Abstract
Aim of the study:
The peripheral nervous system is involved in regulation of bone metabolism via sensory and sympathetic innervation. Substance P (SP) and calcitonin gene-related peptide (CGRP) are two sensory neuropeptides that have been associated with regulation of osteogenic differentiation. However, the interaction between SP and CGRP both with each other and the bone morphogenetic protein 2 (BMP2) in regulation of osteogenic differentiation has not been studied. Therefore, the aim of this study was to investigate the interaction between SP and CGRP on BMP2-induced bone differentiation using model progenitor cells.
Materials and methods:
C2C12 myoblasts and MC3T3 pre-osteoblasts were treated with SP and CGRP, both individually and in combination, in the presence of BMP2. The effects of the neuropeptides on BMP2-induced osteogenic differentiation were assessed by measuring alkaline phosphatase (ALP) activity, mineralization and expression of osteogenic markers.
Results:
Both SP and CGRP enhanced BMP2 signaling, Runx2 mRNA expression, as well as mineralization in vitro. Co-stimulation with SP and CGRP resulted in down-regulation of BMP2-induced bone differentiation, suggesting potential crosstalk between the two neuropeptides in regulation of BMP2 signaling.
Conclusions:
Based on the results shown here, CGRP can mitigate augmenting effects of SP on BMP2 signaling and the three pathways potentially converge on Runx2 to regulate BMP2-induced bone differentiation.
Keywords: Substance P, Calcitonin gene-related peptide, Runx2, BMP2, bone differentiation
INTRODUCTION
Bone tissue is highly innervated by sympathetic and sensory nerve fibers expressing neuropeptides. In addition to their regulatory roles in physiological processes within the bone tissue, numerous clinical and animal studies have also associated these neuropeptides with bone pathologies. Therefore, there is recent interest in understanding the interaction between the neuropeptides and bone metabolism (1–3).
SP and CGRP are among these neuropeptides, which are associated with osteogenic changes inside the bone tissue. For instance, the numbers of SP- or CGRP-positive nerve fibers were increased in metabolically active bone tissues (1, 3). Depletion of SP and CGRP in the sensory nerves of bone, using capsaicin treatment reduced bone integrity and bone mass in animals, accompanied by high numbers of osteoclasts and delayed bone formation (4). Similarly, in patients who had spinal cord injuries, 50–70% bone loss was observed in tibia and femur, the most common fracture sites in these patients (5). Several groups published in vitro results demonstrating osteogenic potential of SP and CGRP, where they can induce osteogenic differentiation of progenitor cells that express receptors for these neuropeptides (6–18).
BMPs are signaling molecules that play crucial roles in bone formation throughout the development and during adulthood. They were first discovered as in the form of protein isolates from demineralized rabbit bone, with the ability to induce ectopic bone at non-skeletal sites in vivo and calcification of shredded muscle tissue in vitro (19). One of the most studied BMPs, BMP2, signals through effector molecules called SMADs after binding to their cell surface receptors. Complexes of SMADs, which is formed by SMAD1/5/8, in the case of BMP2, are activated through site-specific phosphorylation and binding with SMAD4. This complex subsequently translocates to the nucleus to regulate transcription of target genes, including Runx2 and Osterix (Osx) (20). In SMAD-dependent, or canonical, pathways, BMP2 binds to their cell surface receptors and form a hetero-tetrameric complex comprised of two dimers of type I and type II serine/threonine kinase receptors. Non-canonical, or SMAD-independent, BMP signaling goes through the mitogen-associated protein kinase (MAPK) pathways also regulate Runx2 expression to control osteoblast differentiation(20). SP and CGRP have also been associated with the pathophysiology of heterotopic ossification (HO) of soft tissues together with BMP up-regulation (21–24).
In one model of HO that involves exogenous BMP2 delivery, Salisbury et al. observed up-regulation of SP and CGRP in animals with HO. HO volume was decreased by depletion of sensory nerves positive for SP and CGRP in the same model, suggesting regulation of HO formation by SP and CGRP (22). Similarly, Kan et al. showed increased levels of SP during the formation of HO, induced by BMP4-overexpression and trauma. Inhibition of SP signaling reduced the amount of HO with respect to the control levels in this model (21). Our data indicate that SP and CGRP delivery induces HO in murine Achilles, with SP having more significant effects than CGRP. Combination of SP and CGRP can reduce the HO volume, compared to individual treatments of SP and CGRP. In all these cases, we observed up-regulated levels of endogenous BMP2 with delivery of SP and CGRP into the murine Achilles. Therefore, we postulate that SP and CGRP interact with the BMP2 signaling to regulate bone formation in tendon (manuscript in revision).
Building on these results, the study presented here demonstrates direct effects of both individual and combinatorial treatments of SP and CGRP on BMP2 signaling and BMP2-induced osteogenic differentiation of progenitor cells. Particularly, mouse C2C12 myoblasts and mouse calvarial MC3T3–E1 pre-osteoblasts were used since they both have been established as model systems to study osteoblast differentiation in vitro (25, 26). The work we present here adds to the understanding of the crosstalk between the components of the peripheral nervous system and bone tissue, as well as how they interact with each other to regulate bone differentiation in model cells.
MATERIALS AND METHODS
Treatment Preparation and Use
Recombinant human BMP2 (Medtronic, Minneapolis, MN), SP and CGRP (both from Bachem, Torrance, CA) were reconstituted according to manufacturer’s instructions to 1 mg/ml, aliquoted and stored at −80°C. Prior to use, they were freshly diluted to the desired concentration in 100 mM sodium phosphate, pH 7.4. For cell culture experiments, they were further diluted to 100 ng/ml in media.
Cell culture
C2C12 myogenic cells (ATCC, Manassas, VA) were cultured in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin (all from Gibco, Gaithersburg, MD) in 5% CO2 at 37°C. MC3T3–E1 subclone 4 pre-osteoblasts (ATCC, Manassas, VA) were cultured in Alpha Minimum Essential Medium (MEM; Gibco, Gaithersburg, MD), supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin in 5% CO2 at 37°C. C2C12 cells were seeded at an initial density of 1.3×104 cells/cm2 and cultured for 4 days for assessment of alkaline phosphatase (ALP) activity. The treatments (SP and/or CGRP ± BMP2) were replaced in growth medium after 48 hours. Cells were cultured for 18–20 days for assessment of mineralization, replacing the treatments (SP and/or CGRP ± BMP2) in osteogenic media every 72 hours. Osteogenic media includes 50 μg/mL ascorbic acid (Sigma-Aldrich, St Louis, MO) and 10mM β-glycerophosphate (Santa Cruz Biotechnology, Santa Cruz, CA) in growth medium. MC3T3–E1 cells were seeded at an initial density of 5.2×104 cells/cm2 and same protocols were followed for measurement of ALP activity and mineralization. For real-time PCR experiments, C2C12 cells were treated with SP and/or CGRP ± BMP2 for 48 hours and total RNA was isolated as described below.
Osteogenic differentiation assays
Cells were incubated with treatments as described above, washed with 1X PBS to remove culture medium, and fixed for 30 seconds with 10% formalin. As a marker of osteoblastic differentiation, ALP activity was detected after 4 days in culture using the Alkaline Phosphatase, Leukocyte Kit according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO). DIC images were taken using a Zeiss Axiovert 200M microscope (Carl Zeiss Microimaging, Thornwood, NY). ALP staining (blue) on images was quantified on ImageJ (Golden, CO) by measuring % area of the black pixels upon conversion of the RGB images into binary images. Mineralization was assessed by Alizarin red staining (ARS) after 18–20 days in culture. Cells were washed with 1X PBS and fixed for 15–20 min in 10% formalin at room temperature. ARS and quantification of the ARS were performed using an osteogenesis assay kit (Millipore, Billerica, MA) according to the manufacturer’s instructions. Experiments were run in triplicates. Data shown is representative of three independent experiments.
RNA extraction and real-time PCR
Total RNA was extracted from trypsinized C2C12 cells, using an RNeasy total RNA kit (Qiagen, Chatsworth, CA) according to the manufacturers protocol. After DNase I (RNAse-free; Invitrogen, Carlsbad, CA) treatment, cDNAs were synthesized with Super Script IV first-strand synthesis system (Invitrogen, Carlsbad, CA). Real-time quantitative PCRs were performed in 384-well plates, total cDNA 100 ng/well, using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) listed on Table 1. Relative levels of mRNA were reported as mean fold change ± SEM over control mRNA expression (BMP2 treatment), using the ΔΔCt model with Gapdh mRNA as the selected endogenous control. The reactions were run in a 7900HT Fast-time Real-Time PCR System (Applied Biosystems, Foster City, CA) in the Genomics Core at the University of Pittsburgh (Pittsburgh, PA). PCR cycling was performed as 95°C for 12 minutes for one cycle, 95°C for 15 seconds, and 60°C for 60 seconds for 40 cycles. Data analysis was performed on SDS 2.4.2 software (Applied Biosystems, Foster City, CA). Experiments were performed in triplicate. Data shown is representative of three independent experiments.
Table 1 –
TaqMan gene expression assay IDs used for the RT-PCR reactions
| Gene | Assay ID |
|---|---|
| AlpI | Mm00475834_m1 |
| Col1a1 | Mm00801666_g1 |
| Sp7 (Osx) | Mm04209856_m1 |
| Bglap (Ocn) | Mm03413826_mH |
| Runx2 | Mm00501584_m1 |
| Gapdh | Mm99999915_g1 |
Cell Transfection Experiments
pGL3-Bre-Luc was a gift from Martine Roussel & Peter ten Dijke (Addgene plasmid # 45126). C2C12 cells were plated in 96-well plates at an initial density of 3.5×104 cells/cm2 in growth medium the day before transfection. The cells were transfected with >1 μg of the reporter plasmid pGL3-Bre-Luc using X-tremeGene 9 transfection reagent (Roche, Pleasanton, CA) according to manufacturer’s description. In each case, 1 μg of pRL-LUC was co-transfected to provide a means of normalizing the assays for transfection efficiency. Cells were treated with BMP2 in the presence or absence of SP and/or CGRP (each with a final concentration of 100 ng/ml), 4 hours after transfection. Cell lysates were collected 18–24 hours after adding various treatments. Renilla and luciferase activities were measured with the use of a dual-reporter luciferase assay system (Promega, Madison, WI) in a Tecan Safire 2 plate reader (Männedorf, Switzerland).
Statistical analysis
For statistical analysis, all data was subjected to Analysis of Variance (ANOVA) followed by Tukey’s post hoc test for multiple comparison between each treatment group using MATLAB (Natick, MA). Data in figures is presented as mean ± SEM and is representative of 3 independent experiments. Statistical significance was defined at p ≤ 0.05.
RESULTS
Combination of SP and CGRP treatments suppresses BMP2-induced mineralization
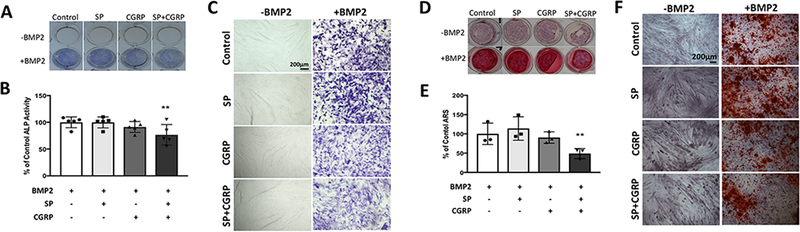
In order to investigate the effects of the neuropeptides on BMP2-induced osteogenic differentiation, early and late markers, ALP activity and mineralization, were measured after treating the cells with SP and CGRP, both individually and in combination. C2C12 cells exhibited a decrease in ALP activity with co-treatment of SP and CGRP (p≤0.01), although no difference was observed with individual treatments (Fig. 1 A–C). The trend observed in mineralization of C2C12 cells was similar, where the combination of SP and CGRP led to a reduction in mineralization compared to BMP2-only controls (p≤0.01). Therefore, combination of SP and CGRP suppressed BMP2 signaling in C2C12 cells (Fig. 1 D–F). As expected, the myoblasts underwent myogenic differentiation in the absence of BMP2 (Fig. 1 F) and the neuropeptides did not have any osteogenic effects on these cells without BMP2.
Figure 1 –
Individual treatments of SP or CGRP did not have any direct effects but the combination of SP and CGRP partially suppressed BMP2-induced ALP activity and mineralization in C2C12 cells. A. Whole well images showing ALP staining (blue) of C2C12 cells upon treatment with SP and/or CGRP ± BMP2 (each 100 ng/ml) after 4 days in culture. B. Quantification of ALP staining using DIC images for each treatment group. Quantification was averaged from 4 independent sets of experiments, each performed in triplicates. 9 regions of interest were quantified per experiment and normalized to BMP2-only treatments. Data is reported as % of control mean value ± SEM. No significant changes were observed across different treatment groups. C. DIC images of cells with respective treatments. Scale bars measure 200 μm. D. Whole well images showing Alizarin red staining (ARS) of C2C12 cells treated with SP and/or CGRP ± BMP2 (100 ng/ml each in osteogenic differentiation medium) after 18 days. E. Quantification of ARS by solubilizing the stain according to manufacturer’s protocol. Data is reported as % of control mean value ± SEM. The neuropeptides did not have marked effects individually but the combination led to a reduction on BMP2-induced mineralization compared to other experimental groups and the controls (p<0.01). Experiments were performed in triplicates. Data is representative of 3 independent experiments. F. DIC images of cells with respective treatments. Scale bars measure 500 μm. C2C12s fused to form myotubes when there is no BMP in culture medium. BMP2 suppressed myogenic differentiation and induced mineralization.
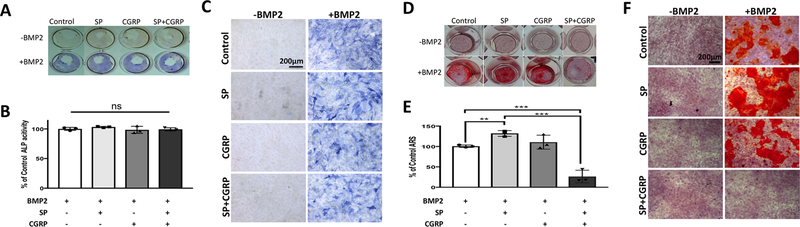
MC3T3–E1 cells did not exhibit any changes in ALP activity across different treatment groups (Fig. 2 A–C). However, BMP2-induced mineralization was enhanced with SP (p≤0.01) and CGRP (p=0.06) in these cells (Fig. 2 D–F). Remarkably, addition of CGRP together with SP led to a complete inhibition of BMP2-induced mineralization in MC3T3–E1 (p≤0.001) (Fig. 2 D–F). Therefore, there is potential crosstalk between SP and CGRP signaling to regulate BMP2-induced mineralization in both cell types but the ALP activity was only affected in M MC3T3–E1 pre-osteoblasts.
Figure 2 –
Neither SP nor CGRP had direct effects on BMP2-induced ALP activity in MC3T3–E1 pre-osteoblasts. Individual treatments of SP and CGRP enhanced and their combination blocked BMP2-induced mineralization in these cells. A. Whole well images showing ALP staining (blue) of MC3T3–E1 pre-osteoblasts upon treatment with SP and/or CGRP ± BMP2 (each 100 ng/ml) after 4 days in culture. B. Quantification of ALP staining using DIC images for each treatment group. Quantification was averaged from 3 independent sets of experiments, each performed in triplicates. 9 regions of interest were quantified per experiment and normalized to BMP2-only treatments. Data is reported as % of control mean value ± SEM. No significant changes were observed across different treatment groups. C. DIC images of cells with respective treatments. Scale bars measure 200 μm. D. Whole well images showing Alizarin Red Staining (ARS) of MC3T3–E1 pre-osteoblasts treated with SP and/or CGRP ± BMP-2 (100 ng/ml each in osteogenic differentiation medium) after 18 days. E. Quantification of ARS by solubilizing the stain according to manufacturer’s protocol. Data is reported as % of control mean value ± SEM. SP enhanced (p<0.01) and the combination of SP and CGRP inhibited BMP2-induced mineralization compared to other experimental groups and the controls (p<0.001). Experiments were performed in triplicates. Data is representative of 3 independent experiments. F. DIC images of cells with respective treatments. Scale bars measure 200 μm.
Combination of SP and CGRP treatments suppresses BMP2 signaling
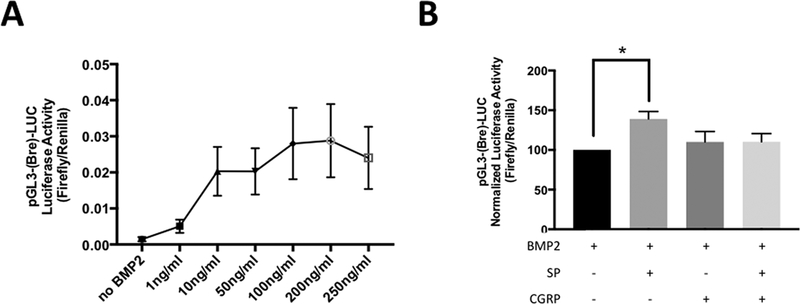
Next, the direct and short-term effects of the neuropeptides on BMP2 signaling were investigated. A reporter construct, pGL3-Bre-LUC, that is specific to the BMP-responsive element (Bre) was used. Bre is found in the Id1 gene, which is directly activated by the SMAD complex upon BMP2 binding to its receptors. In the case of Bre-LUC, Bre activation turns on the luciferase expression in less than 24 hours. This was previously demonstrated in C2C12 cells (27). Consistent with these reports, we observed a dose-dependent effect on Bre-LUC activity with BMP2, starting as low doses as 10ng/ml. Maximal response was observed at 200 ng/ml (Fig. 3 A). Higher doses reduced the luciferase activity suggesting negative regulation of BMP2 signaling by itself in the case of over-activation. For the rest of the Bre-LUC experiments, BMP2 was added with a final concentration of 100 ng/ml. SP and/or CGRP (both 100 ng/ml as final concentration) were then added together with BMP2 to determine their direct effects on BMP2 signaling in C2C12 cells. We observed an increase with SP, but not with CGRP (p≤0.05 and p=0.06, respectively) compared to BMP2-only controls (Fig. 3 B). Combination of SP and CGRP mitigated the effect of SP on BMP2 signaling (p=0.06). Therefore, there is potential crosstalk between SP and CGRP to regulate BMP2 signaling.
Figure 3 –
SP enhanced BMP2 signaling and CGRP modulated its effect when delivered together. C2C12 cells were transiently transfected with pGL3-(Bre)-LUC (Firefly) and pRL-LUC (Renilla) plasmids. After 4 hours, cells were treated with respective treatments. Luciferase activity was measured within 16–20 hours. A. Dose-dependent effects of BMP2 on pGL3-(Bre)-LUC activation. Maximum response was observed at 100 ng/ml with saturation at 200ng/ml. Higher concentrations than 200 ng/ml down-regulated pGL3-(Bre)-LUC activation. B. SP and/or CGRP were added in combination with BMP2 (100ng/each) after the transfection. SP enhanced BMP2 signaling (p<0.05), while there was a clear trend for CGRP to mitigate the effect of SP (p=0.063), when delivered together. The data shown represent 6 independent experiments.
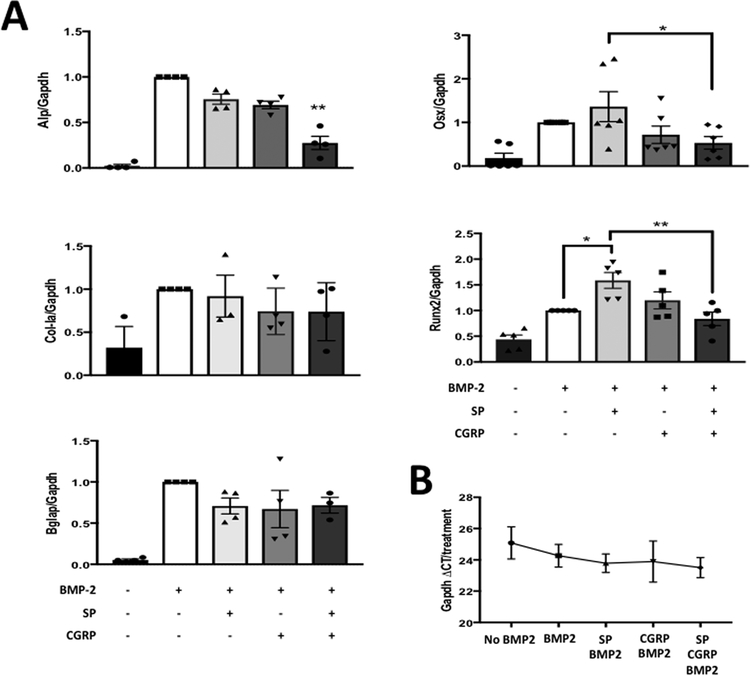
SP enhanced BMP2-induced increase in Osx and Runx2 expression, while co-treatment of SP and CGRP led to a reduction
In order to elucidate where the three pathways converges, we then performed qRT-PCR experiments to measure changes in expression of osteogenic markers with neuropeptide stimulation in C2C12 cells. No significant changes were observed with individual and combinatorial treatments of the neuropeptides compared to BMP2-only controls at 24 hours (data not shown). However, there was an increase in Runx2 expression by SP or CGRP treatment alone (p ≤0.05 and p=0.060, respectively) and a reduction compared to SP-treatment, by the combination of SP and CGRP treatments (p ≤0.01) at 48 hours (Fig. 4 A). Combination of SP and CGRP also led to a down-regulation of Alp expression, resulting in expression below the BMP2-only control levels (p ≤0.01), similar to the effect observed in ALP activity in C2C12 cells (Fig. 4 A). Osx (Sp7) expression was also upregulated by SP (p=0.06) and down-regulated by the combinatorial treatment compared to SP-treated cells (p ≤0.05) (Fig. 4 A). For collagen type I a (Col-Ia) and osteocalcin (Bglap), which are both controlled by the Runx2 protein (19), there was no effect of any treatment at 48 hours (Fig. 4 A). However, it is possible that 48 hours is an early time-point for expression of these markers. Glyceraldehyde 3-phosphate dehydrogenase gene (Gapdh) was used as the internal control for all the qRT-PCR experiments as its expression was not altered by different treatments (Fig. 4 B).
Figure 4 –
Combination of SP and CGRP treatment resulted in downregulation of BMP2-induced expression ostegenic markers in C2C12 cells A. qRT-PCR for expression of osteogenic differentiation markers Alp, Col-Ia, Sp7 (Osx), Bglap (Ocn) and Runx2. Cells were treated with growth medium (GM) that contains neuropeptides SP and/or CGRP with BMP2 (each 100 ng/mL) for 2 days. Each column represents the fold change over the BMP2 control. * indicates p<0.05 and ** indicates p<0.01. Each column also shows average values of the biological replicates in each treatment. B. Gapdh was used as a reference gene since the CT value did not change across different treatment types. Combination of SP and CGRP with BMP2 induced down-regulation of Alp expression (p<0.01), while the individual treatments had no significant effect. Runx2 expression was upregulated by SP compared to BMP2-only control (p<0.05), where the combination mitigated this effect (p<0.01 compared to SP+BMP2). Therefore, there is possible convergence on regulation of Runx2 expression between the two pathways and the BMP2 signaling.
DISCUSSION
The results reported here demonstrated regulatory effects of SP and CGRP on BMP2 signaling and certain aspects of osteogenic differentiation, such as mineralization. CGRP did not have statistically significant effects compared to BMP2-only or SP and BMP2 co-treated cells. However, it could counteract the effect of SP on BMP2 signaling when delivered together with SP. The most remarkable finding of this study was down-regulation of BMP2 signaling and bone differentiation by the combinatorial treatments of SP and CGRP, which potentially converges on Runx2 gene expression.
Runx2 expression and activation is considered as a crucial point incorporating many signals that regulate osteoblast differentiation. Runx2 modulates expression of many other target genes and works in concert with several molecular factors, which are involved in osteogenic differentiation (28, 29). Therefore, it was expected to see reductions in osteogenic markers, including ALP expression or mineralization with reduced Runx2 expression. Consistent with the mRNA expression; ALP staining was down-regulated with co-treatment of SP and CGRP in C2C12 myoblasts. MC3T3 pre-osteoblasts did not show any difference across different treatment groups. This is interesting since we observed marked effects in mineralization with different treatments. It is not clear whether the neuropeptides have any effect on ALP activity since the qRT-PCR analysis was not done with the MC3T3 cells. Therefore, it will be important to repeat similar assays with MC3T3 pre-osteoblasts to elucidate potential mechanisms that regulate the neuropeptide response observed in BMP2-induced mineralization in these cells. Runx2 is potentially involved, as it is also required for proper bone matrix formation in osteoblasts (28, 29). However, it is still interesting to see complete inhibition of BMP2-induced mineralization with the combination of SP and CGRP in both cell types, since it suggests inhibition of BMP2 signaling with activation of SP- and CGRP-induced factors at the same time.
Many in vitro studies reported presence of SP and CGRP receptors in various stem cell populations and up-regulation of osteogenic markers with particularly SP treatment when cultured under conditions required to stimulate osteogenic differentiation (8, 11, 13, 18, 30). However, aside from a few in vivo studies (31, 32), combined effects of the neuropeptides with BMPs in regulation of osteogenic differentiation were not studied in detail. Ma et. al. reported down-regulation of ALP activity in pre-osteoblasts, compared to BMP2-only controls, when SP is combined with BMP2 (10). In this study, although we did not observe any inhibitory effects of SP on BMP2 signaling, we suspect that mitigating effects of CGRP on SP might be a result of a negative feedback mechanism. We observed down-regulation with higher concentrations of BMP2 in Bre-activation-induced luciferase activity, which is potentially due to a similar kind of feedback response. Shorter time-point, i.e. 4–6 hour, stimulation of the C2C12s with the neuropeptides exhibited increased luciferase activity and no marked reduction with the combinatorial treatment, which could support this hypothesis (data not shown).
One potential convergence point between the two pathways and the BMP2 signaling could be the non-canonical BMP2 signaling cascades that involve MAPK activation. MAPK signaling was shown to have contrasting effects on BMP2 signaling, and therefore the interaction between the two pathways was suggested to correlate with the levels of MAPK activation (33). For instance, over-activation of MAPK due to tumor necrosis factor α or interleukin-1β stimulation had inhibitory effects on BMP2 signaling via inhibition of Runx2 expression (34). SP was shown to activate MAPK signaling, particularly p38 and extracellular signal regulated kinase (ERK) 1/2, via protein kinase C (PKC) activation in various cell culture models with osteogenic potential, which could explain the effect of SP on BMP2 signaling (30, 35–37). Additionally, CGRP was shown to both activate (38) and attenuate MAPK signaling through protein kinase (PKA) activation (39). In fact, CGRP was shown to mitigate TGFβ signaling in the latter example (39). While p38 appears to have positive stimulation, which was consistent across many different studies including this one, ERK 1/2 signaling was shown have differential effects on BMP signaling (33). ERK 1/2 phosphorylation was shown to be necessary for osteoblast differentiation in vitro and in skeletal development in vivo (40–42). It appears be involved in phosphorylation of Runx2, which increases its DNA binding capability, and therefore transcriptional activity to regulate osteogenic markers (43, 44). In contrast, ERK1/2 could also phosphorylate SMAD1 on specific sites to attenuate its nuclear translocation and inhibit BMP signaling (45, 46). These differential effects are possibly a result of diverse set of upstream signals that can activate ERK1/2, including the Ras/MEK pathway (33). Since we observed complete inhibition with SP and CGRP on BMP2-induced mineralization, this requires convergence of both pathways at a certain molecule that can suppress BMP2 signaling. This inhibition might also be due to another extracellular signal that has inhibitory effects on BMP2 signaling. More work is warranted to identify the actual mechanisms by which CGRP mitigates the effects of SP on BMP2 signaling. However, here we propose a model that involves potential convergence on MAPK activation, upon activation of SP and CGRP signaling pathways, which subsequently inhibits BMP2 (Fig. 5). Hence this model requires additive effects of SP and CGRP signaling, investigation of dose-dependent and/or temporal effects of SP and CGRP is the next step in elucidating the exact molecular mechanisms, by which the two neuropeptides crosstalk with the BMP2 signaling. Furthermore, since we observed a clear effect on a late marker of osteoblastic differentiation, it is required to study the effects on the neuropeptides on expression of other markers at a longer time course instead of 48 hours. For instance, osteocalcin was shown to be affected by ERK 1/2–induced Runx2 activation, which is an important factor involved BMP2-induced bone mineralization (33). We did not observe any changes across different treatment groups at 48 hours but it is possible that osteocalcin levels would change at a later time-point.
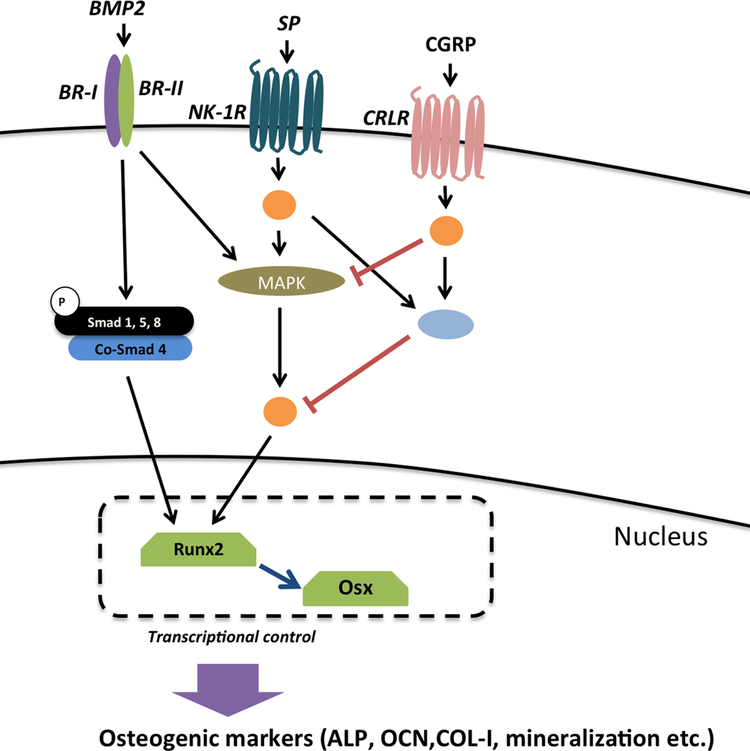
Figure 5 –
Hypothetical model that proposes the molecular interactions involved in the SP-CGRP-BMP2 crosstalk. Orange circles represent intermediate components of the signaling cascades. Blue circle denotes an “AND gate”, which potentially turns on a repressor of a downstream target that is required for ALP expression or mineralization. Since the combination showed down-regulation below the baseline (i.e. in comparison to BMP2-only controls), and CGRP-only had no significant effect on BMP2 signaling, we propose down-regulation of BMP2-induced osteogenic differentiation is due to an interaction between SP and CGRP. CGRP could also mitigate MAPK signaling, which suggests another possible interaction.
In conclusion, the results reported here illustrate regulatory effects of the neuropeptides SP and CGRP on BMP2-induced osteogenic differentiation in osteoprogenitor cells. In addition, this study demonstrates an interaction between SP and CGRP, which when combined can have inhibitory effects on BMP2 signaling, and therefore osteogenic differentiation. These results contribute to the understanding of the interaction between the sensory neuropeptides and bone metabolism in both physiological and pathological conditions.
FUNDING
This work was supported by NIH grant R01004343 (PC).
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Grassel SG. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther. 2014;16(6):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KB, Mollano AV, Morcuende JA, Cooper RR, Saltzman CL. Bone and brain: a review of neural, hormonal, and musculoskeletal connections. Iowa Orthop J 2004;24:123–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Castellano JM, Diaz-Herrera P, Morcuende JA. Is bone a target-tissue for the nervous system? New advances on the understanding of their interactions. Iowa Orthop J. 2000;20:49–58. [PMC free article] [PubMed] [Google Scholar]
- 4.Offley SC, Guo TZ, Wei T, Clark JD, Vogel H, Lindsey DP, et al. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. 2005;20(2):257–67. [DOI] [PubMed] [Google Scholar]
- 5.Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27(2):305–9. [DOI] [PubMed] [Google Scholar]
- 6.Backman LJ, Fong G, Andersson G, Scott A, Danielson P. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PLoS One. 2011;6(11):e27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong G, Backman LJ, Hart DA, Danielson P, McCormack B, Scott A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J Orthop Res. 2013;31(1):91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu S, Mei G, Wang Z, Zou ZL, Liu S, Pei GX, et al. Neuropeptide substance P improves osteoblastic and angiogenic differentiation capacity of bone marrow stem cells in vitro. Biomed Res Int. 2014;2014:596023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Jiang LS, Dai LY. Substance P and its receptors in bone metabolism. Neuropeptides. 2007;41(5):271–83. [DOI] [PubMed] [Google Scholar]
- 10.Ma WH, Liu YJ, Wang W, Zhang YZ. Neuropeptide Y, substance P, and human bone morphogenetic protein 2 stimulate human osteoblast osteogenic activity by enhancing gap junction intercellular communication. Braz J Med Biol Res. 2015;48(4):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei G, Zou Z, Fu S, Xia L, Zhou J, Zhang Y, et al. Substance P activates the Wnt signal transduction pathway and enhances the differentiation of mouse preosteoblastic MC3T3–E1 cells. Int J Mol Sci. 2014;15(4):6224–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian Ming L M, Duan Mengna, Zhang Haiyang, Wu Zhe and Zhou Yanmin. Substance P Induce Osteogenic Differentiation in Human Adipose-Derived Stem Cells. Journal of Animal and Veterinary Advances. 2013;12(8):863–7. [Google Scholar]
- 13.Sun HB, Chen JC, Liu Q, Guo MF, Zhang HP. Substance P stimulates differentiation of mice osteoblast through up-regulating Osterix expression. Chin J Traumatol. 2010;13(1):46–50. [PubMed] [Google Scholar]
- 14.Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T. Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett. 1997;233(2–3):125–8. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Zhao R, Shi X, Wei T, Halloran BP, Clark DJ, et al. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone. 2009;45(2):309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou B, Zhou Y, Tang K. The effects of substance P on pluripotent tendon cells: an in vitro and in vivo study. J Musculoskelet Neuronal Interact. 2014;14(3):349–58. [PubMed] [Google Scholar]
- 17.Mrak E, Guidobono F, Moro G, Fraschini G, Rubinacci A, Villa I. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by beta-catenin stabilization. J Cell Physiol. 2010;225(3):701–8. [DOI] [PubMed] [Google Scholar]
- 18.Tian G, Zhang G, Tan YH. Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol Sin. 2013;34(11):1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12(4):203–21. [DOI] [PubMed] [Google Scholar]
- 20.Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kan L, Lounev VY, Pignolo RJ, Duan L, Liu Y, Stock SR, et al. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112(10):2759–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112(10):2748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lui PP. Histopathological changes in tendinopathy--potential roles of BMPs? Rheumatology (Oxford). 2013;52(12):2116–26. [DOI] [PubMed] [Google Scholar]
- 24.Lui PP, Chan LS, Fu SC, Chan KM. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sports Med. 2010;38(4):757–64. [DOI] [PubMed] [Google Scholar]
- 25.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127(6 Pt 1):1755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiraki Y, Inoue H, Shigeno C, Sanma Y, Bentz H, Rosen DM, et al. Bone morphogenetic proteins (BMP-2 and BMP-3) promote growth and expression of the differentiated phenotype of rabbit chondrocytes and osteoblastic MC3T3–E1 cells in vitro. J Bone Miner Res. 1991;6(12):1373–85. [DOI] [PubMed] [Google Scholar]
- 27.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277(7):4883–91. [DOI] [PubMed] [Google Scholar]
- 28.Long F Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13(1):27–38. [DOI] [PubMed] [Google Scholar]
- 29.Rutkovskiy A, Stenslokken KO, Vaage IJ. Osteoblast Differentiation at a Glance. Med Sci Monit Basic Res. 2016;22:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kook YA, Lee SK, Son DH, Kim Y, Kang KH, Cho JH, et al. Effects of substance P on osteoblastic differentiation and heme oxygenase-1 in human periodontal ligament cells. Cell Biol Int. 2009;33(3):424–8. [DOI] [PubMed] [Google Scholar]
- 31.La WG, Jin M, Park S, Yoon HH, Jeong GJ, Bhang SH, et al. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int J Nanomedicine. 2014;9 Suppl 1:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noh SS, Bhang SH, La WG, Lee S, Shin JY, Ma YJ, et al. A dual delivery of substance P and bone morphogenetic protein-2 for mesenchymal stem cell recruitment and bone regeneration. Tissue Eng Part A. 2015;21(7–8):1275–87. [DOI] [PubMed] [Google Scholar]
- 33.Schindeler A, Little DG. Ras-MAPK signaling in osteogenic differentiation: friend or foe? J Bone Miner Res. 2006;21(9):1331–8. [DOI] [PubMed] [Google Scholar]
- 34.Huang RL, Yuan Y, Tu J, Zou GM, Li Q. Opposing TNF-alpha/IL-1beta- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014;5:e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiebich BL, Schleicher S, Butcher RD, Craig A, Lieb K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. J Immunol. 2000;165(10):5606–11. [DOI] [PubMed] [Google Scholar]
- 36.Tokuda M, Miyamoto R, Sakuta T, Nagaoka S, Torii M. Substance P activates p38 mitogen-activated protein kinase to promote IL-6 induction in human dental pulp fibroblasts. Connect Tissue Res. 2005;46(3):153–8. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008;294(6):C1586–96. [DOI] [PubMed] [Google Scholar]
- 38.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsui S, Yamane T, Kobayashi-Hattori K, Oishi Y. Calcitonin gene-related peptide regulates mitogen-activated protein kinase pathway to decrease transforming growth factor beta1-induced hepatic plasminogen activator inhibitor-1 mRNA expression in HepG2 cells. Biosci Biotechnol Biochem. 2014;78(5):787–90. [DOI] [PubMed] [Google Scholar]
- 40.Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 2007;176(5):709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, et al. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276(17):14443–50. [DOI] [PubMed] [Google Scholar]
- 42.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275(13):9645–52. [DOI] [PubMed] [Google Scholar]
- 43.Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Roca H, et al. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J Biol Chem. 2009;284(47):32533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jun JH, Yoon WJ, Seo SB, Woo KM, Kim GS, Ryoo HM, et al. BMP2-activated Erk/MAP kinase stabilizes Runx2 by increasing p300 levels and histone acetyltransferase activity. J Biol Chem. 2010;285(47):36410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finelli MJ, Murphy KJ, Chen L, Zou H. Differential phosphorylation of Smad1 integrates BMP and neurotrophin pathways through Erk/Dusp in axon development. Cell Rep. 2013;3(5):1592–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reilly GC, Golden EB, Grasso-Knight G, Leboy PS. Differential effects of ERK and p38 signaling in BMP-2 stimulated hypertrophy of cultured chick sternal chondrocytes. Cell Commun Signal. 2005;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]