | 48.5 (15.6–79.3) | 22.9 (14.4–60.9) | 22.7 (8.9–54.9) | 0.33 | 0.48 |
| No. of subjects in age categories (n, %) | ||||||
| 0 to < 12 mo | 42 (26.3%) | 2 (15.4%) | 14 (20.9%) | 26 (32.5%) | ||
| 12 mo to < 24 mo | 43 (26.9%) | 4 (30.8%) | 22 (32.8%) | 17 (21.3%) | 0.45 | 0.30 |
| 24 mo to < 60 mo | 34 (21.3%) | 2 (15.4%) | 14 (20.9%) | 18 (22.5%) | ||
| ≥60 mo | 41 (25.6%) | 5 (38.5%) | 17 (25.4%) | 19 (23.8%) | ||
| Gender (n, %) | ||||||
| Male | 87 (54.4%) | 9 (69.2%) | 35 (52.2%) | 43 (53.8%) | 0.37 | 0.87 |
| Female | 73 (456%) | 4 (30.8%) | 32 (47.8%) | 37 (46.3%) | ||
| Malaria status (n, %)‡ | ||||||
| Positive | 25 (N = 64, 39.1%) | 0/3 (0%) | 5/22 (22.7%) | 20/39 (51.3%) | 0.23 | 0.03 |
| Negative | 39 (N = 64, 60.9%) | 3/3 (100%) | 17/22 (77.3%) | 19/39 (48.7%) | ||
| Laboratory-based malaria diagnosis (n, %) | ||||||
| Positive | 15 (N = 59, 25.4%) | 0/3 (0%) | 2/20 (10.0%) | 13/36 (36.1%) | 0.54 | 0.06 |
| Negative | 44 (N = 59, 74.6%) | 3/3 (100%) | 18/20 (90.0%) | 23/36 (63.9%) | ||
| Baseline median CD4 count (IQR), n | 746 (315–1339), 159 | 438 (104–799), 13 | 722 (332–1339), 66 | 757 (391–1474), 80 | 0.07 | 0.77 |
| Baseline median CD4 percentage (IQR), n | 15.0 (9.0–22.5), 159 | 11.0 (6.0–15.0), 13 | 15.1 (9.0–20.2), 66 | 16.6 (9.7–24.3), 80 | 0.10 | 0.21 |
| Baseline median WAZ§ (IQR), n | −2.66 (−3.7 to −1.6), 154 | −3.31 (−4.9 to −1.7), 12 | −3.32 (−4.3 to −2.3), 65 | −2.01 (−3.1 to −1.1), 77 | 0.03 | <0.01 |
| Baseline median WHZ||(IOR), n | −1.67 (−3.0 to −0.2), 119 | −2.46 (−4.0 to −1.9), 8 | −2.49 (−3.7 to −1.1), 50 | −1.05 (−2.3–0.3), 61 | 0.01 | <0.01 |
| Time-Varying Characteristics |
Total | TB-Confirmed | TB-Unconfirmed | TB Unlikely | TB- Confirmed, P* |
TB- Unconfirmed, P* |
| Median CD4 count over the study period (IQR), n | 1003 (503–1694), 477 | 457 (104–998), 34 | 932 (474–1603), 199 | 1146 (606–1891), 244 | <0.01 | 0.02 |
| Median CD4 percentage over the study period (IQR), n | 19.1 (113–260), 476 | 15.0 (6.0–25.2), 34 | 18.2 (12.2–24.1), 199 | 20.1 (13.5–27.9), 243 | 0.01 | 0.01 |
| Median WAZ§ over the study period (IQR), n | −1.83 (−3.0 to −1.0), 1102 | −2.66 (−4.5 to −1.3), 70 | −2.34 (−3.4 to −1.4), 482 | −1.45 (−2.3 to −0.7), 550 | <0.01 | <0.01 |
| Median WHZ|| over ihe study period (IQR), n | −0.97 (−2.1–0.2), 805 | −2.24 (−3.5 to −0.8), 46 | −1.37 (−2.8 to −0.3), 341 | −0.48 (−1.5–06), 418 | <0.01 | <0.01 |
P values from Fisher exact test reported for categorical variables. Wilcoxon 2-sample test t approximation or Student t test P values reported for continuous variables. Reference group is TB Unlikely.
Interquartile range.
Positive malaria status defined as positive clinical or laboratory smear diagnosis of malaria at enrollment.
WAZ were based on WHO child growth standards.
WHZ were based on WHO child growth standards for children 5 years of age and younger.
In the confirmed TB group, 6 died and 1 was lost to follow-up.
In the unconfirmed TB group, 9 died and 8 were lost to follow-up.
In the unlikely TB group, 15 died and 4 were lost to follow-up.
ACKNOWLEDGEMENTS:
We thank children and caregivers who participated in the study. We also thank the Pediatric Urgent Start of HAART (PUSH) trial staff for their administrative, clinical and data support.
LMC, RKC, and GJS designed the study. RKC and LMC analyzed clinical data. RKC and LMC wrote the manuscript. All authors read the manuscript draft, provided feedback and approved the final submitted manuscript.
This work was supported by the National Institute of Child Health and Human Development (NICHD), National Institute of Allergy and Infectious Diseases (NIAID), Fogarty International Center, and National Center For Advancing Translational Sciences at the National Institutes of Health (NIH) (R01 HD023412 and K24 HD054314–06 to GJS, D43TW009783 to IN, T32 AI007140 to PBP, K12 HD000850 to LMC, K23 AI 120793–01 to SML, and UL1TR000423 for REDCap), University of Washington Center for AIDS Research (P30 AI027757), UW Global Center for Integrated Heath of Women, Adolescents and Children (Global WACh), the Pediatric Scientist Development Program (PSDP) through grants from the American Pediatric Society and American Academy of Pediatrics (LMC), and the Infectious Diseases Society of America Medical Scholars Program (RKC). Portions of these data were presented at the 48th Union World Conference on Lung Health in Guadalajara, Mexico on October 12, 2017.
CONFLICTS OF INTEREST AND SOURCE OF FUNDING:
The authors report no conflicts of interest. Contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The funding sources were not involved in the analyses or interpretation of data. None of the authors were paid to write this article by a pharmaceutical company or other agency.
REFERENCES:
- 1.Cox JA, Lukande RL, Lucas S, Nelson AM, Van Marck E, Colebunders R. Autopsy causes of death in HIV-positive individuals in sub-Saharan Africa and correlation with clinical diagnoses. AIDS Rev. 2010;12(4):183–194. [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2017. November 30, 2017. Available at: http://www.who.int/tb/publications/global_report/en/0.
- 3.Venturini E, Turkova A, Chiappini E, Galli L, de Martino M, Thorne C. Tuberculosis and HIV co-infection in children. BMC infectious diseases. 2014;14 Suppl 1:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Velez CM, Roya-Pabon CL, Marais BJ. A systematic approach to diagnosing intra-thoracic tuberculosis in children. J Infect. 2017;74 Suppl 1:S74–S83. [DOI] [PubMed] [Google Scholar]
- 5.Rozot V, Patrizia A, Vigano S, et al. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis. 2015;60(3):432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portevin D, Moukambi F, Clowes P, et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. Lancet Infect Dis. 2014;14(10):931–938. [DOI] [PubMed] [Google Scholar]
- 7.Anderson ST, Kaforou M, Brent AJ, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med. 2014;370(18):1712–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaforou M, Wright VJ, Oni T, et al. Detection of tuberculosis in HIV-infected and-uninfected African adults using whole blood RNA expression signatures: a case-control study. PLoS Med. 2013;10(10):e1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naranbhai V, Kim S, Fletcher H, et al. The association between the ratio of monocytes:lymphocytes at age 3 months and risk of tuberculosis (TB) in the first two years of life. BMC Med. 2014;12:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naranbhai V, Moodley D, Chipato T, et al. The association between the ratio of monocytes: lymphocytes and risk of tuberculosis among HIV-infected postpartum women. J Acquir Immune Defic Syndr. 2014;67(5):573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naranbhai V, Hill AV, Abdool Karim SS, et al. Ratio of monocytes to lymphocytes in peripheral blood identifies adults at risk of incident tuberculosis among HIV-infected adults initiating antiretroviral therapy. J Infect Dis. 2014;209(4):500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Manna MP, Orlando V, Dieli F, et al. Quantitative and qualitative profiles of circulating monocytes may help identifying tuberculosis infection and disease stages. PLoS One. 2017;12(2):e0171358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Yin Y, Wang X, et al. Ratio of monocytes to lymphocytes in peripheral blood in patients diagnosed with active tuberculosis. Braz J Infect Dis. 2015;19(2):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–298. [PubMed] [Google Scholar]
- 16.Berens-Riha N, Kroidl I, Schunk M, et al. Evidence for significant influence of host immunity on changes in differential blood count during malaria. Malar J. 2014;13:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelliffe DB, Chandra RK. Immunocompetence in undernutrition. The Journal of Pediatrics.81(6):1194–1200. [DOI] [PubMed] [Google Scholar]
- 18.Njuguna IN, Cranmer LM, Otieno VO, et al. Urgent versus post-stabilisation antiretroviral treatment in hospitalised HIV-infected children in Kenya (PUSH): a randomised controlled trial. The lancet HIV. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National AIDS/STI Control Program (NASCOP). Guidelines for antiretroviral therapy in Kenya. 4th ed Ministry of Medical Services, Republic of Kenya: 2011. [Google Scholar]
- 20.Kenya Ministry of Health. Guidelines for Management of Tuberculosis and Leprosy in Kenya. Division of Leprosy Tuberculosis and Lung Disease; 2013.
- 21.WHO Anthro and macros [computer program]. Version 3.2.2 World Health Organization, 2011. Available at: http://www.who.int/childgrowth/software/en/.
- 22.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Children: An Update. Clin Infect Dis. 2015;61 Suppl 3:S179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean AG SK, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 3.01 November 30, 2017. Available at: www.OpenEpi.com.
- 24.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. American journal of epidemiology. 2006;163(7):670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marais BJ, Donald PR, Gie RP, Schaaf HS, Beyers N. Diversity of disease in childhood pulmonary tuberculosis. Ann Trop Paediatr. 2005;25(2):79–86. [DOI] [PubMed] [Google Scholar]
- 26.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clin Infect Dis. 2006;42(8):e69–71. [DOI] [PubMed] [Google Scholar]
- 27.Nicol MP, Spiers K, Workman L, et al. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis. 2013;57(3):e18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walters E, van der Zalm MM, Palmer M, et al. Xpert MTB/RIF on Stool is Useful for the Rapid Diagnosis of Tuberculosis in Young Children with Severe Pulmonary Disease. Pediatr Infect Dis J. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chipinduro M, Mateveke K, Makamure B, Ferrand RA, Gomo E. Stool Xpert(R) MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis at primary clinics in Zimbabwe. Int J Tuberc Lung Dis. 2017;21(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moussa H, Bayoumi FS, Mohamed AM. Gene Xpert for Direct Detection of Mycobacterium Tuberculosis in Stool Specimens from Children with Presumptive Pulmonary Tuberculosis. Ann Clin Lab Sci. 2016;46(2):198–203. [PubMed] [Google Scholar]
- 31.LaCourse SM, Pavlinac PB, Cranmer LM, et al. Stool Xpert MTB/RIF and urine lipoarabinomannan (LAM) for diagnosing tuberculosis in hospitalized HIV-infected children. AIDS (London, England). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zar HJ, Workman L, Isaacs W, Dheda K, Zemanay W, Nicol MP. Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use of Xpert MTB/RIF on respiratory specimens: a prospective study. Lancet Glob Health. 2013;1(2):e97–104. [DOI] [PubMed] [Google Scholar]
- 33.Zar HJ, Workman L, Isaacs W, et al. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clin Infect Dis. 2012;55(8):1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroidl I, Clowes P, Reither K, et al. Performance of urine lipoarabinomannan assays for paediatric tuberculosis in Tanzania. Eur Respir J. 2015;46(3):761–770. [DOI] [PubMed] [Google Scholar]
- 35.Nicol MP, Allen V, Workman L, et al. Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: a prospective study. Lancet Glob Health. 2014;2(5):e278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iskandar A, Nursiloningrum E, Arthamin MZ, Olivianto E, Chandrakusuma MS. The Diagnostic Value of Urine Lipoarabinomannan (LAM) Antigen in Childhood Tuberculosis. J Clin Diagn Res. 2017;11(3):EC32–EC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon C, Chaisson LH, Patel SM, et al. Diagnostic accuracy of C-reactive protein for active pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2017;21(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavan Kumar N, Anuradha R, Andrade BB, et al. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin Vaccine Immunol. 2013;20(5):704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliwa JN, Maina J, Ayieko P, et al. Variability in distribution and use of tuberculosis diagnostic tests in Kenya: a cross-sectional survey. BMC infectious diseases. 2018;18(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clouse K, Blevins M, Lindegren ML, et al. Low implementation of Xpert MTB/RIF among HIV/TB co-infected adults in the International epidemiologic Databases to Evaluate AIDS (IeDEA) program. PLoS One. 2017;12(2):e0171384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rendell NL, Bekhbat S, Ganbaatar G, Dorjravdan M, Pai M, Dobler CC. Implementation of the Xpert MTB/RIF assay for tuberculosis in Mongolia: a qualitative exploration of barriers and enablers. PeerJ. 2017;5:e3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157(3 Pt 1):679–691. [DOI] [PubMed] [Google Scholar]
- 43.Scriba TJ, Coussens AK, Fletcher HA. Human Immunology of Tuberculosis. Microbiol Spectr. 2017;5(1). [DOI] [PubMed] [Google Scholar]
- 44.Naranbhai V, Fletcher HA, Tanner R, et al. Distinct Transcriptional and Anti-Mycobacterial Profiles of Peripheral Blood Monocytes Dependent on the Ratio of Monocytes: Lymphocytes. EBioMedicine. 2015;2(11):1619–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.