Abstract
Cancer genomics research aims to advance personalized oncology by finding and targeting genetic alterations associated with cancers. In genome-driven oncology, treatments are selected for individual patients based on the genomic sequence of their tumor. This personalized oncology approach has prolonged survival for subsets of cancer patients. However, many patients do not respond to the predicted therapies based on genomic profiles of their tumors. Recent studies pairing genomic and proteomic analyses in the same tumors have shown that the proteome encodes novel information that is not discerned through genomic analysis alone. This has led to the concept of “proteogenomics,” in which both types of data are leveraged for a more complete view of tumor biology that may more successfully match cancer patients to efficacious treatments. We discuss the added value of a combined proteogenomics approach over the current genome-centric approach in characterizing human cancers, and summarize current efforts to incorporate targeted proteomic measurements based on multiple reaction monitoring mass spectrometry (MRM) into the clinical laboratory to facilitate clinical proteogenomics.
Genome-driven oncology: missing tumor biology
Cancer is a disease of the genome, evidenced by rampant genomic instability in tumor cells leading to pervasive mutational or chromosomal abnormalities. Large-scale sequencing efforts have generated comprehensive catalogs of the key genomic changes in many types of cancer, identifying potentially actionable abnormalities1. Because of these findings, we now see an increased use of clinical sequencing on cancer patients, especially through genome-driven clinical trials designed to select a targeted therapy based on an individual patient’s tumor genomic profile (“personalized oncology”)2,3.
The use of genomic biomarkers to guide a personalized oncology approach has been highly successful for subsets of patients4, such as CML patients harboring the BCR-ABL translocation, breast cancer patients with HER2 amplification, melanoma patients with BRAF mutations, and lung cancer patients with EGFR mutations or ALK rearrangements. However, significant challenges remain5–10, most notably the lower-than-expected response rates to targeted therapies in patients predicted to be responsive based on genomic profiles of their tumors, and the ultimate emergence of resistant disease in the vast majority of patients. Thus, while genome-driven oncology has prolonged survival for subsets of cancer patients, significant tumor biology, not apparent from the genomic profiles of tumors, must be elucidated before personalized oncology can become broadly applicable and efficacious10.
Proteins connect genotype to cancer phenotype
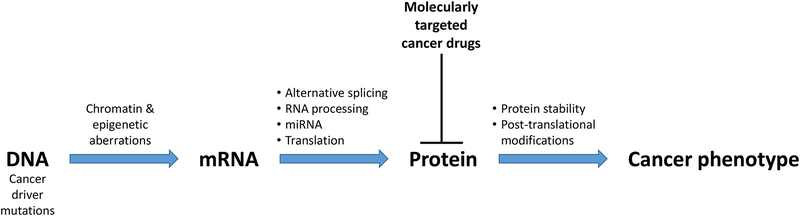
Based on first principles, it is not surprising that the exclusive use of tumor genomic profiles is insufficient to guide the reliable selection of targeted therapies. As summarized in Figure 1, many cellular processes downstream of the genome
Figure 1. Many processes downstream of the genome affect the cancer phenotype.
Proteins execute the genome to control tumor phenotype, and proteins are most frequently targeted in precision oncology.
determine which aspects of the cancer genome actually affect the phenotype of cancer cells. For example, epigenetic changes are common in human cancers and affect the expression of critical cancer genes11–14, such as oncogenes, tumor suppressors, and microRNAs (miRNAs), with major effects on cell signaling and homeostasis13. Histone modifications play a role in alternative splicing15, which helps to drive hallmarks of cancer16,17. Genomic, epigenomic, and transcriptomic programs are eventually executed at the protein level, which is further regulated by protein translation, post-translational modifications such as phosphorylation, glycosylation, and acetylation, and protein degradation. Thus, tumor mutation profiles are only one of many potential determinants of patient response to targeted therapies, and an exclusive focus on genomic profiles omits important aspects of tumor biology that are downstream of the genome and affect response to therapies.
From the clinical perspective, the majority of molecularly targeted therapies do not target the cancer genome, but rather target proteins in cancer cells, such as kinase inhibitors, PARP inhibitors, and therapies targeting immunomodulatory proteins. Thus, it is critically important to quantify proteins throughout all phases of personalized oncology, from drug development to patient selection. For example, we need to determine if the target protein is expressed in the target tissue, and at what level. We need to know if the compound engages the target, and what is the exposure-response relationship. We need to understand cross-talk amongst signaling pathways, as these can determine drug resistance and synergies. Finally, we need to understand the variability among patients in target protein expression and cellular responses to the therapy to improve patient selection.
Characterizing tumors with mass-spectrometry-based proteomics
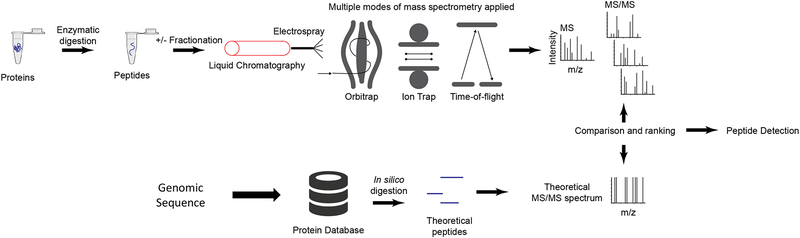
During the past decade, untargeted (“shotgun”) mass-spectrometry (MS) based proteomics has evolved as a powerful technology for protein detection and quantification in complex samples (Figure 2). In these analyses, proteins extracted from biological samples, such as tumor specimens, are enzymatically digested into peptides and then analyzed by liquid chromatography−tandem mass spectrometry (LC−MS/MS). To reduce sample complexity, the peptides may be fractionated prior to LC-MS/MS, which serves to increase proteome coverage but has the drawback of decreasing sample throughput and increasing costs. Alternatively, recent advances in instrumentation and methods using long chromatographic columns have allowed analysis of unfractionated samples with only modest reductions in protein detection compared with pre-fractionated samples18,19. These advances have the potential to significantly increase the number of samples that can be profiled in a reasonable timeframe. Following LC-MS/MS data acquisition, computational algorithms are used to analyze resulting precursor (peptide) ion spectra and tandem mass spectra (MS-MS spectra) to produce peptide and protein detection and quantitation20. Relative quantitation is performed by two methods: label-free and isobaric labeling approaches. In label-free MS analysis, each sample is analyzed separately, and proteins are quantified on the basis of precursor ion intensity or the number of associated fragment spectra. In contrast, isobaric labeling methods21 (e.g., iTRAQ and TMT) enable sample multiplexing, and quantification is based on relative intensities of the reporter ions in fragment spectra. Advances in both instrumentation and sample preparation protocols have enabled near proteome-wide quantitative measurements at single amino acid resolution, which enables proteomics to join forces with genomics for comprehensive molecular characterization of human tumors (i.e., proteogenomics)22.
Figure 2. Untargeted (i.e. “shotgun”) discovery proteomics.
In untargeted (i.e. ‘shotgun’) proteomics, proteins are converted to peptides through proteolytic digestion (typically using trypsin). The peptides are fractionated by liquid chromatography and introduced to a mass spectrometer through electrospray ionization. For detection of low abundance proteins, an additional fractionation or enrichment step may be performed. Multiple modes of mass spectrometry are used, depending on available instrumentation and the design of the experiment. For example, an Orbitrap instrument uses image current detection to measure ions oscillating around the central electrode. Fourier transformation converts the signal from the time domain to the frequency domain, producing the mass spectrum. The Orbitrap is capable of high resolution and mass accuracy with a trade-off of relatively longer acquisition times. A linear ion trap uses static and RF (radiofrequency) fields to confine ions within the trap. The RF voltage is adjusted to confine/eliminate desired ions for detection. The ion trap is fast, but the spectra have relatively lower resolution and mass accuracy. Finally, a time-of-flight instrument measures the drift time of ions as they pass through a field-free region. The drift time is proportional to the mass-to-charge ratio. Time-of-flight acquisition is relatively fast, and resolution varies depending on instrument model. Regardless of instrument type, the most common structure of an untargeted proteomics dataset is data-dependent, i.e. it consists of a survey MS scan followed by MS/MS spectra of individual ions (peptides). The MS/MS spectra are searched against a database of known sequences to detect the peptide sequence.
Proteogenomics-driven target discovery: added value of proteomics
Cancer proteogenomics is the fusion of proteomics and genomics, in which hypotheses generated by genomic observations are tested at the protein level, interpretations of protein abundance and modification are guided by knowledge of the actual genome in the specific tumors under investigation, and genomic and proteomic measurements are integrated to understand and predict cancer phenotypes. The National Cancer Institute (NCI) has funded a major effort in cancer proteogenomics through its Clinical Proteomic Tumor Analysis Consortium (CPTAC). The three CPTAC flagship reports on colorectal23, breast24, and ovarian25 cancers, respectively, have provided a resource to the cancer research community, both by indicating which of the genomic and transcriptomic features associated with these cancers was recapitulated at the protein level, and by providing new insights into the substantial effects of post-translational modifications, specifically phosphorylation and acetylation, on the functional activities associated with DNA repair, proliferation, and survival. The proteogenomic approach has also been applied to other cancer studies based on tumor specimens, patient-derived xenograft (PDX) models, or cell lines26–33. There have been multiple reviews on the concept and methods of proteogenomics and its application to cancer research34–42. This section will focus on the added value of proteomics in proteogenomics-driven target discovery.
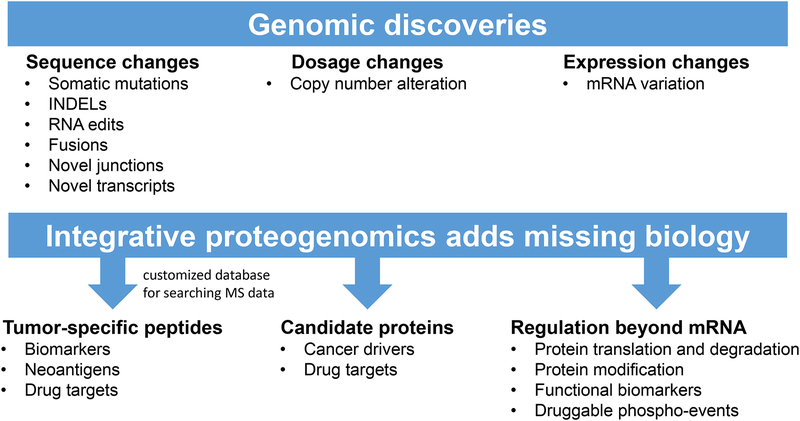
Thoughtful integration of genomic and proteomic data enhances our knowledge of cancer biology and biomarker and drug target discovery in multiple ways (Figure 3). First, proteogenomics is becoming a powerful approach for validation of novel peptide sequences inferred from genomic studies43. Cancer genomic studies have identified a large number of tumor-specific DNA or RNA sequences, including somatic mutations, fusions, novel junctions, etc. Proteins or peptides translated from these sequences are promising candidates for biomarkers, drug targets, and neoantigens in immunotherapy protocols. The first step toward clinical translation of these genomic discoveries is validation of their expression at the protein level. Shotgun LC-MS/MS allows proteome sequencing44. However, traditional proteomics data analysis workflows28,45 interpret MS/MS spectra using a reference protein sequence database (e.g., RefSeq or Ensembl) and cannot identify any novel, cancer-specific sequences. The proteogenomic approach incorporates candidate protein sequences derived from exome sequencing or RNA-Seq data into a customized database for MS/MS spectra interpretation, allowing the identification of sample-specific peptides that are missing in the reference databases27,46. Using this approach, the CPTAC colorectal23, breast24, and ovarian25 cancer studies provided proteomic validation for 64, 89, and 14 somatic mutations. Proteomic evidence has also been found for other genomic alterations, such as gene fusions25. Although a proteogenomic approach for discovering tumor-specific peptides is promising, there are several caveats to consider. For example, sequence coverage of the current shotgun proteomic platforms is not comprehensive. Using genomic, transcriptomic, and proteomic data from two PDX breast cancer models, it has been shown that only 10% of the single nucleotide variants (SNVs) detected by both DNA and RNA sequencing were observed as peptides, even with substantial chromatographic fractionation of the tumor lysates and 30 process replicates47. Therefore, although proteomics data provide the ultimate verification of novel, cancer-specific coding sequences, lack of proteomic evidence does not exclude the existence of the genomically predicted novel peptides. Using multiple proteases with distinct cleavage preferences to generate peptides for analysis may increase sequence coverage48. It is important to control false identifications. When possible, a strict sample-specific customized database derived from matched DNA or RNA sequencing data from the same sample should be used, and only highly confident novel genomic events should be included in the database. A step-wise FDR control scheme could be used, as novel peptide identifications tend to have higher FDRs than identifications of known peptides36. Other interpretations of a spectrum supporting a novel peptide, such as contaminants or post-translational modification of known peptides, should be carefully excluded. Eventually, novel peptides with high confidence should be validated by targeted proteomic assays (discussed below).
Figure 3. Integrative proteogenomic data analysis adds missing biology.
Genomic data identify somatic changes in tumors, and enable the generation of custom databases for searching MS data. Proteomic data confirm the translation of genomic results and also uncover additional biology (e.g. post-translational modifications).
Recent advances in mass spectrometry sensitivity and throughput have enabled the direct interrogation of immunopeptides released from affinity-enriched MHC complexes49 rather than the previous laborious approach requiring analysis of tumor infiltrating lymphocytes42,50. Putative neoantigens identified by proteogenomic approaches that combine whole-exome sequencing, RNA-Seq, and direct mass spectrometric analysis of the MHC peptidome have proven successful as vaccines promoting an anti-tumor immune response, and are approaching clinical trial status49,51.
Second, integrative proteogenomic analysis reveals that for many genes, global mRNA abundance variation across tumors is poorly correlated with protein abundance variation23–25. Although some genes have high mRNA-protein correlation, 30–70% genes showed no significant positive correlation23,25. Thus, for many genes, global mRNA measurements are poor predictors of protein abundance variations. Although this may be partially explained by technical noise associated with different platforms, it has been shown that protein profiling data is more closely aligned with function than mRNA profiling data52, suggesting that protein translation and degradation are tightly regulated and play a critical role in determining gene function.
Many studies identified mRNAs as cancer prognosis biomarkers by associating mRNA variations with patient survival, but very few mRNA biomarkers have been translated into clinical practice53. One possibility is that mRNA biomarkers may lack a direct link to protein activity and gene function, thus limiting their clinical applicability. Using clinical, somatic copy-number alteration, DNA methylation, mRNA, microRNA and Reverse Phase Protein Array (RPPA) protein expression data from four TCGA cancer types, Yuan et al.54 showed that among all molecular data-based prognosis models, only the protein expression model for lung squamous cell carcinoma had a performance similar to that of the model based on clinical variables, highlighting the promise of protein measurements in clinical applications. In a recent study on 44 colorectal cancer cell lines31, MS-based global proteomics data better reveal known drug-target associations and known drug-pathway associations as compared to mutation, DNA copy number, and mRNA expression data. In a formal evaluation using random forest and 5-fold cross-validation, proteomics data demonstrated better performance for predicting sensitivity to four major drugs used in the treatment of human colorectal cancer (5-FU, oxaliplatin, SN-38, regorafenib, and the EGFR inhibitor erlotinib) in 11 out of 15 pair-wise comparisons against other omics modalities. Similarly, analysis using MS-based global proteome profiling data from the CPTAC25,55 showed that proteomic profiles of ovarian serous carcinoma responses were strongly correlated with patient overall survival; the prognostic performance of these signatures is currently under evaluation. These studies demonstrate the potential of global proteome profiling in the discovery of prognostic and predictive cancer biomarkers. However, careful evaluation of the potential clinical utility of global proteome profiling data will require more datasets with matched clinical and proteomic data and sufficient sample size.
Third, protein modifications provide critical information on protein activity that is difficult to predict based on genomic or even global proteomic data. Sustained proliferative signaling is arguably the most important hallmark of cancer56, and protein phosphorylation plays a central role in proliferation-associated signal transduction. Many computational methods have been developed to infer signaling pathway activity by mapping cancer genomic data to pre-curated signaling networks and pathways57. Although useful, the accuracy of such inference is not easy to evaluate. The RPPA platform provides an antibody‐based assay to quantify protein abundance and post-translational modifications for preselected proteins, and the value of this functional assay has been demonstrated in the TCGA studies58. MS-based phosphoproteomic platforms provide deep coverage of the phosphoproteome. The CPTAC breast and ovarian cancer studies respectively identified a total of 62,679 and 24,464 phosphosites, representing on average 26,310 and 21,298 phosphosites per tumor. Most of the phosphosites were not included in existing knowledge bases such as PhosphoSitePlus59, thus adding new knowledge to protein signaling. One limitation of these studies is that the number of clinical specimens in the studies was too small to support conclusive clinical correlations.
A proteogenomic analysis of breast cancer PDX models validated some genomically predicted receptor tyrosine kinase targets; however, some phosphorylation events such as overexpression of ARAF, BRAF, and HSP90AB1 phosphosites cannot be explained by genomic data, suggesting that druggable phosphorylation events may require direct proteomic detection32. In addition to analyses based on individual phosphosites or phosphoproteins, integrating global phosphorylation data with known kinase-target relationships or signaling pathways and networks enables the inference of kinase or pathway activities60. More importantly, existing knowledge of phosphosites and kinase-target relationships is far from complete, and signaling pathways and networks are not personalized. Deep phosphoproteome assays on a large number of samples may eventually enable the construction of cancer type-, subtype- or tumor-specific signaling pathways and networks, which are critical for precision medicine.
Fourth, proteogenomic integration provides a means to prioritize candidate cancer drivers. For example, a subset of one or more protein-coding genes in the same copy number amplification region may be implicated as cancer drivers based on concordant overexpression of mRNA, protein, and/or phosphoprotein23,24.
Validating and translating proteomic markers into the clinic
The studies described above clearly demonstrate the power of integrative proteogenomics in generating novel hypotheses on tumor biomarkers and drug targets. However, this approach suffers from the “large p, small n” problem, in which the number of mutations, genes, proteins, and modification sites analyzed in a study far exceeds the number of samples that can be analyzed in a reasonable timeframe using global “discovery” platforms (e.g., shotgun LC-MS/MS). Therefore, putative candidates identified from these studies must be further tested in much larger sample sets before eventual clinical implementation. These biomarker qualification/verification studies61–63 require precise and specific protein quantification, preferably using assays that can be readily harmonized across laboratories and run at moderate-to-high throughput in multiplex (such that many candidate biomarkers can be tested while consuming a minimum amount of precious clinical biospecimens).
Next Generation Sequencing (NGS) provides a powerful tool for both unbiased mutation discovery and targeted clinical assay of preselected mutations. In contrast, because of technological limitations, the translation of protein-based biomarkers has lagged behind, resulting in a surprising dearth of novel tumor biomarkers for use in clinical practice despite tremendous investments over the past few decades towards biomarker discovery64. The primary platform for clinical translation of tumor protein biomarkers is immunohistochemistry (IHC). Despite their wide deployment in diagnostics and recent technological advances65, IHC assays are associated with analytical issues (e.g., specificity, reproducibility, subjectivity, semi-quantification, difficulties multiplexing) that have rendered them unreliable as companion diagnostic tests66–73. The cost and complexity of IHC assay development is also prohibitive for use of IHC as an efficient platform for testing large numbers of candidate biomarkers emanating from genome-scale proteogenomic studies. Likewise, shotgun LC-MS/MS used in biomarker discovery is not transferable to clinical laboratories due to analytical performance characteristics, low throughput, and high complexity64. While RPPA has been deployed in clinical trials74, the utility of this platform for biomarker verification and validation is greatly limited by the low availability of highly specific antibodies compatible with the RPPA platform. To address this unmet need for a platform to bridge the gap from proteomic and proteogenomic biomarker candidate discovery to clinical validation, targeted MS approaches have been developed to provide multiplexed, precise quantification with high dynamic range and sample throughput61–63.
Targeted mass spectrometry-based assays: bridge to clinical validation
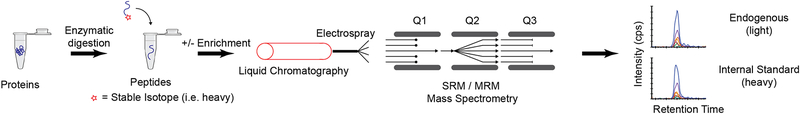
A suitable solution for biomarker qualification at moderate to high throughput is the use of targeted modes of mass spectrometry (MS) to quantify proteins61–63,75–77. In contrast to the shotgun MS approach, targeted MS focuses the full analytic capacity of the instrument on a discrete number of analytes of interest identified from proteomic and/or genomic discovery data61,62,78. By being directed and selective in the proteins to be measured, targeted MS addresses the limitations imposed in conventional shotgun-based methods (i.e. reliance on annotated sequence databases, lack of repeatability in identifications, poor consistency in quantification, and decreased sensitivity for low abundance analytes). There are multiple mass spectrometry-based acquisition schemes capable of accomplishing this goal79. The most common approach, termed Selected Reaction Monitoring (SRM), also commonly referred to as Multiple Reaction Monitoring (MRM), employs isolation and fragmentation of the targeted molecule with detection of specific fragments, confirmed by the addition of internal standards80,81 (Figure 4). While specificity in SRM/MRM can be very high, triple quadrupole mass spectrometers employed in the SRM/MRM mode are not high resolution instruments, imposing some limitations on the absolute specificity. Recent advances in high resolution and high mass accuracy instrumentation have given rise to a similar approach called Parallel Reaction Monitoring (PRM)82,83. Using PRM has shown benefits for measuring low abundance analytes in very complex matrices, where the combination of high resolution / mass accuracy and acquisition of all fragment ions can provide improved sensitivity and selectivity84. While PRM will likely continue to grow in use, the sensitivity, availability in clinical laboratories, relative low cost, and robustness of SRM/MRM instrumentation make it the most common technique, and the approach most likely to have immediate impact in clinical proteogenomic applications.
Figure 4. Targeted MS-based assays.
In targeted proteomics, proteins are converted to peptides through enzymatic (typically trypsin) digestion. The peptides are separated by liquid chromatography and introduced to a mass spectrometer through electrospray ionization. For low abundance proteins, a fractionation or enrichment step may be performed, such as immunoaffinity enrichment (e.g. immuno-MRM assay). Stable isotope-labeled peptides are added as internal standards and SRM/MRM analysis uses a triple quadrupole mass spectrometer (QQQ). The first and third quadrupoles (Q1, Q3) are used as mass filters separated by a collision cell for fragmentation (Q2). Q1 is tuned to pass the m/z of the target ion (i.e. the precursor ion), while filtering all other ions. The targeted precursor ion is fragmented by collision-induced dissociation in Q2, producing fragment ions (i.e. product ions). Q3 is used as a mass filter to pass a single analyte-specific product ion for detection. The combination of a specific precursor / product ion pair is termed a “transition.” The data output is a peak area ratio of the endogenous analyte peptide relative to the internal standard. By monitoring transitions, sensitivity is improved over untargeted methods by removing chemical noise and increasing the signal-to-noise ratio for the analyte. Specificity is also high by incorporating multiple levels of isolation and choosing highly specific transitions. Multiplexing is achieved by sequentially stepping through a list of transitions. Acquisition of multiple transitions is typically performed on a timescale that is tens to hundreds of times faster than the chromatographic peak widths of eluting peptides, enabling multiple points to be measured across a peak. The measurement of up to several hundred peptides are readily multiplexed into a single analytical run.
SRM/MRM has been used extensively in the quantification of small molecules, including measurements performed in clinical reference laboratories of metabolites that accumulate as a result of inborn errors of metabolism85,86. The same general principles of quantification of small molecules are applicable to proteomic applications87. Proteotypic peptides (i.e. peptide sequences that are unique to the protein of interest and “ionize well” in the mass spectrometer) are selected to represent the protein of interest. Selection of proteotypic peptides is an important step in method development, and selection rules and considerations have been published80,88,89. Quantification is performed using internal standards, such as synthetic peptides with identical sequences to the analyte peptides but incorporating heavy stable isotope labeled amino acids. Typically, stable isotope labels are incorporated at the C-terminal Arg and Lys residues of tryptic peptides (e.g. 13C6 15N4 for Arg, 13C6 15N2 for Lys), and the absolute quantity of the material is determined using orthogonal techniques (e.g. amino acid analysis)89. Thus, the internal standards feature chemically and physically identical behavior to the endogenous analytes, but are distinguished by their m/z in the mass spectrometer. The use of synthetic tryptic peptides does not control for performance of the enzymatic digestion step, thus larger, ‘extended’ peptides and recombinant proteins have also been used as internal standards to improve accuracy or provide common reference materials90–92. The availability and renewability of the internal standards used in targeted proteomics renders the approach highly amenable to harmonization and transfer among laboratories93–96. The approach is scalable for the generation of large numbers of assays96,97, with much less time and costs required in comparison to traditional immunoassays. Additionally, due to the high specificity of the MS detector, targeted MS assays can be highly multiplexed, enabling the measurement of up to several hundred peptides in a single analytical run96,98–101, reducing costs and sparing precious clinical sample.
To produce actionable biomarker information that would affect a tumor board discussion, targeted MRM assay panels must be sufficiently sensitive to quantify protein networks in real-word clinical specimens, especially core needle biopsies (CNB) and 5–10 micron sections cut from formalin-fixed, paraffin-embedded (FFPE) tumor blocks. Many clinical biomarkers will require enrichment upstream of MRM analysis, to attain sufficient sensitivity for quantification from limiting amounts of clinical material. There are a variety of options for enrichment of analytes employing physicochemical or affinity-based approaches. Physicochemical approaches typically use chromatography-based fractionation or the enrichment of broad analyte groups (e.g. IMAC for phosphorylated peptides), whereas affinity based approaches typically employ antibodies targeting a specific sequence or motif (e.g. phosphorylated tyrosine or ubiquitination). Affinity-based approaches are preferred due to higher enrichment factors, more streamline sample preparation requirements, and higher throughput capabilities. Obviously, affinity enrichment is limited to those targets where high affinity antibodies are available or can be generated. Another consideration in enriching target analytes is the point of enrichment. Enrichment can be performed at the protein or peptide level (i.e. pre- or post-digestion). Choice of protein or peptide enrichment is largely dependent on the affinity reagent available. Note that the de novo generation of antibodies to short, linear sequences (including post-translational modifications) has shown a relatively high success rate for making antibodies that work in peptide immunoaffinity enrichment97. Immuno-affinity enrichment of the target protein/peptide, coupled with MRM detection, produces highly sensitive and specific “immuno-MRM” assays102,103.
Unlike conventional antibody-based immunoassays (e.g., immunohistochemistry, ELISA, Western blotting), immuno-MRM couples antibody-based enrichment of targets with a detection system, mass spectrometry, that provides highly specific information about the amino acid sequence of the peptide. Protein lysates from biospecimens (e.g., biopsies) are proteolyzed with trypsin, and reliably released tryptic peptide “analytes” are enriched using anti-peptide antibodies and detected using a mass spectrometer. Because the instrument is “tuned” to detect only the analyte of interest (based on its mass-to-charge ratio), its full analytical capacity is focused on analytes of interest, resulting in significant improvements in sensitivity and selectivity compared to untargeted “shotgun” modes of mass spectrometry that are widely used in proteomic experiments. Furthermore, because the detector (mass spectrometer) provides single amino acid resolution, and each analyte has a unique mass fingerprint, the inevitable nonspecific binding of proteins to reagent antibodies (“interferences”) can be excluded from further analysis. This feature of MRM also makes it relatively straightforward to develop anti-peptide antibodies for immuno-MRM assays97,104.
The benefits of using a targeted SRM/MRM approach have enabled a broad range of applications in recent years, though it has especially gained traction as a tool in biomarker verification studies63,75–77,105,106; this use represents an important synergy with more traditional protein immunoassays, as the MRM assays can be used to down select only those candidate biomarkers that meet appropriate performance metrics, sparing the cost in time and resources of developing traditional immunoassays to scores of candidates that may not verify. The technique is amenable to a wide range of biological samples, including serum/plasma76,77,107,108, urine109,110, secretions111, tissue aspirates112, frozen tissue113, archived (i.e. formalin-fixed and paraffin-embedded) specimens114–116, and immortalized and primary cells117,118. One of the most appealing aspects is the versatility in assay development. If a suitable proteotypic peptide can be identified, the approach can be used to quantify a wide range of proteoforms, including isoforms119, post-translational modifications117,118,120–122, and protein variants123,124.
Limitations of targeted mass spectrometry-based assays
The many benefits of targeted MS-based assays described above (precision, harmonization, multiplexing, specificity) need to be weighed against the limitations of this platform. For example, while coupling immuno-affinity enrichment of peptides to targeted MS provides enhanced sensitivity for detection of proteins in clinical samples, the sensitivity of immuno-MS assays is currently not sufficient to support single cell analyses, although progress is being made towards this goal125,126. Unlike single cell techniques (e.g., immunohistochemistry, flow cytometry), MS-based assays quantify the average amount of peptides and proteins across thousands of cells, similar to other extraction-based techniques such as ELISA and Western blotting. Thus, it is not yet feasible to apply targeted MS assays to scant biospecimens, such as small numbers of circulating tumor cells, nor is it feasible to quantify cell-by-cell differences in protein expression. Additionally, while many proteins and posttranslational modifications of high clinical interest will be detectable in clinical samples by immuno-MRM, some are likely to remain below the lower limit of quantification of the assay. In those cases where a sufficiently high affinity anti-peptide antibody cannot be developed, there are alternative approaches involving extensive sample fractionation that also provide highly sensitive, reproducible results127–129, albeit at lower throughput using high complexity protocols. Second, subcellular localization or spatial resolution of protein expression (e.g., in different regions of a tumor with clonal heterogeneity, invasive edge vs hypoxic center of tumor) is not possible without additional sample processing such as macro- or micro-dissection of biospecimens. Third, to develop an assay there must be a reliable proteotypic peptide available for measurement. For the majority of proteins this is not an issue. However, in some cases, proteotypic peptides can be very limited. For example, in designing assays for specific post-translational modifications, the modification must reside on a peptide that is released reproducibly using a protease and is detectable by the mass spectrometer. Fourth, inter-laboratory studies of MRM measurements have identified the protease (e.g. trypsin) digestion step as the largest source of variation93. Because variation in digestion can occur on a sample-level basis and is analyte-dependent, intra-sample quality controls for monitoring digestion are most effective, such as use of a cleavable internal standard that is digested in situ with the native protein130–132. Fifth, immuno-MRM assays require antibodies to enrich the target analyte, and generating antibodies can be costly and time-consuming; however, the high specificity of the mass spectrometer makes it easier to develop antibodies97 for MS assays compared to traditional immunoassay platforms, and many commercially available antibodies on the market are likely to work in the immuno-MRM platform133.
Resources and recommendations to support development and use of targeted mass spectrometry-based assays
Software and database resources are available to support the development, analysis, and dissemination of targeted SRM/MRM assays. When designing assays, peptide selection is aided by searching online proteomics databases, such as those in the ProteomeXchange consortium134 and Global Proteome Machine135. Tools like PeptidePicker136 and MRMAssayDB137 can be used to compile results and aid in selecting the best surrogate peptides. Efforts to profile the SRM/MRM parameters for peptides covering the entire human proteome have recently been made public138,139, with results available in SRMAtlas140. These and other datasets provide utility in selecting proteotypic peptides and coordinates for transition selection and optimization. Skyline141, a vendor-neutral tool designed specifically for targeted proteomics (though it also is applied to the analysis of small molecules), contains a variety of features and plug-in modules that enable researchers to identify peptides for assay development, optimize instrumental parameters, and analyze targeted data.
Finally, there are several online repositories designed for targeted proteomics data. Panorama142 is a database for experimental results and functions closely with Skyline. PASSEL143 is a repository for published experiments employing targeted data where the contents can be mined for existing assay coordinates. Looking beyond databases of SRM/MRM transitions, the broader quantitative utility of assays is assembled in two online resources, the Quantitative Assay Database (QuAD)144 and the CPTAC Assay Portal145. QuAD contains characterization data of standard peptides, including response curves, in addition to assay coordinates and optimized parameters. The CPTAC Assay Portal currently contains the most highly characterized targeted assays, with fit-for-purpose validated figures of merit, links to the characterization data, and downloadable standard operating procedures to aid assay transferability. The description of multiple tiers of validation using fit-for-purpose validation criteria has been described146. Generally, traditional analytical figures of merit (e.g. linearity, limits of detection, repeatability, selectivity, and stability) are also applicable to the validation of targeted proteomics assays based on SRM/MRM. A consensus document has been published summarizing recommendations for the generation, quantification, storage, and handling of peptides used for MS-based assays89.
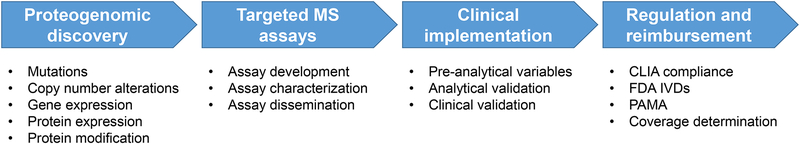
Clinical implementation of targeted MS assays: a work in process
Development and translation of a targeted MRM-based assay into the clinical laboratory is a multi-step process (Figure 5), as discussed in the thyroglobulin example below. Despite a decade of extensive literature on preclinical applications, targeted MS proteomic assays are only now beginning to be deployed in clinical laboratories or in clinical trials, since a critical mass of well characterized, targeted MS assays is becoming available for quantifying panels of cancer proteins145. The NCI is supporting efforts to drive these NextGen protein assays into clinical trials, through CPTAC’s Proteogenomic Translational Research Centers as well as the Applied Proteogenomics OrganizationaL Learning and Outcomes (APOLLO) Network147.
Figure 5. Workflow for clinical implementation of proteogenomics.
One of the earliest translations of a targeted MS proteomic assay to the clinical environment was the measurement of serum thyroglobulin, an important cancer biomarker used in monitoring patients treated for differentiated thyroid carcinoma148. The motivation for the development of a targeted MS assay for the quantification of serum thyroglobulin was the high prevalence of autoantibodies in thyroid cancer patients that interfere with FDA-cleared serum thyroglobulin immunoassays149. For example, false negative results can occur in the ~25% of patients that have autoantibodies to thyroglobulin, which interfere with the formation of sandwiches in immunometric assays. False positive results are also possible for the same patients when competitive radioimmunoassays are used. Naturally occurring anti-reagent antibodies also interfere with immunoassays and cause incorrect results. These errors have clinical consequences for patients, since false positive results can lead to unnecessary imaging or treatment, and false negative results inevitably lead to a delay in diagnosis and therapy. Fortuitously, the digestion of serum with trypsin in preparation for MRM analysis degrades interfering autoantibodies, and the enrichment of thyroglobulin-derived peptides using peptide-specific antibodies allows for the detection of very low concentrations of peptide, which serves as a surrogate for the protein150,151.
The greatest hurdle in quantification of thyroglobulin by MRM was the very low concentration of thyroglobulin in human serum. Indeed, for each peptide liberated from thyroglobulin in a patient’s sample, there are more than 40,000,000 peptides from albumin in the same complex mixture (based on the average mass concentration of albumin vs. thyroglobulin in human serum, 4 g/dL vs. 1 ng/mL, respectively). While liquid chromatography-tandem mass spectrometry is able to resolve an impressive number peptides from one another, it was not sufficient in this case. The use of an affinity-purified polyclonal anti-peptide antibody targeting a proteotypic peptide from thyroglobulin provided 10,000-fold enrichment, resulting in a lower limit of quantification ~3 ng/mL148. Affinity reagents and sample preparation conditions were optimized and the assay was replicated in other clinical laboratories152–155. The assay is now sufficiently sensitive (i.e., lower limit of detection of 0.15 ng/mL) and efficient to replace immunoassays for the measurement of thyroglobulin in patient samples and is now offered by 6 clinical reference laboratories in North America. This marks an important advance for the field of clinical mass spectrometry.
One of the hopes of targeted proteomics was the improved concordance between laboratories151. In order to evaluate the agreement of thyroglobulin measurements, a group of clinical laboratories collaborated on a small project in which leftover patient samples with and without autoantibodies were analyzed by four immunoassays and four LC-MS/MS assays156. The results demonstrated that even though the methods used for digestion and calibration were different in each laboratory, the LC-MS/MS assays agreed with one another much better than the four immunoassays that were run in the same laboratory. Additional collaboration demonstrated that the clinical performance of two LC-MS/MS assays in predicting outcomes for patients was essentially equivalent to one another and that LC-MS/MS assays are at least as good as immunoassays in the care of patients157.
The road from hypothesis to disseminated technology in the form of clinical measurement procedures was predictably long. More than 10 years elapsed between the first proof-of-principle experiments to the most recent version of the assay. During that time, the assay was iteratively improved and tested using the pre-validation experiments now outlined in the Clinical and Laboratory Standards Institute (CLSI) document C62-A158,159 and elsewhere145,158,160. The lessons learned from the success of thyroglobulin will undoubtedly shorten the time it takes to get future measurement procedures into the clinical laboratory.
Beyond thyroglobulin, additional targeted MS assays are making their way towards clinical use. For example, within breast cancer patients whose tumors tested HER2-positive (HER2+) by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH), a targeted MRM assay detected a wide dynamic range of HER2 protein expression, ranging multiple orders of magnitude161,162. Quantitative HER2 measurement by MRM is predictive of response to anti-HER2 therapy and survival in the supposedly homogeneous subgroup of HER2-positive (by standard IHC/FISH) breast cancer patients162. Importantly, the MRM assay allows the identification of FISH-positive cases that express low levels of HER2 and respond poorly to anti-HER2 therapy. In another recent example, MRM was used to measure two plasma proteins in the PANOPTIC (Pulmonary Nodule Plasma Proteomic Classifier) Trial to evaluate the accuracy of an integrated proteomic classifier in identifying benign lung nodules in patients with a pretest probability of cancer (pCA) ≤ 50%108. Additionally, clinical trials are combining genomics and proteomics as a component. NantHealth is currently recruiting for a clinical trial of its ‘GPS Cancer’ assay, a combination of whole genome and transcriptome sequencing and quantitative proteomics [NCT03073473].
The high multiplex capacity of MRM-based measurements will further enable the cost-effective development and deployment of panels of proteomic assays to complement Next-Generation Sequencing (NGS) panels in clinical trials. Also, FDA-cleared immunoassays often do not agree well with one another149. Even though assays are initially cleared as “substantially equivalent,” calibration drifts over time until assays are no longer harmonized. It is possible that by directly detecting peptides using targeted mass spectrometry (rather than the indirect assay signals observed in traditional immunoassays) laboratories could be more successful in calibrating their laboratory-developed tests (LDTs) to agree with one another. In a head to head comparison of PSA measurements using either a CLIA-approved ELISA assay or a targeted mass spectrometry-based assay (PRISM-SRM-MS), the mass spectrometry-based assay had greater sensitivity and equivalent reproducibility compared to the ELISA assay163.
Regulatory considerations for targeted MS assays
In the Unites States, translation of novel assays into patient care is overseen by the Centers for Medicare and Medicaid Services (CMS) and the Food and Drug Administration (FDA)164. While CMS oversees the accreditation of laboratories under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), FDA evaluates the safety and effectiveness of in vitro diagnostic medical devices (IVDs) for use in patient care. The FDA has recently recognized a standards guideline, C62-A158, from the Clinical and Laboratory Standards Institute (CLSI) that describes analytical validation studies for LC-MS assays, and is primarily focused on small molecule detection using LC-MS (metabolites165,166 and therapeutic drugs165).
Currently, all targeted proteomic MS assays run in clinical laboratories are laboratory developed tests (LDTs), which are subset of IVDs that are designed, manufactured, and used in a single laboratory167,168. No LDTs using targeted proteomics and LC-MS have yet been cleared or approved by the FDA. Although there are currently no FDA guidance documents that describe the analytical study requirements for targeted LC-MS-based proteomic IVDs, the analytical validation studies required for clearance or approval for LC-MS IVDs will follow the standard template for other proteomic IVDs64, with some additional technology-specific studies and information that will need to be provided. FDA and the National Cancer Institute’s CPTAC held a public workshop on analytical validation of protein and peptide LC-MS assays169,170. More recently, FDA held two public workshops on proteomics technologies in the clinic and analytical validation of protein and peptide LC-MS assays. The transcripts, presentations, and a discussion paper from these workshops are publicly available171,172.
Reimbursement for use of targeted MS assays
Potential reimbursement mechanisms for clinical proteomic testing differ country by country. Because the US is a major market for such tests, reimbursement mechanisms in the US will be covered here. The pathway to getting a novel proteomic assay covered by a US payer, either public or private, requires significant investments of the performing laboratory’s time and/or money to satisfy the multiple stakeholders, including insurance companies, benefits management companies, and federal regulatory bodies such as the FDA and the American Medical Association (the body that issues the Current Procedural Terminology [CPT] codes required for payment). To satisfy all of these stakeholders that a new test is safe and effective, medically necessary, and moreover, worthy of payment in an ever-increasing climate of cost-consciousness, is a daunting task.
Payments made by private insurers in the US are often set, at least initially, in relation to levels paid by the federal government’s Center of Medicare and Medicaid Services (CMS), which now sets payment levels according to the Protecting Access to Medicare Act (PAMA). PAMA’s provisions relevant to laboratory reimbursement include defining Advanced Diagnostic Laboratory Test (ADLT) as “…an analysis of multiple biomarkers of DNA, RNA or proteins combined in a unique algorithm to yield a single patient specific result.” An ADLT must also be performed by only one laboratory. Pricing for ADLTs will be set at the performing laboratory’s list price initially, followed by a shift to the weighted-median of private payer rates later. For newer tests that report actual analyte concentrations, and not the result(s) of an algorithm, CMS will set prices either by “cross-walking” (establishing that the new test is similar to an old test with a set payment rate, and using that rate with or without a multiplier), or “gap-filling” (a more holistic analysis by CMS that may consider charges for the test, resources required to perform the test, etc.). The effort expended by laboratories to lobby for favorable payment decisions from CMS, private payers, and laboratory benefits management companies is considerable.
Title XVIII of the Social Security Act (SSA) §1862(a)(1)(A) states that no Medicare payment shall be made for items or services that “are not reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of malformed body member.” Services not considered medically necessary are often referred to as experimental and/or investigational. Local coverage determinations from one specific Medicare administrator (Novitas) for oncologic biomarker panels are summarized in Box 1.
Box 1. Local coverage determinations from one specific Medicare administrator (Novitas) for oncologic biomarker panels generally require that 4 criteria are met to demonstrate medical necessity.
First, the biomarkers must have proven clinical validity/utility (CVU).
Second, to support the medical necessity of the service, there must be acceptance/uptake of specific testing into patient management. It is essential that physicians be familiar enough with all specific biomarkers, which they order, such that all test results may become clinically actionable.
Third, providers managing oncological conditions must demonstrate that the use of biomarkers will be used to assist in the management/treatment of the beneficiary.
-
Peer-reviewed full manuscript evidence is required to support combination panels for multiple biomarkers, particularly regarding their alleged composite clinical validity/utility. For example; such potential billing for multiple, diverse biomarkers (e.g., diagnostic/monitoring/prognostic/predictive) can only achieve medical necessity when it is clearly evident how each requested biomarker can be individually contributory.
Unsurprisingly, novel tests are usually not considered medically necessary when first offered. Publication of a single study showing analytical accuracy or clinical benefit is usually not enough for a test to be considered Medically Necessary, yet vexingly, some tests have been covered for years despite ample evidence of clinical obsolescence173. Sources that Novitas uses to support a local coverage determination are summarized in Box 2.
Box 2. Sources that Novitas uses to support a local coverage determination.
FDA labeling documentation.
National Comprehensive Cancer Network (NCCN) Biomarkers Compendium recommendations, particularly where Category 1 evidence is noted.
Findings from well-established, independent technology assessments (e.g., Evaluation of Genomic Applications in Practice and Prevention (EGAPP), Agency for Healthcare Research and Quality (AHRQ), Blue Cross and Blue Shield Association Technology Evaluation Center (BCBSA TEC) and the Cochrane Collaboration).
- Other independent, objective evaluations or systematic literature reviews, which can substantively contribute to the evidence base, including, but not restricted to, emerging National Institutes of Health (National Cancer Institute) guidelines for the accrual of genomics/proteomics clinical validity/utility evidence. Although there is not a prescriptive format for such systematic reviews, the documentation (submitted to Novitas) for reconsideration purposes should include the following three elements:
- Some type of recurring/periodic Committee structure, which is comprised of at least qualified biomathematicians/methodologists, molecular pathology laboratory specialists and relevant clinicians (e.g., oncologists).
- Evidence of active sharing of the critical evaluations in a manner that enables sufficiently broad input into this process, and a feasibly wide acceptance of this process by representative molecular pathology stakeholders. There is no preference between such a Committee being based at a single site, or even rotating among several sites.
-
Transparency of the biomarker evaluations via minutes (or a summary of minutes).
The Veristrat test offered by Biodesix in Boulder, Colorado, USA, can be considered as a proteomic test that has navigated a variety of systemic hurdles to win reimbursement from many insurers. This test is a MALDI mass spectrometric assay of serum that uses a proprietary algorithm to generate a score that directs appropriate therapy in non-small cell lung cancer. Evidence cited in several of the insurers’ medical policies (links to evidence on http://www.biodesix.com/coverage-and-reimbursement/) include both prospective/randomized/controlled trials and retrospective studies published in peer-reviewed literature and a cost-utility analysis. Policies generally limit the use of the test to a very narrow clinical indication.
Conclusion
While precision oncology approaches based on tumor genomes have benefitted a subset of cancer patients, many patients do not respond to the therapies selected based on their tumor genome (and most initial responders develop resistance). Therefore, we need a deeper readout of tumor biology to predict tumor phenotype with respect to drug response. Towards this end, tumor proteomic data are being used to annotate tumor genomes (with respect to which genomic features are expressed) and to identify tumor biology not discernable using genomic profiles alone (e.g., posttranslational modifications, non-mutational “epigenetic” causes of inactivation). It is expected that the integration of personalized genome information, coupled with data regarding its execution at the protein level, will soon become an accepted component in the practice of precision oncology.
Targeted mass spectrometry-based assays have matured technically over the past decade and are now capable of achieving analytical metrics comparable to those of FDA-cleared immunoassays and other affinity-reagent based assays. These assays provide a bridge from discovery of candidate biomarkers to biomarker verification studies, and are now beginning to make their way into the clinical laboratory as well. The value of targeted MS assays, and the additional information provided regarding protein expression and post-translational modifications, needs to be established through the results of clinical trials, and this is an area of intense activity.
Acknowledgments
This research was supported by U01 CA214114 (A.G.P. & A.N.H.), R50 CA211499 (J.R.W.), U01 CA214116 (K.D.R), and U24 CA210954 (B.Z.).
Competing financial interests
A.N.H. receives grant funding and instrument support from Waters, Inc., a mass spectrometry company. G.S.B. is a consultant for “Avalon Healthcare Solutions” in the area of laboratory benefits management. A.G.P receives instrument support from Sciex, a mass spectrometry company, and is founder of Precision Assays.
Contributor Information
Bing Zhang, Department of Molecular and Human Genetics, Lester and Sue Smith Breast Center, Baylor College of Medicine, Houston, Texas 77030.
Jeffrey R Whiteaker, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, Washington 98109.
Andrew N. Hoofnagle, Departments of Laboratory Medicine and Medicine, University of Washington, Seattle, WA 98195,
Geoffrey S Baird, Departments of Laboratory Medicine and Pathology, University of Washington, Seattle, WA 98195.
Karin D. Rodland, Biological Sciences Division, Pacific Northwest National Laboratory, Richland WA 99354 Department of Cell, Development, and Cancer Biology, Oregon Health & Sciences University, Portland OR 97221.
Amanda G. Paulovich, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, Washington 98109 Division of Medical Oncology, University of Washington School of Medicine, Seattle, Washington 98195.
BIBLIOGRAPHY
- 1.Vogelstein B et al. Cancer genome landscapes. Science 339, 1546–1558, doi: 10.1126/science.1235122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon R Critical Review of Umbrella, Basket, and Platform Designs for Oncology Clinical Trials. Clin Pharmacol Ther 102, 934–941, doi: 10.1002/cpt.814 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Maitland ML & Schilsky RL Clinical trials in the era of personalized oncology. CA Cancer J Clin 61, 365–381, doi: 10.3322/caac.20135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbiah V & Kurzrock R Debunking the Delusion That Precision Oncology Is an Illusion. Oncologist 22, 881–882, doi: 10.1634/theoncologist.2017-0040 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyman DM, Taylor BS & Baselga J Implementing Genome-Driven Oncology. Cell 168, 584–599, doi: 10.1016/j.cell.2016.12.015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad V Perspective: The precision-oncology illusion. Nature 537, S63, doi: 10.1038/537S63a (2016). [DOI] [PubMed] [Google Scholar]
- 7.West HJ No Solid Evidence, Only Hollow Argument for Universal Tumor Sequencing: Show Me the Data. JAMA Oncol 2, 717–718, doi: 10.1001/jamaoncol.2016.0075 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Vasan N et al. A targeted next-generation sequencing assay detects a high frequency of therapeutically targetable alterations in primary and metastatic breast cancers: implications for clinical practice. Oncologist 19, 453–458, doi: 10.1634/theoncologist.2013-0377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sholl LM et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 1, e87062, doi: 10.1172/jci.insight.87062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letai A Functional precision cancer medicine-moving beyond pure genomics. Nat Med 23, 1028–1035, doi: 10.1038/nm.4389 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Flavahan WA, Gaskell E & Bernstein BE Epigenetic plasticity and the hallmarks of cancer. Science 357, doi: 10.1126/science.aal2380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baer C, Claus R & Plass C Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res 73, 473–477, doi: 10.1158/0008-5472.CAN-12-3731 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Rupaimoole R, Calin GA, Lopez-Berestein G & Sood AK miRNA Deregulation in Cancer Cells and the Tumor Microenvironment. Cancer Discov 6, 235–246, doi: 10.1158/2159-8290.CD-15-0893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SH et al. Widespread intronic polyadenylation inactivates tumour suppressor genes in leukaemia. Nature 561, 127–131, doi: 10.1038/s41586-018-0465-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luco RF et al. Regulation of alternative splicing by histone modifications. Science 327, 996–1000, doi: 10.1126/science.1184208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oltean S & Bates DO Hallmarks of alternative splicing in cancer. Oncogene 33, 5311–5318, doi: 10.1038/onc.2013.533 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Chen J & Weiss WA Alternative splicing in cancer: implications for biology and therapy. Oncogene 34, 1–14, doi: 10.1038/onc.2013.570 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Hebert AS et al. Comprehensive Single-Shot Proteomics with FAIMS on a Hybrid Orbitrap Mass Spectrometer. Anal Chem 90, 9529–9537, doi: 10.1021/acs.analchem.8b02233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier F, Geyer PE, Virreira Winter S, Cox J & Mann M BoxCar acquisition method enables single-shot proteomics at a depth of 10,000 proteins in 100 minutes. Nat Methods, doi: 10.1038/s41592-018-0003-5 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Bantscheff M, Lemeer S, Savitski MM & Kuster B Quantitative mass spectrometry in proteomics: critical review update from 2007 to the present. Anal Bioanal Chem 404, 939–965, doi: 10.1007/s00216-012-6203-4 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Rauniyar N & Yates JR 3rd. Isobaric labeling-based relative quantification in shotgun proteomics. J Proteome Res 13, 5293–5309, doi: 10.1021/pr500880b (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis MJ et al. Connecting genomic alterations to cancer biology with proteomics: the NCI Clinical Proteomic Tumor Analysis Consortium. Cancer Discov 3, 1108–1112, doi: 10.1158/2159-8290.CD-13-0219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B et al. Proteogenomic characterization of human colon and rectal cancer. Nature 513, 382–387, doi: 10.1038/nature13438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mertins P et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534, 55–62, doi: 10.1038/nature18003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H et al. Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell 166, 755–765, doi: 10.1016/j.cell.2016.05.069 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharpnack MF et al. Proteogenomic Analysis of Surgically Resected Lung Adenocarcinoma. J Thorac Oncol, doi: 10.1016/j.jtho.2018.06.025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lobas AA et al. Proteogenomics of Malignant Melanoma Cell Lines: The Effect of Stringency of Exome Data Filtering on Variant Peptide Identification in Shotgun Proteomics. J Proteome Res 17, 1801–1811, doi: 10.1021/acs.jproteome.7b00841 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Alfaro JA et al. Detecting protein variants by mass spectrometry: a comprehensive study in cancer cell-lines. Genome Med 9, 62, doi: 10.1186/s13073-017-0454-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L et al. Novel RNA-Affinity Proteogenomics Dissects Tumor Heterogeneity for Revealing Personalized Markers in Precision Prognosis of Cancer. Cell Chem Biol 25, 619–633 e615, doi: 10.1016/j.chembiol.2018.01.016 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Komor MA et al. Identification of Differentially Expressed Splice Variants by the Proteogenomic Pipeline Splicify. Mol Cell Proteomics 16, 1850–1863, doi: 10.1074/mcp.TIR117.000056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J et al. Colorectal Cancer Cell Line Proteomes Are Representative of Primary Tumors and Predict Drug Sensitivity. Gastroenterology 153, 1082–1095, doi: 10.1053/j.gastro.2017.06.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang KL et al. Proteogenomic integration reveals therapeutic targets in breast cancer xenografts. Nat Commun 8, 14864, doi: 10.1038/ncomms14864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granados DP et al. Proteogenomic-based discovery of minor histocompatibility antigens with suitable features for immunotherapy of hematologic cancers. Leukemia 30, 1344–1354, doi: 10.1038/leu.2016.22 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Boja ES & Rodriguez H Proteogenomic convergence for understanding cancer pathways and networks. Clin Proteomics 11, 22, doi: 10.1186/1559-0275-11-22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggles KV et al. Methods, Tools and Current Perspectives in Proteogenomics. Mol Cell Proteomics 16, 959–981, doi: 10.1074/mcp.MR117.000024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesvizhskii AI Proteogenomics: concepts, applications and computational strategies. Nat Methods 11, 1114–1125, doi: 10.1038/nmeth.3144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfaro JA, Sinha A, Kislinger T & Boutros PC Onco-proteogenomics: cancer proteomics joins forces with genomics. Nat Methods 11, 1107–1113, doi: 10.1038/nmeth.3138 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Sheynkman GM, Shortreed MR, Cesnik AJ & Smith LM Proteogenomics: Integrating Next-Generation Sequencing and Mass Spectrometry to Characterize Human Proteomic Variation. Annu Rev Anal Chem (Palo Alto Calif) 9, 521–545, doi: 10.1146/annurev-anchem-071015-041722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subbannayya Y, Pinto SM, Gowda H & Prasad TS Proteogenomics for understanding oncology: recent advances and future prospects. Expert Rev Proteomics 13, 297–308, doi: 10.1586/14789450.2016.1136217 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Dimitrakopoulos L et al. Proteogenomics: Opportunities and Caveats. Clin Chem 62, 551–557, doi: 10.1373/clinchem.2015.247858 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Menschaert G & Fenyo D Proteogenomics from a bioinformatics angle: A growing field. Mass Spectrom Rev 36, 584–599, doi: 10.1002/mas.21483 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Creech AL et al. The Role of Mass Spectrometry and Proteogenomics in the Advancement of HLA Epitope Prediction. Proteomics 18, e1700259, doi: 10.1002/pmic.201700259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y et al. Discovery of coding regions in the human genome by integrated proteogenomics analysis workflow. Nat Commun 9, 903, doi: 10.1038/s41467-018-03311-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aebersold R & Mann M Mass spectrometry-based proteomics. Nature 422, 198–207, doi: 10.1038/nature01511 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Eng JK, McCormack AL & Yates JR An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5, 976–989, doi: 10.1016/1044-0305(94)80016-2 (1994). [DOI] [PubMed] [Google Scholar]
- 46.Wang X et al. Protein identification using customized protein sequence databases derived from RNA-Seq data. J Proteome Res 11, 1009–1017, doi: 10.1021/pr200766z (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruggles KV et al. An Analysis of the Sensitivity of Proteogenomic Mapping of Somatic Mutations and Novel Splicing Events in Cancer. Mol Cell Proteomics 15, 1060–1071, doi: 10.1074/mcp.M115.056226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choudhary G, Wu SL, Shieh P & Hancock WS Multiple enzymatic digestion for enhanced sequence coverage of proteins in complex proteomic mixtures using capillary LC with ion trap MS/MS. J Proteome Res 2, 59–67 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Yadav M et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572–576, doi: 10.1038/nature14001 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Bassani-Sternberg M & Coukos G Mass spectrometry-based antigen discovery for cancer immunotherapy. Curr Opin Immunol 41, 9–17, doi: 10.1016/j.coi.2016.04.005 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Gubin MM et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581, doi: 10.1038/nature13988 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J et al. Proteome Profiling Outperforms Transcriptome Profiling for Coexpression Based Gene Function Prediction. Mol Cell Proteomics 16, 121–134, doi: 10.1074/mcp.M116.060301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cronin M et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem 53, 1084–1091, doi: 10.1373/clinchem.2006.076497 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Yuan Y et al. Assessing the clinical utility of cancer genomic and proteomic data across tumor types. Nat Biotechnol 32, 644–652, doi: 10.1038/nbt.2940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu KH et al. Predicting Ovarian Cancer Patients’ Clinical Response to Platinum-Based Chemotherapy by Their Tumor Proteomic Signatures. J Proteome Res 15, 2455–2465, doi: 10.1021/acs.jproteome.5b01129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674, doi: 10.1016/j.cell.2011.02.013 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Creixell P et al. Pathway and network analysis of cancer genomes. Nat Methods 12, 615–621, doi: 10.1038/nmeth.3440 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoadley KA et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158, 929–944, doi: 10.1016/j.cell.2014.06.049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hornbeck PV et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 43, D512–520, doi: 10.1093/nar/gku1267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weidner C, Fischer C & Sauer S PHOXTRACK-a tool for interpreting comprehensive datasets of post-translational modifications of proteins. Bioinformatics 30, 3410–3411, doi: 10.1093/bioinformatics/btu572 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Wang P, Whiteaker JR & Paulovich AG The evolving role of mass spectrometry in cancer biomarker discovery. Cancer Biol Ther 8, 1083–1094 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gillette MA & Carr SA Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat Methods 10, 28–34, doi: 10.1038/nmeth.2309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez H et al. Reconstructing the pipeline by introducing multiplexed multiple reaction monitoring mass spectrometry for cancer biomarker verification: an NCI-CPTC initiative perspective. Proteomics Clin Appl 4, 904–914, doi: 10.1002/prca.201000057 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuzery AK, Levin J, Chan MM & Chan DW Translation of proteomic biomarkers into FDA approved cancer diagnostics: issues and challenges. Clin Proteomics 10, 13, doi: 10.1186/1559-0275-10-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camp RL, Chung GG & Rimm DL Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med 8, 1323–1327, doi: 10.1038/nm791 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Toki MI, Cecchi F, Hembrough T, Syrigos KN & Rimm DL Proof of the quantitative potential of immunofluorescence by mass spectrometry. Lab Invest 97, 329–334, doi: 10.1038/labinvest.2016.148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quinn M Newfoundland launches judicial inquiry. CMAJ 177, 24–25, doi: 10.1503/cmaj.070741 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wasielewski R, Hasselmann S, Ruschoff J, Fisseler-Eckhoff A & Kreipe H Proficiency testing of immunohistochemical biomarker assays in breast cancer. Virchows Arch 453, 537–543, doi: 10.1007/s00428-008-0688-4 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Allred DC et al. NCCN Task Force Report: Estrogen Receptor and Progesterone Receptor Testing in Breast Cancer by Immunohistochemistry. J Natl Compr Canc Netw 7 Suppl 6, S1–S21; quiz S22–23 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Carvajal-Hausdorf DE, Schalper KA, Neumeister VM & Rimm DL Quantitative measurement of cancer tissue biomarkers in the lab and in the clinic. Lab Invest 95, 385–396, doi: 10.1038/labinvest.2014.157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolff AC et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31, 3997–4013, doi: 10.1200/JCO.2013.50.9984 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Buttner R et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol 35, 3867–3876, doi: 10.1200/JCO.2017.74.7642 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Vani K, Sompuram SR, Fitzgibbons P & Bogen SA National HER2 proficiency test results using standardized quantitative controls: characterization of laboratory failures. Arch Pathol Lab Med 132, 211–216, doi: 10.1043/1543-2165(2008)132[211:NHPTRU]2.0.CO;2 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Mueller C, Liotta LA & Espina V Reverse phase protein microarrays advance to use in clinical trials. Mol Oncol 4, 461–481, doi: 10.1016/j.molonc.2010.09.003 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whiteaker JR et al. Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J Proteome Res 6, 3962–3975, doi: 10.1021/pr070202v (2007). [DOI] [PubMed] [Google Scholar]
- 76.Addona TA et al. A pipeline that integrates the discovery and verification of plasma protein biomarkers reveals candidate markers for cardiovascular disease. Nat Biotechnol 29, 635–643, doi: 10.1038/nbt.1899 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whiteaker JR et al. A targeted proteomics-based pipeline for verification of biomarkers in plasma. Nat Biotechnol 29, 625–634, doi: 10.1038/nbt.1900 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Picotti P, Bodenmiller B & Aebersold R Proteomics meets the scientific method. Nat Methods 10, 24–27, doi: 10.1038/nmeth.2291 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Vidova V & Spacil Z A review on mass spectrometry-based quantitative proteomics: Targeted and data independent acquisition. Anal Chim Acta 964, 7–23, doi: 10.1016/j.aca.2017.01.059 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Lange V, Picotti P, Domon B & Aebersold R Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol 4, 222, doi: 10.1038/msb.2008.61 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan S et al. Mass spectrometry based targeted protein quantification: methods and applications. J Proteome Res 8, 787–797, doi: 10.1021/pr800538n (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peterson AC, Russell JD, Bailey DJ, Westphall MS & Coon JJ Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol Cell Proteomics 11, 1475–1488, doi: 10.1074/mcp.O112.020131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bourmaud A, Gallien S & Domon B Parallel reaction monitoring using quadrupole-Orbitrap mass spectrometer: Principle and applications. Proteomics 16, 2146–2159, doi: 10.1002/pmic.201500543 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Ronsein GE et al. Parallel reaction monitoring (PRM) and selected reaction monitoring (SRM) exhibit comparable linearity, dynamic range and precision for targeted quantitative HDL proteomics. J Proteomics 113, 388–399, doi: 10.1016/j.jprot.2014.10.017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chace DH & Kalas TA A biochemical perspective on the use of tandem mass spectrometry for newborn screening and clinical testing. Clin Biochem 38, 296–309, doi: 10.1016/j.clinbiochem.2005.01.017 (2005). [DOI] [PubMed] [Google Scholar]
- 86.Want EJ, Cravatt BF & Siuzdak G The expanding role of mass spectrometry in metabolite profiling and characterization. Chembiochem 6, 1941–1951, doi: 10.1002/cbic.200500151 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Brun V, Masselon C, Garin J & Dupuis A Isotope dilution strategies for absolute quantitative proteomics. J Proteomics 72, 740–749, doi: 10.1016/j.jprot.2009.03.007 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Keerthikumar S & Mathivanan S Proteotypic Peptides and Their Applications. Methods Mol Biol 1549, 101–107, doi: 10.1007/978-1-4939-6740-7_8 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Hoofnagle AN et al. Recommendations for the Generation, Quantification, Storage, and Handling of Peptides Used for Mass Spectrometry-Based Assays. Clin Chem 62, 48–69, doi: 10.1373/clinchem.2015.250563 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brun V et al. Isotope-labeled protein standards: toward absolute quantitative proteomics. Mol Cell Proteomics 6, 2139–2149, doi: 10.1074/mcp.M700163-MCP200 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Konopka A et al. Improving the precision of quantitative bottom-up proteomics based on stable isotope-labeled proteins. Anal Bioanal Chem 404, 1079–1087, doi: 10.1007/s00216-012-6007-6 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Scott KB, Turko IV & Phinney KW Quantitative performance of internal standard platforms for absolute protein quantification using multiple reaction monitoring-mass spectrometry. Anal Chem 87, 4429–4435, doi: 10.1021/acs.analchem.5b00331 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Addona TA et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol 27, 633–641, doi: 10.1038/nbt.1546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prakash A et al. Interlaboratory reproducibility of selective reaction monitoring assays using multiple upfront analyte enrichment strategies. J Proteome Res 11, 3986–3995, doi: 10.1021/pr300014s (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abbatiello SE et al. Large-Scale Interlaboratory Study to Develop, Analytically Validate and Apply Highly Multiplexed, Quantitative Peptide Assays to Measure Cancer-Relevant Proteins in Plasma. Mol Cell Proteomics 14, 2357–2374, doi: 10.1074/mcp.M114.047050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kennedy JJ et al. Demonstrating the feasibility of large-scale development of standardized assays to quantify human proteins. Nat Methods 11, 149–155, doi: 10.1038/nmeth.2763 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whiteaker JR et al. Evaluation of large scale quantitative proteomic assay development using peptide affinity-based mass spectrometry. Mol Cell Proteomics 10, M110 005645, doi: 10.1074/mcp.M110.005645 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huttenhain R et al. Reproducible quantification of cancer-associated proteins in body fluids using targeted proteomics. Sci Transl Med 4, 142ra194, doi: 10.1126/scitranslmed.3003989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Surinova S et al. Prediction of colorectal cancer diagnosis based on circulating plasma proteins. EMBO Mol Med 7, 1166–1178, doi: 10.15252/emmm.201404873 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abelin JG et al. Reduced-representation Phosphosignatures Measured by Quantitative Targeted MS Capture Cellular States and Enable Large-scale Comparison of Drug-induced Phenotypes. Mol Cell Proteomics 15, 1622–1641, doi: 10.1074/mcp.M116.058354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ippoliti PJ et al. Automated Microchromatography Enables Multiplexing of Immunoaffinity Enrichment of Peptides to Greater than 150 for Targeted MS-Based Assays. Anal Chem 88, 7548–7555, doi: 10.1021/acs.analchem.6b00946 (2016). [DOI] [PubMed] [Google Scholar]
- 102.Whiteaker JR & Paulovich AG Peptide immunoaffinity enrichment coupled with mass spectrometry for peptide and protein quantification. Clin Lab Med 31, 385–396, doi: 10.1016/j.cll.2011.07.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson NL et al. Mass spectrometric quantitation of peptides and proteins using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA). J Proteome Res 3, 235–244 (2004). [DOI] [PubMed] [Google Scholar]
- 104.Schoenherr RM et al. Automated screening of monoclonal antibodies for SISCAPA assays using a magnetic bead processor and liquid chromatography-selected reaction monitoring-mass spectrometry. J Immunol Methods 353, 49–61, doi: 10.1016/j.jim.2009.11.017 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chambers AG, Percy AJ, Simon R & Borchers CH MRM for the verification of cancer biomarker proteins: recent applications to human plasma and serum. Expert Rev Proteomics 11, 137–148, doi: 10.1586/14789450.2014.877346 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Faria SS et al. A Timely Shift from Shotgun to Targeted Proteomics and How It Can Be Groundbreaking for Cancer Research. Front Oncol 7, 13, doi: 10.3389/fonc.2017.00013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Percy AJ, Chambers AG, Yang J & Borchers CH Multiplexed MRM-based quantitation of candidate cancer biomarker proteins in undepleted and non-enriched human plasma. Proteomics 13, 2202–2215, doi: 10.1002/pmic.201200316 (2013). [DOI] [PubMed] [Google Scholar]
- 108.Silvestri GA et al. Assessment of Plasma Proteomics Biomarker’s Ability to Distinguish Benign From Malignant Lung Nodules: Results of the PANOPTIC (Pulmonary Nodule Plasma Proteomic Classifier) Trial. Chest, doi: 10.1016/j.chest.2018.02.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duriez E et al. Large-Scale SRM Screen of Urothelial Bladder Cancer Candidate Biomarkers in Urine. J Proteome Res 16, 1617–1631, doi: 10.1021/acs.jproteome.6b00979 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Kentsis A et al. Urine proteomics for discovery of improved diagnostic markers of Kawasaki disease. EMBO Mol Med 5, 210–220, doi: 10.1002/emmm.201201494 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim Y et al. Targeted proteomics identifies liquid-biopsy signatures for extracapsular prostate cancer. Nat Commun 7, 11906, doi: 10.1038/ncomms11906 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martinez-Garcia E et al. Targeted Proteomics Identifies Proteomic Signatures in Liquid Biopsies of the Endometrium to Diagnose Endometrial Cancer and Assist in the Prediction of the Optimal Surgical Treatment. Clin Cancer Res 23, 6458–6467, doi: 10.1158/1078-0432.CCR-17-0474 (2017). [DOI] [PubMed] [Google Scholar]
- 113.Song E et al. Targeted proteomic assays for quantitation of proteins identified by proteogenomic analysis of ovarian cancer. Sci Data 4, 170091, doi: 10.1038/sdata.2017.91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hembrough T et al. Application of selected reaction monitoring for multiplex quantification of clinically validated biomarkers in formalin-fixed, paraffin-embedded tumor tissue. J Mol Diagn 15, 454–465, doi: 10.1016/j.jmoldx.2013.03.002 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Sprung RW et al. Precision of multiple reaction monitoring mass spectrometry analysis of formalin-fixed, paraffin-embedded tissue. J Proteome Res 11, 3498–3505, doi: 10.1021/pr300130t (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kennedy JJ et al. Optimized Protocol for Quantitative Multiple Reaction Monitoring-Based Proteomic Analysis of Formalin-Fixed, Paraffin-Embedded Tissues. J Proteome Res 15, 2717–2728, doi: 10.1021/acs.jproteome.6b00245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kennedy JJ et al. Immobilized Metal Affinity Chromatography Coupled to Multiple Reaction Monitoring Enables Reproducible Quantification of Phospho-signaling. Mol Cell Proteomics 15, 726–739, doi: 10.1074/mcp.O115.054940 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Whiteaker JR et al. Peptide Immunoaffinity Enrichment and Targeted Mass Spectrometry Enables Multiplex, Quantitative Pharmacodynamic Studies of Phospho-Signaling. Mol Cell Proteomics 14, 2261–2273, doi: 10.1074/mcp.O115.050351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu X et al. Constrained selected reaction monitoring: quantification of selected post-translational modifications and protein isoforms. Methods 61, 304–312, doi: 10.1016/j.ymeth.2013.03.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wolf-Yadlin A, Hautaniemi S, Lauffenburger DA & White FM Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proc Natl Acad Sci U S A 104, 5860–5865, doi: 10.1073/pnas.0608638104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Whiteaker JR et al. Targeted mass spectrometry enables robust quantification of FANCD2 mono-ubiquitination in response to DNA damage. DNA Repair (Amst) 65, 47–53, doi: 10.1016/j.dnarep.2018.03.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whiteaker JR et al. A Multiplexed Mass Spectrometry-Based Assay for Robust Quantification of Phosphosignaling in Response to DNA Damage. Radiat Res 189, 505–518, doi: 10.1667/RR14963.1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang P et al. A novel, multiplexed targeted mass spectrometry assay for quantification of complement factor H (CFH) variants and CFH-related proteins 1–5 in human plasma. Proteomics 17, doi: 10.1002/pmic.201600237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin A et al. Novel allele-specific quantification methods reveal no effects of adult onset CAG repeats on HTT mRNA and protein levels. Hum Mol Genet 26, 1258–1267, doi: 10.1093/hmg/ddx033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu Y et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10–100 mammalian cells. Nat Commun 9, 882, doi: 10.1038/s41467-018-03367-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi T et al. Facile carrier-assisted targeted mass spectrometric approach for proteomic analysis of low numbers of mammalian cells. Communications Biology 1, 103, doi: 10.1038/s42003-018-0107-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nie S et al. Deep-Dive Targeted Quantification for Ultrasensitive Analysis of Proteins in Nondepleted Human Blood Plasma/Serum and Tissues. Anal Chem 89, 9139–9146, doi: 10.1021/acs.analchem.7b01878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shi T et al. Advances in targeted proteomics and applications to biomedical research. Proteomics 16, 2160–2182, doi: 10.1002/pmic.201500449 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shi T et al. Sensitive targeted quantification of ERK phosphorylation dynamics and stoichiometry in human cells without affinity enrichment. Anal Chem 87, 1103–1110, doi: 10.1021/ac503797x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Faria M et al. Comparison of a stable isotope labeled (SIL) peptide and an extended SIL peptide as internal standards to track digestion variability of an unstable signature peptide during quantification of a cancer biomarker, human osteopontin, from plasma using capillary microflow LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 1001, 156–168, doi: 10.1016/j.jchromb.2015.05.040 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Nouri-Nigjeh E et al. Effects of calibration approaches on the accuracy for LC-MS targeted quantification of therapeutic protein. Anal Chem 86, 3575–3584, doi: 10.1021/ac5001477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bronsema KJ, Bischoff R & van de Merbel NC High-sensitivity LC-MS/MS quantification of peptides and proteins in complex biological samples: the impact of enzymatic digestion and internal standard selection on method performance. Anal Chem 85, 9528–9535, doi: 10.1021/ac4015116 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Schoenherr RM et al. Commercially available antibodies can be applied in quantitative multiplexed peptide immunoaffinity enrichment targeted mass spectrometry assays. Proteomics 16, 2141–2145, doi: 10.1002/pmic.201500540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deutsch EW et al. The ProteomeXchange consortium in 2017: supporting the cultural change in proteomics public data deposition. Nucleic Acids Res 45, D1100–D1106, doi: 10.1093/nar/gkw936 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Craig R, Cortens JP & Beavis RC Open source system for analyzing, validating, and storing protein identification data. J Proteome Res 3, 1234–1242, doi: 10.1021/pr049882h (2004). [DOI] [PubMed] [Google Scholar]
- 136.Mohammed Y et al. PeptidePicker: a scientific workflow with web interface for selecting appropriate peptides for targeted proteomics experiments. J Proteomics 106, 151–161, doi: 10.1016/j.jprot.2014.04.018 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Bhowmick P, Mohammed Y & Borchers CH MRMAssayDB: an integrated resource for validated targeted proteomics assays. Bioinformatics, doi: 10.1093/bioinformatics/bty385 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kusebauch U et al. Human SRMAtlas: A Resource of Targeted Assays to Quantify the Complete Human Proteome. Cell 166, 766–778, doi: 10.1016/j.cell.2016.06.041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mohammed Y & Borchers CH An extensive library of surrogate peptides for all human proteins. J Proteomics 129, 93–97, doi: 10.1016/j.jprot.2015.07.025 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Kusebauch U et al. Using PeptideAtlas, SRMAtlas, and PASSEL: Comprehensive Resources for Discovery and Targeted Proteomics. Curr Protoc Bioinformatics 46, 13 25 11–28, doi: 10.1002/0471250953.bi1325s46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pino LK et al. The Skyline ecosystem: Informatics for quantitative mass spectrometry proteomics. Mass Spectrom Rev, doi: 10.1002/mas.21540 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma V et al. Panorama: a targeted proteomics knowledge base. J Proteome Res 13, 4205–4210, doi: 10.1021/pr5006636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Farrah T et al. PASSEL: the PeptideAtlas SRMexperiment library. Proteomics 12, 1170–1175, doi: 10.1002/pmic.201100515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Remily-Wood ER et al. A database of reaction monitoring mass spectrometry assays for elucidating therapeutic response in cancer. Proteomics Clin Appl 5, 383–396, doi: 10.1002/prca.201000115 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Whiteaker JR et al. CPTAC Assay Portal: a repository of targeted proteomic assays. Nat Methods 11, 703–704, doi: 10.1038/nmeth.3002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carr SA et al. Targeted peptide measurements in biology and medicine: best practices for mass spectrometry-based assay development using a fit-for-purpose approach. Mol Cell Proteomics 13, 907–917, doi: 10.1074/mcp.M113.036095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fiore LD, Rodriguez H & Shriver CD Collaboration to Accelerate Proteogenomics Cancer Care: The Department of Veterans Affairs, Department of Defense, and the National Cancer Institute’s Applied Proteogenomics OrganizationaL Learning and Outcomes (APOLLO) Network. Clin Pharmacol Ther 101, 619–621, doi: 10.1002/cpt.658 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Hoofnagle AN, Becker JO, Wener MH & Heinecke JW Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem 54, 1796–1804, doi: 10.1373/clinchem.2008.109652 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoofnagle AN & Wener MH The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods 347, 3–11, doi: 10.1016/j.jim.2009.06.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Whiteaker JR et al. Antibody-based enrichment of peptides on magnetic beads for mass-spectrometry-based quantification of serum biomarkers. Anal Biochem 362, 44–54, doi: 10.1016/j.ab.2006.12.023 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoofnagle AN & Roth MY Clinical review: improving the measurement of serum thyroglobulin with mass spectrometry. J Clin Endocrinol Metab 98, 1343–1352, doi: 10.1210/jc.2012-4172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Netzel BC, Grebe SK & Algeciras-Schimnich A Usefulness of a thyroglobulin liquid chromatography-tandem mass spectrometry assay for evaluation of suspected heterophile interference. Clin Chem 60, 1016–1018, doi: 10.1373/clinchem.2014.224816 (2014). [DOI] [PubMed] [Google Scholar]
- 153.Shuford CM et al. Absolute Protein Quantification by Mass Spectrometry: Not as Simple as Advertised. Anal Chem 89, 7406–7415, doi: 10.1021/acs.analchem.7b00858 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Kushnir MM et al. Measurement of thyroglobulin by liquid chromatography-tandem mass spectrometry in serum and plasma in the presence of antithyroglobulin autoantibodies. Clin Chem 59, 982–990, doi: 10.1373/clinchem.2012.195594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Clarke NJ, Zhang Y & Reitz RE A novel mass spectrometry-based assay for the accurate measurement of thyroglobulin from patient samples containing antithyroglobulin autoantibodies. J Investig Med 60, 1157–1163, doi: 10.2310/JIM.0b013e318276deb4 (2012). [DOI] [PubMed] [Google Scholar]
- 156.Netzel BC et al. First Steps toward Harmonization of LC-MS/MS Thyroglobulin Assays. Clin Chem 62, 297–299, doi: 10.1373/clinchem.2015.245266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Netzel BC et al. Thyroglobulin (Tg) Testing Revisited: Tg Assays, TgAb Assays, and Correlation of Results With Clinical Outcomes. J Clin Endocrinol Metab 100, E1074–1083, doi: 10.1210/jc.2015-1967 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lynch KL CLSI C62-A: A New Standard for Clinical Mass Spectrometry. Clin Chem 62, 24–29, doi: 10.1373/clinchem.2015.238626 (2016). [DOI] [PubMed] [Google Scholar]
- 159.Approved guideline CLSI document C62-A. (Clinical and Laboratory Standards Institute, 2014). [PubMed] [Google Scholar]
- 160.Grant RP & Hoofnagle AN From lost in translation to paradise found: enabling protein biomarker method transfer by mass spectrometry. Clin Chem 60, 941–944, doi: 10.1373/clinchem.2014.224840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.An E et al. Quantitative proteomic analysis of HER2 expression in the selection of gastric cancer patients for trastuzumab treatment. Ann Oncol 28, 110–115, doi: 10.1093/annonc/mdw442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nuciforo P et al. High HER2 protein levels correlate with increased survival in breast cancer patients treated with anti-HER2 therapy. Mol Oncol 10, 138–147, doi: 10.1016/j.molonc.2015.09.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi T et al. Antibody-free, targeted mass-spectrometric approach for quantification of proteins at low picogram per milliliter levels in human plasma/serum. Proc Natl Acad Sci U S A 109, 15395–15400, doi: 10.1073/pnas.1204366109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lathrop JT, Jeffery DA, Shea YR, Scholl PF & Chan MM US Food and Drug Administration Perspectives on Clinical Mass Spectrometry. Clin Chem 62, 41–47, doi: 10.1373/clinchem.2015.244731 (2016). [DOI] [PubMed] [Google Scholar]
- 165.510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE, <https://www.accessdata.fda.gov/cdrh_docs/reviews/K083130.pdf> (
- 166.EVALUATION OF AUTOMATIC CLASS III DESIGNATION FOR Vitamin D 200M Assay DECISION SUMMARY, <https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN170019.pdf> (
- 167.Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories: Framework for Regulatory Oversight of Laboratory Developed Tests, <https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm416685.pdf> (2014).
- 168.Laboratory Developed Tests, <https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/LaboratoryDevelopedTests/default.htm> (
- 169.Regnier FE et al. Protein-based multiplex assays: mock presubmissions to the US Food and Drug Administration. Clin Chem 56, 165–171, doi: 10.1373/clinchem.2009.140087 (2010). [DOI] [PubMed] [Google Scholar]
- 170.Rodriguez H et al. Analytical validation of protein-based multiplex assays: a workshop report by the NCI-FDA interagency oncology task force on molecular diagnostics. Clin Chem 56, 237–243, doi: 10.1373/clinchem.2009.136416 (2010). [DOI] [PubMed] [Google Scholar]
- 171.Public Workshop – Proteomics in the Clinic, <http://wayback.archive-it.org/7993/20170112091100/http://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm392858.htm> (
- 172.Public Workshop - Mass Spectrometry in the Clinic: Regulatory Considerations Surrounding Validation of Liquid Chromatography-Mass Spectrometry Based Devices, <https://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm486484.htm> (
- 173.Wu AH et al. Antiquated tests within the clinical pathology laboratory. Am J Manag Care 16, e220–227 (2010). [PubMed] [Google Scholar]