Abstract
REDD+ projects primarily focus on reducing carbon emissions from deforestation and forest degradation in developing countries. These projects are regularly evaluated against their core objective of conserving carbon stocks, but their contribution to biodiversity conservation has rarely been assessed. To assess the conservation value of the area and the relative performance of a REDD+ land use plan in Yaeda Valley, a semi-arid savannah ecosystem in northern Tanzania, we implemented an annual wildlife monitoring scheme. Based on direct sightings and indirect signs of wildlife, obtained from stratified walking transects conducted annually from 2015–2018, we estimated annual trends of mammal species richness and wildlife densities in three REDD+ and three non-REDD+ land-use strata. Our surveys document a near complete mammal community in the area. Species accumulation curves, and subsequent statistical comparisons, indicated highest mammal species richness in the woodland habitats (both REDD+ and non REDD+ strata) as compared to more human and livestock impacted areas, and suggested constant species richness from 2015–2018. To estimate stratum- and year-specific livestock and wildlife densities (cattle, donkey, goat and sheep combined, Thomson’s gazelle, Kirk’s dik-dik) and wildlife sign densities (aardvark, bushbuck, bushpig, Kirk’s dik dik, eland, elephant, Maasai giraffe, greater kudu, hyena, impala, lesser kudu, warthog, wildebeest, Plains zebra), we fitted species-specific detection functions in a distance sampling framework. Species-specific densities varied between 2015 and 2018 and showed substantial increases and occasional declines in other species-stratum combinations. However, population growth rates were not systematically associated with specific land-use strata. Although our results do not explicitly provide evidence that REDD+ land-use plans directly co-benefit wildlife conservation, they show that REDD+ areas have the potential to maintain intact wildlife assemblages. To ensure effective long-term conservation outcomes, we advocate for a more formal integration of wildlife conservation goals in the REDD+ scheme.
Introduction
Worldwide, habitat loss, habitat fragmentation, homogenization of ecosystems, invasive organisms, climate change, and direct exploitation cause reductions of most wild animal populations and succeeding (local) extinctions of species [1–5]. Among mammals, both large herbivores and carnivores are declining in most parts of the world [2,6–8] with East Africa closely mirroring this global trend [9,10]. Yet, East Africa still supports an impressive variety of large mammal populations and substantial wildlife assemblages persist outside fully protected areas, mainly in semi-arid rangelands [11–15]. Despite their critical role for many wildlife species as migratory and seasonal ranges, as well as permanent habitat [16–20], and associated ecosystem services provided by resident and migratory species [2], these rangelands often lose productivity, decline in size (often due to expanding subsistence agriculture and other human-caused land use changes), and experience accelerated rates of wildlife-livestock competition and other forms of conflict between humans and wildlife [21–26].
To strengthen wildlife conservation, Tanzania considerably increased the size of fully protected national parks in the last 20 years [27], but given the current human population growth rate and ensuing demand for space it is unlikely that fully protected areas can be substantially expanded in size in the future. Therefore, effectively conserving wildlife in increasingly human-dominated landscapes requires identification and implementation of conservation and land-use models that mitigate anthropogenic effects while allowing sustainable natural resource utilization by humans [28,29]. In Tanzania, these multiple-use areas include state-run game controlled areas, forest reserves, nature reserves, and game reserves [27], as well as community-based wildlife management areas [30,31]. A rather novel community-based approach of protecting potential wildlife habitat is the REDD+ scheme (“Reducing emissions from deforestation and forest degradation and the role of conservation, sustainable management of forests and enhancement of forest carbon stocks in developing countries”) which was developed by the United Nations Framework Convention on Climate Change (UNFCCC). Under this scheme, carbon offsets generated by avoided deforestation are sold to local and international buyers, and revenue is distributed among participating communities. The incentive based program is conditional, i.e. payments are only distributed if carbon stocks are monitored and conserved effectively [32]. Although focused on protecting forest resources and forest management, an added “co-benefit” of preserving forests may [33], or may not [34], be the conservation of wildlife species.
Assessing or comparing this co-benefit of specific land-uses and land management schemes across space or time requires implementing effective monitoring schemes which allow estimation of species richness and population trends [12,35–38]. Unfortunately, this crucial monitoring component is often missing in community-based conservation schemes [39,40]. Since baseline estimates before conservation implementations are often missing (and before-after control-impact studies hence not feasible), assessments of conservation approaches often rely on spatial comparisons and ideally use the rate of change in species richness or population density as metrics to gauge conservation effectiveness [12,41,42].
To assess how different land-uses and the REDD+ conservation approach affect wildlife distribution, species richness and population trends over time, we monitored wildlife species richness and relative densities by walking transects in three land-use strata inside, and three land-use strata outside, a REDD+ driven land-use plan in Yaeda Valley, northern Tanzania. Walking transects allow systematic distribution of transects and therefore reduce design-based biases of inferred density estimates, but are relatively labor- and cost-intensive [43]. Additionally, walking transects provide an opportunity to incorporate local people in monitoring activities [44,45] and permits assessing direct and indirect signs of animal presence. Recording indirect signs of wildlife can be an effective method to estimate species richness and relative densities, especially if animal signs can be reliably identified by incorporating indigenous knowledge during field work [46–48].
Here we provide baseline information on the value of different land-use and conservation forms in Yaeda Valley for wildlife conservation in northern Tanzania. First, we assessed overall mammal species richness, a crucial state variable for assessing biodiversity [49,50], and evaluated the completeness of the mammal community against an expected mammal species list derived from current distribution maps [51]. Secondly, and more specifically, we estimated trends of species richness and relative densities of mammalian wildlife species over time [49,52,53] within the six distinct sampled habitat strata (three strata under a REDD+ land-use plan and three others without a specific land-use plan). We hypothesized that areas within the REDD+ land-use plan would be characterized by stable wildlife population communities and show stable or increasing trends in population density and that wildlife populations in areas outside the REDD+ land-use plan would be more likely to decline. In addition, we estimated trends of livestock populations because this allowed us to assess if the land-use policies were effectively implemented, and if livestock populations–which potentially affect wildlife distribution and density [14,54,55]–changed over time.
Methods
Study area
Yaeda Valley is located in the Mbulu district of Tanzania, south-east of Lake Eyasi and Ngorongoro Conservation Area [56,57]. This semi-arid region experiences three distinct seasons; the short rains from November—December, the long rains from February—May, and the dry season from June—October. On average, the region receives 450 mm of rain per year with a mean monthly temperature range of 25–30°C [58]. The area is mainly inhabited by agro-pastoralist (Iraqw, Isanzu, Nyiramba) and pastoralist (Datoga) ethnicities, and forms the core distribution area of the Hadza, a hunter-gatherer ethnicity [59]. Carbon Tanzania, a Tanzania based social enterprise, has implemented a project within the REDD+ framework in Domanga and Mongo Wa Mono village lands. The REDD+ project has been designed under the Plan Vivo Standard which supports local stakeholders, particularly pastoralist Datoga and hunter gatherer Hadza in natural resource management [60,61].
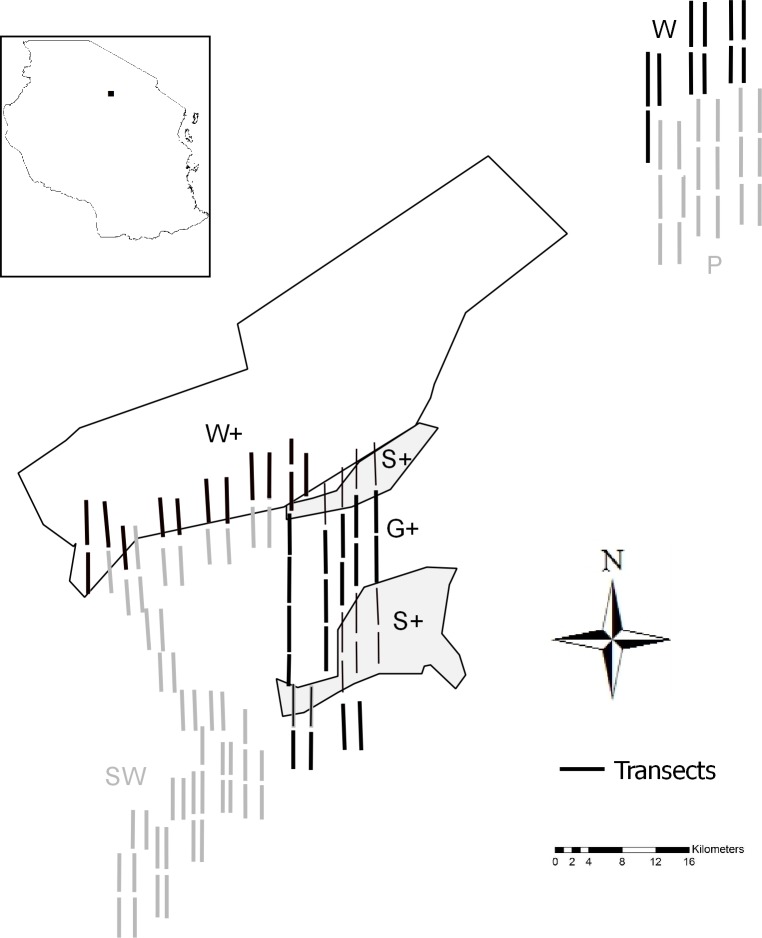
This study was conducted in six different strata that were delineated according to main vegetation cover and whether or not they were part of the REDD+ land-use plan (Fig 1). Stratification was initially based on main vegetation forms only, and was post-stratified according to management regimes for this analysis. Hence, a few transects dissect multiple land-use strata. In these cases we assigned transects to specific land-use strata if ≥ 50% of the transect fell in the corresponding stratum. Stratified sampling allowed direct assessments of spatial differences in the state, and temporal trends of species richness and (relative) densities of wildlife species.
Fig 1. Map of Yaeda Valley and delineated land-use strata.
The map shows the distribution of the walking transects in three land use strata defined in the REDD+ scheme [Woodland (W+), Grazing (G+), Settlement (S+)] and three non-REDD+ strata (Woodland (W), Plains (P), and Southern woodland (SW)]. The inset on the top left indicates the location of Yaeda Valley in Tanzania. For clarity, transects were bolded and are hence not to scale.
The three sampled land-use strata that were defined in the REDD+ scheme were Woodland (W+), Grazing (G+), and Settlement (S+). W+ is located in the northwest of the valley along the Kideru ridge, and is dominated by baobab (Adansonia digitata) and acacia (Vachellia seyal and Senegalia brevispica) woodland, interspersed with bushland. The Hadza mainly utilize this area for hunting and foraging. Livestock keeping is not allowed in this area. S+ and G+ are located in the Kideru plains, in the central part of the valley. G+ is designated for livestock grazing and mainly consists of grasslands, seasonal swamps, and acacia woodland. Settlements are not allowed in this area. Areas designated for settlement (S+) are mainly located at slightly higher elevations than the floodplain. Here, settlements, subsistence agriculture and woodland remnants are the main landscape features, and livestock keeping is allowed.
For the control (non-REDD+) strata, we selected three land-use forms that are similar in vegetation structure to the REDD+ land-use strata. W, located in the northwestern corner of our study area, consists of baobab and acacia woodlands on the slopes of the Kideru ridge; this area is also traditionally used by Hadza. The plains (P) to the east of W are also seasonally flooded and constitute a mix of grassland, bushland, and acacia woodland. The Southern woodland (SW), adjacent to W+, G+ and S+, is characterized by relatively flat terrain, a mix of acacia woodland and grasslands and contains small interspersed patches of agriculture established by residents of agro-pastoralist ethnicities. Settlements and livestock keeping occur in this area as well.
Field sampling
In 2015, we established systematic walking transects using the existing roads as start and endpoints. The same set of 115 transects was completed across all six strata in the short rains (early November) of 2015, 2016, 2017, and 2018. The total effort was 880.3 km (few transects were slightly shorter than 2 km), consisting of 13 transects in W+, 17 in G+, 12 in S+, 11 in W, 18 in P, and 44 in SW (Fig 1). We walked transects in the north-south or south-north direction for 2 km, and we separated consecutive transects by 500 m (north-south). The east-west distance between parallel transects was 1 km to avoid double counting of animals (Fig 1). We used a compass and handheld GPS unit with pre-determined start- and endpoints for bearings and orientation in the field. Three people walked each transect (6 teams per day), with one person being either a village game scout (VGS) or employee of the Mbulu District Game Office. All guides were long-term residents of the study area (from Isanzu, Datoga and Hadza ethnicities) and were knowledgeable in local fauna and sign identification due to formal training and traditional knowledge. Each year we spent the first day training all survey participants in field orientation, sign identification (facilitated by a species list in three languages: English, Swahili, Hadza) and consistent data measurement and recording. Upon seeing a mammal or mammal sign, we identified and recorded detections to species level. In a few cases, unambiguous identification to species level was not possible; therefore we combined closely related species to one species name (e.g. both hyena species, all three potentially occurring hyrax and jackal species, and solitary, similarly-sized mongoose species; Table 1). For each sighting, we recorded the GPS coordinates, cluster size (either herd size or aggregation of animal signs), and perpendicular distances between transect and sighting. Perpendicular distances to animals were measured using a laser range finder (Bushnell Elite 1500), and perpendicular distances to signs were measured using a measuring tape.
Table 1. Detected mammal species in Yaeda Valley during walking transects conducted from 2015–2018.
Mammal species presence (assessed via signs and/or direct sightings) in the three REDD+ (W+, G+, and S+) and control land-use strata (W, P, and SW) of the Yaeda Valley in 2015 (´15), 2016 (´16), 2017 (´17), and 2018 (´18). Wildlife species expected to be present but not observed are included at the bottom; completeness of the mammal community was calculated as the proportion of medium-large sized mammal species expected to occur in the area. We estimated sign or animal densities for species highlighted in bold only.
| W+ | G+ | S+ | W | P | SW | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common | Scientific | ´15 | ´16 | ´17 | ´18 | ´15 | ´16 | ´17 | ´18 | ´15 | ´16 | ´17 | ´18 | ´15 | ´16 | ´17 | ´18 | ´15 | ´16 | ´17 | ´18 | ´15 | ´16 | ´17 | ´18 |
| Livestock | |||||||||||||||||||||||||
| Cattle | Bos taurus | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Domestic dog | Canis lupus familiaris | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| Donkey | Equus africanus asinus | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Domestic cat | Felis catus | X | X | ||||||||||||||||||||||
| Sheep & goat | Ovis aries & Capra aegagrus hircus | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Wildlife | |||||||||||||||||||||||||
| Cheetah | Acinonyx jubatus | X | X | ||||||||||||||||||||||
| Impala | Aepyceros melampus | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Jackal (Side-striped, Golden, Black-backed) | Canis adustus, C. aureus, C. mesomelas | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Vervet monkey | Chlorocebus pygerythrus | X | X | X | X | X | X | X | |||||||||||||||||
| African civet | Civettictis civetta | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Wildebeest | Connochaetes taurinus | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Plains zebra | Equus quagga | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Thomson's gazelle | Eudorcas thomsonii | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Caracal | Caracal caracal | X | X | ||||||||||||||||||||||
| African wildcat | Felis lybica | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Serval | Leptailurus serval | X | X | X | X | X | X | X | X | ||||||||||||||||
| Greater bushbaby | Otolemur crassicaudatus | X | X | X | |||||||||||||||||||||
| Genet (Small-spotted, Large-spotted) | Genetta genetta, G. maculata | X | X | X | X | X | X | X | X | X | |||||||||||||||
| Maasai giraffe | Giraffa camelopardalis | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Dwarf Mongoose | Helogale parvula | X | X | X | X | X | X | ||||||||||||||||||
| Mongoose (Egyptian, Slender) | Herpestes ichneumon, Galerella sanguinea | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Hyena (Striped, Spotted) | Hyaena hyaena, Crocuta crocuta | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Porcupine | Hystrix cristata | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| White-tailed mongoose | Ichneumia albicauda | X | X | X | X | X | X | X | X | ||||||||||||||||
| Zorilla | Ictonyx striatus | X | X | X | |||||||||||||||||||||
| Hare (Cape, Scrub, Spring) | Lepus capensis, L. saxatilis, Pedetes capensis | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Elephant | Loxodonta africana | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Wild dog | Lycaon pictus | X | X | ||||||||||||||||||||||
| Kirk's dik-dik | Madoqua kirkii | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Honey badger | Mellivora capensis | X | X | X | X | X | X | X | X | ||||||||||||||||
| Banded mongoose | Mungos mungo | X | X | X | X | X | X | ||||||||||||||||||
| Klipspringer | Oreotragus oreotragus | X | X | X | X | X | X | ||||||||||||||||||
| Aardvark | Orycteropus afer | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Bat-Eared Fox | Otocyon megalotis | X | X | X | X | X | X | ||||||||||||||||||
| African lion | Panthera leo | X | X | X | |||||||||||||||||||||
| Leopard | Panthera pardus | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Olive baboon | Papio anubis | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Warthog | Phacochoerus africanus | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Bushpig | Potamochoerus larvatus | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Hyrax (Tree, Bush, Rock) | Dendrohyrax arboreus, Heterohyrax brucei, Procavia johnstoni | X | X | X | X | ||||||||||||||||||||
| Steenbok | Raphicerus campestris | X | X | X | X | ||||||||||||||||||||
| Bohor reedbuck | Redunca redunca bohor | X | X | ||||||||||||||||||||||
| Bush duiker | Sylvicapra grimmia | X | X | X | X | ||||||||||||||||||||
| Buffalo | Syncerus caffer | X | X | X | X | X | X | ||||||||||||||||||
| Lesser kudu | Tragelaphus imberbis | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Eland | Tragelaphus oryx | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Bushbuck | Tragelaphus scriptus | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Greater kudu | Tragelaphus strepsiceros | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Potential species | |||||||||||||||||||||||||
| Hartebeest | Alcelaphus buselaphus | ||||||||||||||||||||||||
| Roan antelope | Hippotragus equinus | ||||||||||||||||||||||||
| Aardwolf | Proteles cristata | ||||||||||||||||||||||||
| Pangolin | Smutsia temminckii | ||||||||||||||||||||||||
| Wildlife species richness | 24 | 24 | 22 | 25 | 19 | 19 | 15 | 18 | 13 | 12 | 13 | 15 | 26 | 24 | 24 | 22 | 18 | 19 | 16 | 22 | 27 | 30 | 30 | 30 | |
| Proportion of expected mammal community | .51 | .51 | .47 | .53 | .40 | .40 | .32 | .38 | .28 | .26 | .28 | .32 | .55 | .51 | .51 | .47 | .38 | .40 | .34 | .47 | .57 | .64 | .64 | .64 | |
Data analyses
Completeness of overall mammal species richness in Yaeda Valley was evaluated by comparing confirmed species presence to a list of expected mammal species in the area, which was derived from a recent field guide for mammals in Tanzania [51]. Mammal species richness estimates were calculated for all study year-stratum combinations using the first order Jackknife estimates generated in EstimateS 9.1 [62]. Species accumulation curves as functions of sampling effort (number of walked transects) were graphed using R 3.3.2 [63]. To compare species richness between strata and years, we ran a general linear model (glm) on estimated species richness at highest common sampling effort (11 transects). Since the response variable was normally distributed (Shapiro-Wilk test: W = 0.962, p = 0.484), we used the Gaussian error distribution for the glm. We first fitted the most complex model (interaction of stratum x year) to explain differences in species richness, then generated all subsets of this model using the dredge function of the MUMIn package and finally selected the most supported model based on the second order AIC-score [64].
Mammal densities based on direct sightings and relative densities based on signs in each stratum and year were estimated using DISTANCE 6.0 [65]. For each species, four different global detection models (uniform, half-normal, hazard-rate, and negative-exponential) were fitted to species-specific data using conventional distance sampling. Chi-squared goodness of fit values of the detection functions were frequently significant, which implied poor fit of detection functions [66,67]. Because half-normal detection functions had a general acceptable visual fit, we selected half-normal detection functions for all species. Since relatively few direct mammal sightings were recorded, and distance sampling methodology requires a relatively high number of observations (>60 observations) to reliably estimate detection functions [67], detection functions were based on the entire dataset, and densities were only estimated for the five most frequently directly encountered species, three of which were livestock. Livestock and Thomson’s gazelle (Eudorcas thomsonii) exceeded the recommended observation threshold, and Kirk’s dik-dik (Madoqua kirkii) was included despite only 40 direct sightings. Fourteen species were selected for sign density estimations, all of which exceeded the threshold of 60 observations. Stratum- and year-specific densities were estimated using the post-stratification option in the distance software and were inferred based on mean cluster sizes in each stratum. We assessed temporal density differences in each stratum between 2015 and 2018, testing the null hypothesis that density did not change over time, using a generalized linear model with log transformed error distribution [68]. If a species was not detected in a given year-stratum combination, we converted the estimated zero density to 0.01 to allow log transformation. Given that the test power for 4 years of monitoring is likely weak, we did not strictly focus on p-value testing but instead graphically compared yearly regression coefficients (i.e. yearly population growth rates) across strata. For this comparison, we excluded species with only one density estimate in time that exceeded zero signs or individuals per km2. This was done to avoid assigning strong weight to possible outliers in population growth rates that may have been caused by observations of species that do not regularly occupy a given stratum.
Results
Mammal species richness
Over the course of four years, we detected a total of 9007 independent observations of signs and 346 direct sightings of wildlife species. We observed signs from 43 wildlife species (groups), with direct sightings of 27 species (S1 Data). Compared to 47 expected terrestrial mammal species (groups) in the region, our survey confirmed that a near complete (91.5%) mammal species assemblage occurred in the area.
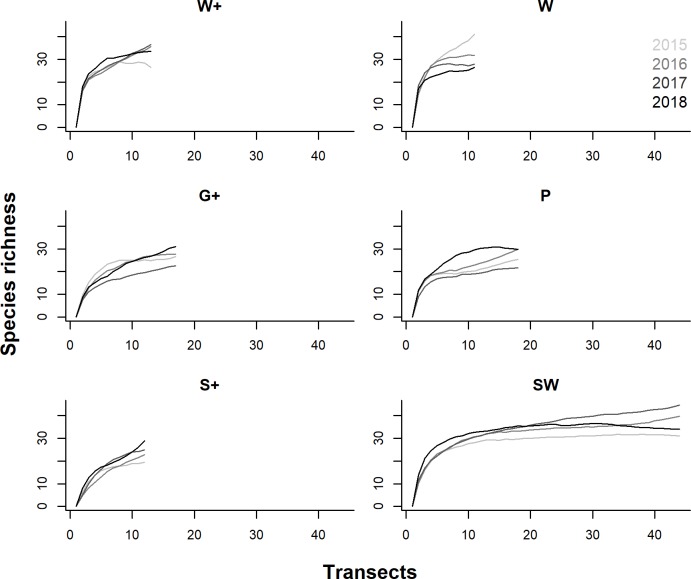
Stratum-specific species rarefaction curves were mostly asymptotic (Fig 2), suggesting that sampling efforts in most strata were sufficient. Estimated mammal species richness was highest in W+, W, and SW compared to other strata in all survey years, and stratum-specific species richness estimates exhibited variability across years (Fig 2).
Fig 2. First-order Jackknife estimates of mammal species richness in Yaeda Valley.
Species richness estimates were plotted against sampling effort in the three REDD+ and the three control strata in 2015, 2016, 2017 and 2018.
Model selection suggested that the most supported model to explain variation in species richness (at smallest common sampling effort of 11 transects) contained the factor “stratum” only (Table 2A). This analysis confirmed that mammal species richness was highest in the three woodland strata (W+, W, SW), and was significantly lower in the plains habitats (G+, P) and in the settlement area (S+) (Table 2B). Incorporating survey year in the model was not supported based on information-theoretic model weights, suggesting that species richness did not differ substantially across survey years (Table 2A). Although not statistically significant, mammal species richness appears to have declined linearly over time in stratum W (Fig 2).
Table 2. (a) Model selection table and (b) regression coefficients of the most supported general linear model to explain spatio-temporal variation in mammal species richness in Yaeda Valley.
Species richness was estimated in six strata [three REDD+ strata (W+, G+, and S+) three control land-use strata (W, P, and SW) in 2015, 2016, 2017, and 2018. The response variable was estimated for a sampling effort of n = 11 transects in each stratum.
| (a) | Intercept | Stratum | Year | Stratum*Year | df | logLik | AICc | delta AICc | weight |
| 30.54 | + | 7 | -63.438 | 147.9 | 0 | 0.831 | |||
| -757.2 | + | 0.3907 | 8 | -63.238 | 152.1 | 4.2 | 0.102 | ||
| 27.25 | 2 | -74.433 | 153.4 | 5.56 | 0.051 | ||||
| -760.5 | 0.3907 | 3 | -74.354 | 155.9 | 8.03 | 0.015 | |||
| -2631 | + | 1.32 | + | 13 | -49.447 | 161.3 | 13.42 | 0.001 | |
| (b) | Estimate | Std. error | P-value | ||||||
| Intercept (SW) | 30.535 | 1.964 | ≤0.001 | ||||||
| Stratum P | -7.618 | 2.778 | 0.013 | ||||||
| Stratum W | 1.247 | 2.778 | 0.659 | ||||||
| Stratum W+ | 1.292 | 2.778 | 0.647 | ||||||
| Stratum G+ | -6.818 | 2.778 | 0.025 | ||||||
| Stratum S+ | -7.825 | 2.778 | 0.011 |
Mammal population and sign densities
Stratum- and year-specific population densities were estimated for livestock species and two of the most frequently encountered wildlife species (S1 Data) using selected detection functions summarized in S2 Data and displayed S1 Fig Sign densities of wildlife species were estimated using half-normal detection functions (S2 Data) which are displayed in the S2 Fig Frequent significant signals of chi-square goodness of fit tests (direct sightings: 2/5 species; signs: 15/15 species) suggested relatively poor statistical fit of the selected detection functions (S2 Data). However, apart from a spike of observations near the transect lines (which likely caused significant goodness of fit results), visual fit of the fitted detection function appeared to match the observed frequencies of observations (S1 and S2 Figs).
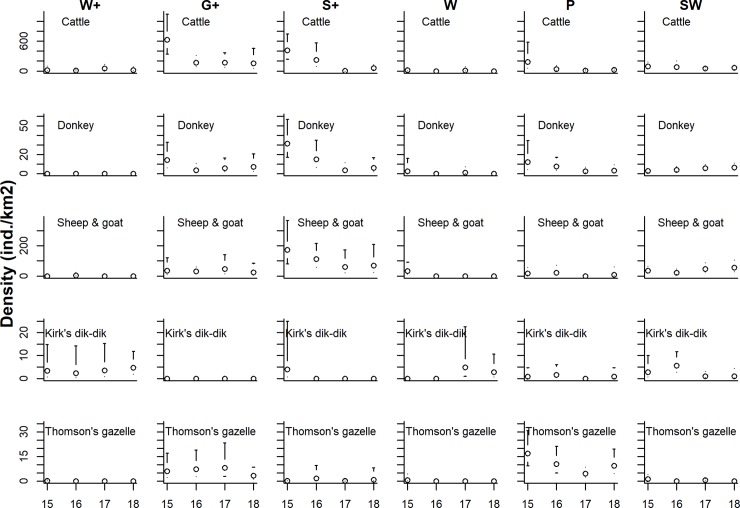
Densities of directly sighted mammal species were highest for livestock species, particularly for cattle in G+ and S+, and sheep and goats in S+ (Fig 3). Densities of Kirk’s dik-dik were particularly high in W+ and Thomson’s gazelle mainly occurred at high densities in the grassland strata (G+, P).
Fig 3. Population density estimates of livestock species, Thomson’s gazelle, and Kirk’s dik- dik in Yaeda Valley, Tanzania.
Density estimates were stratified by stratum [three REDD+ strata (W+, G+, and S+), three control land-use strata (W, P, and SW)], and year. Error bars indicate 95% confidence intervals.
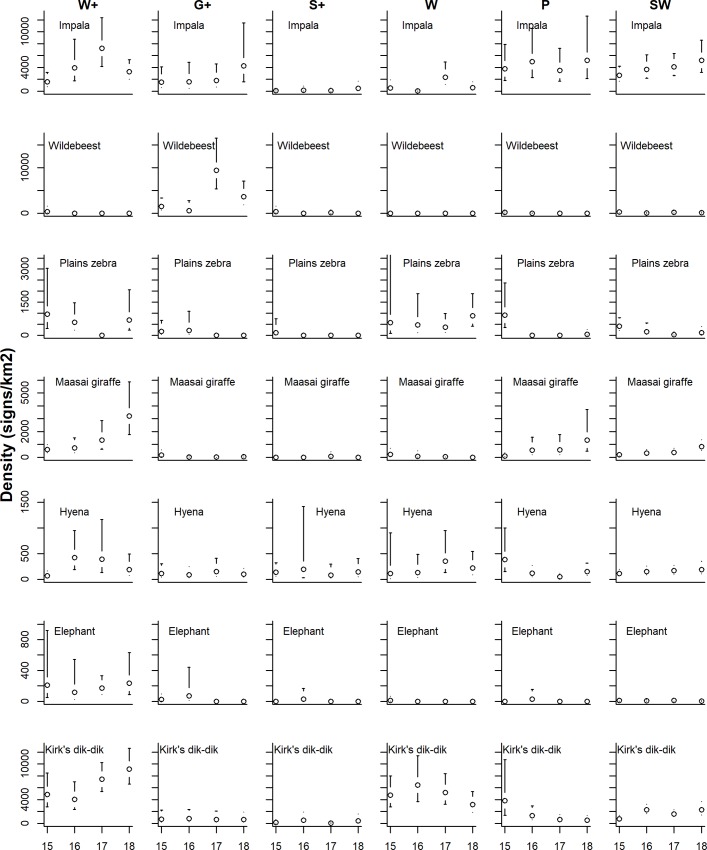
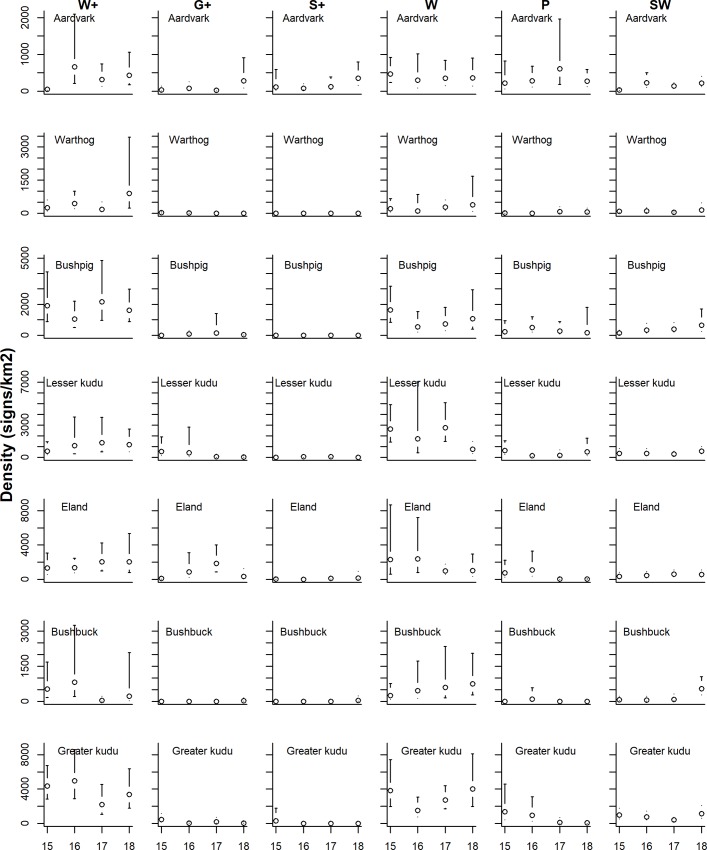
Estimated sign densities indicated that several species occurred at relatively high densities across the landscape. For example impala sign densities were relatively high in most strata except for S+ (Fig 4; S3 Data), and hyena signs were relatively high in all strata (Fig 4). Several species (e.g. Plains zebra, greater and lesser kudu, eland) occurred at relatively high densities in multiple, but not all, strata (Fig 4, Fig 5) whereas other species had rather restricted distributions. For example, wildebeest solely occurred in G+, elephants mainly in W+ and SW, Maasai giraffe primarily occurred in W+ and P (Fig 4), and bushbuck sign densities were high in W+ and W but absent or very low in other strata (Fig 4, Fig 5).
Fig 4. Sign density estimates of impala, wildebeest, Maasai giraffe, hyena, elephant and Kirk’s dik-dik in Yaeda Valley, Tanzania.
Density estimates were stratified by stratum [three REDD+ strata (W+, G+, and S+), three control land-use strata (W, P, and SW)], and year. Error bars indicate 95% confidence intervals.
Fig 5. Sign density estimates of aardvark, warthog, bushpig, lesser kudu, eland, bushbuck, and greater kudu in Yaeda Valley, Tanzania.
Density estimates were stratified by stratum [three REDD+ strata (W+, G+, and S+), and three control land-use strata (W, P, and SW)] and year. Error bars indicate 95% confidence intervals.
Wildlife population trends
Population densities of observed livestock and wildlife species as well as sign densities of the more commonly encountered wildlife species exhibited relatively strong temporal patterns over the last four years (Figs 3–6). In particular, declines in livestock densities in the G+ and S+ strata are noteworthy (Fig 3; S4 Data). Estimates for yearly population growth rates were variable across species and strata (Fig 6, S4 Data). The variation in growth rates was, however not significantly associated with land-use stratum (Kruskal-Wallis Anova: Χ2 = 8.77; df = 5; p = 0.12). Among species-stratum combinations that demonstrated strong temporal patterns (using p ≤ 0.1 as criteria), most of the wildlife population growth rates (9/12) were positive and occurred both in the core REDD+ stratum (W+: n = 2) as well as in non-REDD+ strata (W: n = 1; P: n = 1; SW: n = 5). Particularly interesting is the population trajectory of Maasai giraffe which apparently increased in W+, P, SW but declined in W (S4 Data).
Fig 6. Boxplot showing regression coefficients of annual wildlife population growth rates (β) in Yaeda Valley, Tanzania.
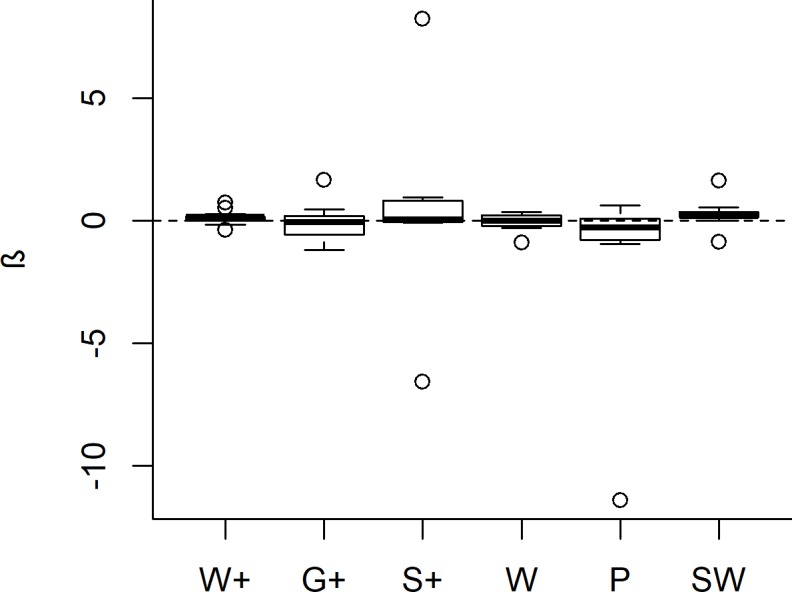
Species-specific regression coefficients of β were estimated using a generalized linear model with log-link and were estimated independently for each land-use stratum [three REDD+ strata (W+, G+, and S+) and three control land-use strata (W, P, and SW)]. The dashed line indicates zero (i.e. no population change).
Discussion
Our ground-based monitoring results reveal that Yaeda Valley, an area partially protected via a REDD+ project, supports a near complete mammal community including charismatic species of conservation concern such as African elephants [69], African lions [70,71], cheetahs [72] and wild dogs [73]. Results of the stratified sampling approach confirm relatively strong habitat-species associations and suggest that wildlife populations in our study area are mostly stable or increasing. Although the REDD+ land-use plan may have contributed to this positive conservation outcome, rates of change in wildlife populations were not strictly associated with REDD+ land-use strata as they occurred in other land-use strata as well.
Patterns of mammal species richness
Habitat protection via REDD+ mechanisms likely plays a role in sustaining a near complete savanna mammal community in Yaeda Valley. This is demonstrated particularly by the highest species richness estimates in the W+, a near pristine (basically no land conversion) dryland savanna habitat where the main land use is foraging (hunting, honey collection, fruit and tuber gathering) by Hadza people [56,74]. Other strata (particularly S+; G+; P) had substantially higher human influences, indicated by higher livestock densities (Fig 4) and consequently -similar to other studies along conservation gradients in northern Tanzania—lower mammal species richness [75,76]. Interestingly, and in line with mammal diversity surveys conducted in Malaysia [77], our study suggests that wooded habitats (W+, W, SW) yield the highest conservation value in terms of mammal species richness. However, this finding contrasts a recent study conducted in Botswana, which found grasslands supporting the greatest large mammal species richness [78]. Possibly, the discrepancy between these studies may be explained by presumably higher overall livestock and human presence in grassland habitats in our study and a rather degraded state of the grasslands during the time of our surveys compared to the (possibly more protected) study sites in Botswana [78].
Beyond effective habitat conservation in parts of our study area, several other factors may promote high mammal species richness in Yaeda Valley. The area is relatively large in size (~2500 km2), and contains multiple habitat types which likely promotes large mammal persistence [79–81]. In line with this argument, the area is structurally connected to Maswa Game Reserve and Ngorongoro Conservation Area [20] and thus likely facilitates the movement of wide ranging animals such as wild dogs, cheetahs and elephants in and out of the area which may additionally ensure the persistence of even rare species [17].
Certain mammal species, which were not detected (pangolin and aardwolf) may actually be present in the study area but probably occur at very low densities and are thus very difficult to detect with sample-based field methods [82]. Historically, the area also contained black rhinoceros, roan antelope and hartebeest [56]. While black rhinoceros most likely went locally extinct due to illegal hunting (and indeed rhinoceros do not occur outside selected fully protected areas in Tanzania and were thus not listed as potentially occurring in the area), one can only speculate as to why roan antelope and hartebeest apparently went locally extinct in this area.
Patterns of wildlife densities
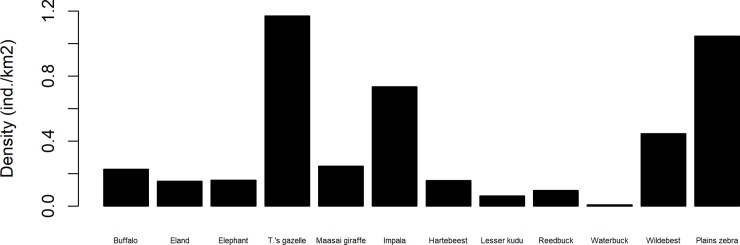
Despite intensive field efforts each year, most of the mammal species were either rarely seen directly (S1 Data) or only indirectly detected via spoor, dung, or feeding signs. This overall pattern may be explained by two mutually non-exclusive hypotheses: actual low wildlife density and/or evasive animal behavior towards humans. Indeed, wildlife may be expected to occur at low densities in this semi-arid area given the close relationships between rainfall, primary productivity, and herbivore and carnivore density [83,84]. However, anecdotal information suggest that wildebeest, zebra and elephant densities were previously much greater than nowadays [56]. Unfortunately, directly comparing density estimates derived from aerial surveys conducted from 1978–1980 in the general area of Yaeda Valley (Fig 7) is not feasible due to methodological and coverage differences, yet the data highlight the historical dimensions of wildlife populations in the area.
Fig 7. Mean wildlife population densities in Yaeda Valley, Tanzania estimated from three systematic reconnaissance flight surveys conducted in 1977, 1978 and 1980.
Data on population densities have been obtained from [85].
The survey set-up did not allow inferring actual animal densities from densities of animal signs [86,87] due to the logistical problems associated with estimating species-specific sign (tracks, dung, feeding signs) production and decomposition rates [88–90] for an entire mammal community. However, based on naive sign densities, several ungulate species appear to occur at relatively high densities, particularly in the W+ and W (Figs 4 and 5), but were rarely or never seen directly (S1 Data). This pattern points to the idea that many animal species are shy and avoid being detected by humans, especially in human-dominated areas. Provided that animals in the area have been hunted by Hadza people with bows and arrows for millennia [59,74], behavioral adaptations in response to this form of persecution have likely evolved [91] and may explain low direct encounter rates of wildlife.
Albeit monitoring indirect signs has substantial advantages in this setting, the monitoring techniques could be improved. For example, in the future (and already implemented in the 2018 survey), randomization of the transect line (e.g. by dropping a walking stick on the transect line upon encountering a wildlife sign) could be used to avoid the observed spike near the transect line and to increase the statistical fit of detection functions (S1 and S2 Figs [92]).
Wildlife population trends
Analyses of repeated surveys suggest that livestock densities varied considerably across years. In particular, cattle densities in the plains habitats (G+, S+) were substantially higher in 2015 compared to 2017. Most likely, this can be explained by invasions of nomadic pastoralists from other regions to the Yaeda area for grazing during 2015. Government interventions likely led to a reduction in this external influx of livestock. Clearly, such added livestock densities may increase grazing pressure on resident livestock populations and on grazing wildlife species [93], and create tension between nomadic pastoralists, residents of the area, and overall conservation goals. On the whole, our surveys confirm that land-use policies are largely implemented, albeit livestock grazing is occasionally observed in W+, where land-use plans do not permit this practice (Fig 3).
Yearly density fluctuations in wildlife (sign) densities were observed in multiple wildlife species (Figs 3–6, S4 Data). These shifts may have been caused by temporal distribution shifts of wildlife species in response to variable resource availability and/or pressures exerted by livestock and humans. This temporal variability in wildlife densities may further suggest that spill-over effects from one area to another are likely. Thus, when using mammals (which can move over large distances) as indicators of conservation effectiveness, large-scale and repeated wildlife monitoring surveys are required for making meaningful assessments [94].
Eventually, long-term monitoring may indicate which species benefit (species with increasing population trends) and which may not be sufficiently protected by REDD+ mechanisms [33]. Among apparent “winners” of REDD+ conservation schemes, are Kirk’s dik dik and Maasai giraffe which appear to thrive particularly well in the core REDD+ area W+. Both species are strict browsers [95] and actively conserving woody plants is likely to benefit these species [53]. However, giraffe populations appear to increase in other strata as well (P and SW; Fig 4) which may point to the idea that additional causal factors may underlie this apparent population increase. Beyond habitat protection, the REDD+ project employs village game scouts, which patrol the area on foot and ensure adherence to land-use policies (i.e. enforcing livestock restrictions, illegal hunting—which in this area is all hunting carried out by non-Hadza ethnicities). It is possible that these anti-poaching efforts have contributed to the overall conservation of the area and have substantially contributed to stable or increased population densities of most wildlife species as well. Our study did not identify apparent “loser species” of REDD+ conservation schemes but by design, the REDD+ scheme does not directly benefit species that rely primarily on grass (e.g. warthogs, wildebeest, Plains zebra), yet these species may benefit indirectly from the REDD+ enforced land-use plans which include designated grazing areas (where settlements are not allowed), moderate livestock densities, and areas where livestock are excluded.
REDD+ and other forest-oriented conservation schemes are now mainstream, global conservation approaches and yet they frequently lack wildlife monitoring schemes [33,77,96]. Although four years of wildlife monitoring may not be sufficient to detect major population changes in large mammal populations, given the often slow, and often time-lagged responses of large mammal species to changes in the environment and conservation policies [53], our results imply that REDD+ based land-use plans can—at least—contribute to sustaining species rich wildlife assemblages in East African rangelands.
Conservation implications
Beyond being of intrinsic value, large mammals provide a multitude of quantifiable ecosystem services, including protein supply for indigenous hunting and gatherer societies, and are thus of critical socio-economic importance [74]. In addition, mammal species are crucial for nutrient cycling and seed dispersal up to the point that defaunation (i.e. the loss of large mammals) has the potential of reducing landscape levels of carbon stocks [97]. Henceforth, investment in conserving intact animal communities directly benefits carbon storage and thus supports the principal goal of REDD+. The few existing wildlife assessments conducted in REDD+ areas [33,96] suggest that wildlife conservation in REDD+ areas is feasible, but may require additional efforts to explicitly address threats to wildlife populations beyond habitat loss such as competition with livestock during times of resource scarcity, and illegal and unsustainable hunting. In Yaeda Valley, village game scouts hired under the REDD+ scheme also enforce anti-poaching laws when patrolling the area on foot and received additional law-enforcement training in 2017. However, the effectiveness of these patrols is likely limited due to the lack of logistical support such as the lack of modern transportation means. Hence, a more formal and specific integration of biodiversity conservation goals under the REDD+ scheme, including specific monitoring programs and incentive based payments for achieving clearly defined wildlife and overall biodiversity conservation goals may substantially improve the ability of REDD+ projects to directly address threats to biodiversity conservation beyond habitat loss [98,99].
Supporting information
(DOCX)
Reported parameters for each model include number of encounters within truncation distance (n), global detection probability (Pa) and associated 95% confidence intervals (Pa-lower—Pa-upper), estimated strip width, and corresponding chi-squared goodness of fit (GOF)–p-value.
(DOCX)
Densities based on sightings were indicated by “S” following the species name, other densities indicate sign densities. Columns with the letter “L” following the species name indicate the lower 95% confidence interval for the density estimate, columns with the letter “U” following the species name, indicate the upper 95%-confidence intervals of the density estimates.
(DOCX)
Population growth rates were estimated from 2015 to 2018 for all six strata [three REDD+ strata (W+, G+, and S+) three control land-use strata (W, P, and SW)] using generalized linear models with log-link. Empty cells denote that the species were not detected in this stratum. Regression coefficients for species highlighted in grey were not utilized for comparisons across land-use strata (main manuscript Fig 6) because those species were either livestock species, or had only one estimate in time that exceeded 0 signs or individuals per km2. For Kirk’s dik-dik we used the sign density, because signs were observed in all strata.
(DOCX)
Histograms (blue bars) represent sighting frequency and the red line is the fitted detection function.
(TIFF)
Histograms (blue bars) represent sighting frequency and the red line is the fitted detection function.
(TIFF)
Acknowledgments
We sincerely thank all participating village game scouts and SFS students who tirelessly collected data while walking in difficult terrain and A. Shanny and E. Nyangisa for help with organizing the surveys. This research was permitted by TAWIRI / COSTECH (permits: 2015-169-ER-2013-192; 2016-349-NA-2013-191; 2017-288-ER-2013-191) and subsequent letters from the Mbulu district. We thank all communities for access to their land. D. Peterson, M. Baker, J. Anderson. B. Wood and L. E. Smith are thanked for sharing valuable information and constructive discussion.
Data Availability
The underlying data have been made available as supporting documents.
Funding Statement
The authors received no specific funding for this work.
References
- 1.McKinney ML, Lockwood JL. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol Evol. 1999;14(11):450–3. [DOI] [PubMed] [Google Scholar]
- 2.Ripple WJJ, Newsome TM, Wolf C, Dirzo R, Everatt KT, Galetti M, et al. Collapse of the world’s largest herbivores. Sci Adv. 2015;1(4):e1400103–e1400103. 10.1126/sciadv.1400103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ducatez S, Shine R. Drivers of extinction risk in terrestrial vertebrates. Conserv Lett. 2017;10:186–94. [Google Scholar]
- 4.Barnosky AD, Matzke N, Tomiya S, Wogan GOU, Swartz B, Quental TB, et al. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471(7336):51–7. 10.1038/nature09678 [DOI] [PubMed] [Google Scholar]
- 5.Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci Adv. 2015;1:e1400253 10.1126/sciadv.1400253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, et al. Trophic downgrading of planet Earth. Science (80-). 2011;333(6040):301–6. [DOI] [PubMed] [Google Scholar]
- 7.Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, et al. Status and ecological effects of the world’s largest carnivores. Science (80-). 2014;343:1241484. [DOI] [PubMed] [Google Scholar]
- 8.Ripple WJ, Chapron G, López-Bao JV, Durant SM, Macdonald DW, Corlett RT, et al. Saving the World’s terrestrial megafauna. Bioscience. 2016;66(10):807–812. 10.1093/biosci/biw092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craigie ID, Baillie JEM, Balmford A, Carbone C, Collen B, Green RE, et al. Large mammal population declines in Africa’s protected areas. Biol Conserv. 2010;143(9):2221–8. [Google Scholar]
- 10.Riggio J, Jacobson A, Dollar L, Bauer H, Becker M, Dickman A, et al. The size of savannah Africa: A lion’s (Panthera leo) view. Biodivers Conserv. 2013;22(1):17–35. [Google Scholar]
- 11.Western D, Russell S, Cuthil I. The status of wildlife in protected areas compared to non-protected areas of Kenya. PLoS One. 2009;4(7):e6140 10.1371/journal.pone.0006140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoner C, Caro T, Mduma S, Mlingwa C, Sabuni G, Borner M. Assessment of effectiveness of protection strategies in Tanzania based on a decade of survey data for large herbivores. Conserv Biol. 2007;21(3):635–46. 10.1111/j.1523-1739.2007.00705.x [DOI] [PubMed] [Google Scholar]
- 13.Kiffner C, Nagar S, Kollmar C, Kioko J. Wildlife species richness and densities in wildlife corridors of Northern Tanzania. J Nat Conserv. 2016;31:29–37. [Google Scholar]
- 14.Ogutu JO, Piepho H-P, Said MY, Ojwang GO, Njino LW, Kifugo SC, et al. Extreme wildlife declines and concurrent increase in livestock numbers in Kenya: What are the causes? PLoS One. 2016;11(9):e0163249 10.1371/journal.pone.0163249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rannestad OT, Danielsen F, Moe SR, Stokke S. Adjacent pastoral areas support higher densities of wild ungulates during the wet season than the Lake Mburo National Park in Uganda. Trop Ecol. 2006;22:675–83. [Google Scholar]
- 16.Fynn RWS, Bonyongo MC. Functional conservation areas and the future of Africa’s wildlife. Afr J Ecol. 2011;49(2):175–88. [Google Scholar]
- 17.Newmark WD. Isolation of African protected areas. Front Ecol Environ. 2008;6(6):321–8. [Google Scholar]
- 18.Morrison TA, Link WA, Newmark WD, Foley CAH, Bolger DT. Tarangire revisited: Consequences of declining connectivity in a tropical ungulate population. Biol Conserv. 2016;197:53–60. [Google Scholar]
- 19.Bond ML, Bradley CM, Kiffner C, Morrison TA, Lee DE. A multi-method approach to delineate and validate migratory corridors. Landsc Ecol. 2017;32(8):1705–21. [Google Scholar]
- 20.Riggio J, Caro T. Structural connectivity at a national scale: Wildlife corridors in Tanzania. PLoS One. 2017;12(11):e0187407 10.1371/journal.pone.0187407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkemade R, Reid RS, van den Berg M, de Leeuw J, Jeuken M. Assessing the impacts of livestock production on biodiversity in rangeland ecosystems. Proc Natl Acad Sci. 2013;110(52):20900–5. 10.1073/pnas.1011013108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.du Toit JT, Kock R, Deutsch JC. Wild rangelands: conserving wildlife while maintaining livestock in semi-arid ecosystems. du Toit JT, Kock R, Deutsch J, editors. Chichester: Wiley-Blackwell; 2010. 1–424 p. [Google Scholar]
- 23.Milton SJ, Dean WRJ, du Plessis MA, Siegfried WR. A conceptual model of arid rangeland degradation: the escalating cost of declining productivity. Bioscience. 1994;44(2):70–6. [Google Scholar]
- 24.Bedunah DJ, Angerer JP. Rangeland degredation, poverty, and conflict: how can rangeland scientists contribute to effective responses and solutions? Rangel Ecol Manag. 2012;65(6):606–12. [Google Scholar]
- 25.Msoffe FU, Said MY, Ogutu JO, Kifugo SC, De J, Gardingen P Van, et al. Spatial correlates of land-use changes in the Maasai- Steppe of Tanzania: Implications for conservation and environmental planning. Int J Biodivers Conserv. 2011;3(7):280–90. [Google Scholar]
- 26.Woodroffe R, Donnelly CA. Risk of contact between endangered African wild dogs Lycaon pictus and domestic dogs: Opportunities for pathogen transmission. J Appl Ecol. 2011;48(6):1345–54. [Google Scholar]
- 27.Caro T, Davenport TRB. Wildlife and wildlife management in Tanzania. Conserv Biol. 2016;30(4):716–23. 10.1111/cobi.12658 [DOI] [PubMed] [Google Scholar]
- 28.Kiss A. Living with wildlife: wildlife resource management with local participation in Africa [Internet]. Kiss A, editor. Washington; 1990. Available from: http://documents.worldbank.org/curated/en/247611468742847173/Living-with-wildlife-wildlife-resource-management-with-local-participation-in-Africa
- 29.Hodgson JA, Moilanen A, Wintle BA, Thomas CD, Hodgson JA, Moilanen A, et al. Habitat area, quality and connectivity: striking the balance for efficient conservation. J Appl Ecol. 2011;48(1):148–52. [Google Scholar]
- 30.Wilfred P. Towards sustainable Wildlife Management Areas in Tanzania. Trop Conserv Sci. 2010;3(1):103–16. [Google Scholar]
- 31.Bluwstein J, Moyo F, Kicheleri R. Austere conservation: Understanding conflicts over resource governance in Tanzanian wildlife management areas. Conserv Soc. 2016;14(3):218–31. [Google Scholar]
- 32.UNFCCC. UNFCCC REDD+ Web Platform [Internet]. 2017 [cited 2017 Dec 21]. Available from: http://redd.unfccc.int/
- 33.Collins MM, Milner-Gulland EJJ, Macdonald EAA, Macdonald DWW. Pleiotropy and charisma determine winners and losers in the REDD+ game: all biodiversity is not equal. Trop Conserv Sci. 2011;4(3):261–6. [Google Scholar]
- 34.Caro T, Borgerhoff Mulder M. Species loss: climate plan saves only trees. Nature. 2016;537:617. [DOI] [PubMed] [Google Scholar]
- 35.Caro T, Gardner TA, Stoner C, Fitzherbert E, Davenport TRB. Assessing the effectiveness of protected areas: paradoxes call for pluralism in evaluating conservation performance. Divers Distrib. 2009;15(1):178–82. [Google Scholar]
- 36.Ogutu JO, Kuloba B, Piepho HP, Kanga E. Wildlife population dynamics in human- dominated landscapes under community-based conservation: the example of Nakuru wildlife conservancy, Kenya. PLoS One. 2017;12(1):e0169730 10.1371/journal.pone.0169730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kremen C, Merenlender AM, Murphy DD. Ecological monitoring: A vital need for integrated conservation and development programs in the tropics. Conserv Biol. 1994;8:388–97. [Google Scholar]
- 38.Geldmann J, Coad L, Barnes M, Craigie ID, Hockings M, Knights K, et al. Changes in protected area management effectiveness over time: A global analysis. Biol Conserv. 2015;191:692–9. [Google Scholar]
- 39.Newmark WD, Hough JL. Conserving Wildlife in Africa: Integrated Conservation and Development Projects and Beyond. Bioscience. 2000;50(7):585–92. [Google Scholar]
- 40.Greene K, Bell D, Kioko J, Kiffner C. Performance of ground-based and aerial survey methods for monitoring wildlife assemblages in a conservation area of northern Tanzania. Eur J Wildl Res. 2017;63(5):77. [Google Scholar]
- 41.Geldmann J, Barnes M, Coad L, Craigie ID, Hockings M, Burgess ND. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol Conserv. 2013;161:230–8. [Google Scholar]
- 42.Barnes MD, Craigie ID, Harrison LB, Geldmann J, Collen B, Whitmee S, et al. Wildlife population trends in protected areas predicted by national socio-economic metrics and body size. Nat Commun. 2016;7:12747 10.1038/ncomms12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waltert M, Meyer B, Shanyangi MW, Balozi JJ, Kitwara O, Qolli S, et al. Foot Surveys of large mammals in woodlands of western Tanzania. J Wildl Manage. 2008;72:603–10. [Google Scholar]
- 44.Danielsen F, Skutsch M, Burgess ND, Jensen PM, Andrianandrasana H, Karky B, et al. At the heart of REDD+: a role for local people in monitoring forests? Conserv Lett. 2011;4(2):158–67. [Google Scholar]
- 45.Danielsen F, Burgess ND, Balmford A. Monitoring matters: examining the potential of locally-based approaches. Biodivers Conserv. 2005;14:2507–42. [Google Scholar]
- 46.Stander PE. Spoor counts as indices of large carnivore populations: the relationship between spoor frequency, sampling effort and true density. J Appl Ecol. 1998;35(3):378–85. [Google Scholar]
- 47.Kohler F, Brondizio ES. Considering the needs of indigenous and local populations in conservation programs. Conserv Biol. 2016;31(2):245–51. 10.1111/cobi.12843 [DOI] [PubMed] [Google Scholar]
- 48.Keeping D, Burger JH, Keitsile AO, Gielen M-C, Mudongo E, Wallgren M, et al. Can trackers count free-ranging wildlife as effectively and efficiently as conventional aerial survey and distance sampling? Implications for citizen science in the Kalahari, Botswana. Biol Conserv. 2018;223:156–69. [Google Scholar]
- 49.Yoccoz NG, Nichols JD, Boulinier T. Monitoring of biological diversity in space and time. Trends Ecol Evol. 2001;16:446–53. [Google Scholar]
- 50.Schmeller DS, Weatherdon L V, Loyau A, Bondeau A, Brotons L, Brummitt N, et al. A suite of essential biodiversity variables for detecting critical biodiversity change. Biol Rev. 2018;93(1):55–71. 10.1111/brv.12332 [DOI] [PubMed] [Google Scholar]
- 51.Foley C, Foley L, Lobora A, De Luca D, Msuha M, Davenport TRB, et al. A Field Guide to Larger Mammals of Tanzania. Princeton University, USA; 2014. [Google Scholar]
- 52.Georgiadis NJ, Olwero JGN, Ojwang GO, Romañach SS, Ojwang’ G, Romañach SS. Savanna herbivore dynamics in a livestock-dominated landscape: I. Dependence on land use, rainfall, density, and time. Biol Conserv. 2007;137(3):461–72. [Google Scholar]
- 53.Kiffner C, Rheault H, Miller E, Scheetz T, Enriquez V, Swafford R, et al. Long-term population dynamics in a multi-species assemblage of large herbivores in East Africa. Ecosphere. 2017;8(12):e02027. [Google Scholar]
- 54.Kinnaird MF, O’Brien TG. Effects of Private-Land Use, Livestock Management, and Human Tolerance on Diversity, Distribution, and Abundance of Large African Mammals. Conserv Biol. 2012;26(6):1026–39. 10.1111/j.1523-1739.2012.01942.x [DOI] [PubMed] [Google Scholar]
- 55.Du Toit JT, Cross PC, Valeix M. Managing the Livestock–Wildlife Interface on Rangelands In: Briske D, editor. Rangeland Systems. Cham: Springer; 2017. p. 395–425. [Google Scholar]
- 56.Peterson D, Baalow R, Cox J. Hadzabe: By The Light of a Million Fires. Dar es Salaam, Tanzania: Mikuki na Nyota Publishers; 2013. [Google Scholar]
- 57.Armitage DR. Environmental management and policy in a dryland ecozone: the Eyasi-Yaeda basin, Tanzania. Ambio. 1996;25(6):396–402. [Google Scholar]
- 58.Yanda PZ, Madulu NF. Water resource management and biodiversity conservation in the Eastern Rift Valley Lakes, Northern Tanzania. Phys Chem Earth. 2005;30:717–25. [Google Scholar]
- 59.Marlowe F. Why the Hadza are still hunter-gatherers In: Kent S, editor. Ethnicity, hunter-gatherers and the “other”: Association or assimilation in Africa. Smithonian Institution Press; 2002. p. 247–75. [Google Scholar]
- 60.Anderson J, Baker M, Bede J. Reducing emissions from deforestation and forest degradation in the Yaeda Valley, Northern Tanzania [Internet]. 2015. Available from: http://www.planvivo.org/docs/Yaeda_REDD_PDD_Jan15.pdf [Google Scholar]
- 61.Jodoin S. Forest preservation in a changing climate: REDD+ and indigenous and community rights in Indonesia and Tanzania. Cambridge: Cambridge University Press; 2017. 1–252 p. [Google Scholar]
- 62.Colwell RK. EstimateS: Statistical estimation of species richness and shared species from samples. 2016. (User’s Guide and application).
- 63.R Core Team. R: A language and environment for statistical computing [Internet]. Vienna: R Foundation for Statistical Computing; 2016. Available from: http://www.r-project.org/ [Google Scholar]
- 64.Bárton K. Model selection and model averaging based on information criteria (AICc and alike) [Internet]. 2013 [cited 2017 Dec 23]. Available from: https://cran.r-project.org/web/packages/MuMIn/index.html
- 65.Thomas L, Buckland ST, Rexstad EA, Laake JL, Strindberg S, Hedley SL, et al. Distance software: Design and analysis of distance sampling surveys for estimating population size. J Appl Ecol. 2010;47(1):5–14. 10.1111/j.1365-2664.2009.01737.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL. Introduction to Distance Sampling Estimating Abundance of Biological Populations. Oxford: Oxford University Press; 2001. 448 p. [Google Scholar]
- 67.Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L. Advanced Distance Sampling: Estimating abundance of biological populations. New York: Oxford University Press; 2004. 416 p. [Google Scholar]
- 68.Thomas. Monitoring long-term population change: why are there so many analysis methods? Ecology. 1996;77(1):49–58. [Google Scholar]
- 69.Chase MJ, Schlossberg S, Griffin CR, Bouché PJC, Djene SW, Elkan P, et al. Continent-wide survey reveals massive decline in African savannah elephants. PeerJ. 2016;4:e2354 10.7717/peerj.2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauer H, Chapron G, Nowell K, Henschel P, Funston P, Hunter LTB, et al. Lion (Panthera leo) populations are declining rapidly across Africa, except in intensively managed areas. Proc Natl Acad Sci. 2015. December 1;112(48):14894–9. 10.1073/pnas.1500664112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riggio J, Caro T, Dollar L, Durant SM, Jacobson AP, Kiffner C, et al. Lion populations may be declining in Africa but not as Bauer et al. suggest. Proc Natl Acad Sci. 2015;113(2):201521506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weise FJ, Vijay V, Jacobson AP, Schoonover RF, Groom RJ, Horgan J, et al. The distribution and numbers of cheetah (Acinonyx jubatus) in southern Africa. PeerJ. 2017;5:e4096 10.7717/peerj.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodroffe R, Sillero-Zubiri C. Lycaon pictus [Internet]. 2012 [cited 2017 Dec 26]. Available from: www.iucnredlist.org
- 74.Wood BM, Marlowe FW. Toward a reality-based understanding of Hadza men’s work. Hum Nat. 2014;25(4):620–30. 10.1007/s12110-014-9218-z [DOI] [PubMed] [Google Scholar]
- 75.Kiffner C, Wenner C, Laviolet A, Yeh K, Kioko J. From savannah to farmland: Effects of land-use on mammal communities in the Tarangire-Manyara ecosystem, Tanzania. Afr J Ecol. 2015;53(2):156–66. [Google Scholar]
- 76.Msuha MJ, Carbone C, Pettorelli N, Durant SM. Conserving biodiversity in a changing world: Land use change and species richness in northern Tanzania. Biodivers Conserv. 2012;21(11):2747–59. [Google Scholar]
- 77.Sollmann R, Mohamed A, Niedballa J, Bender J, Ambu L, Lagan P, et al. Quantifying mammal biodiversity co-benefits in certified tropical forests. Divers Distrib. 2017;23(3):317–28. [Google Scholar]
- 78.Rich LN, Miller DAW, Robinson HS, McNutt JW, Kelly MJ. Using camera trapping and hierarchical occupancy modelling to evaluate the spatial ecology of an African mammal community. J Appl Ecol. 2016;53(4):1225–35. [Google Scholar]
- 79.Andrén H. Effects of Habitat Fragmentation on Birds and Mammals in Landscapes with Different Proportions of Suitable Habitat: A Review. Oikos. 1994;71(3):355–66. [Google Scholar]
- 80.Cromsigt JPGM Prins HHT, Olff H. Habitat heterogeneity as a driver of ungulate diversity and distribution patterns: Interaction of body mass and digestive strategy. Divers Distrib. 2009;15(3):513–22. [Google Scholar]
- 81.Cook WM, Lane KT, Foster BL, Holt RD. Island theory, matrix effects and species richness patterns in habitat fragments. Ecol Lett. 2002;5:619–23. [Google Scholar]
- 82.Steinbeiser CM, Kioko J, Aresi A, Kaitilia R, Kiffner C. Relative abundance and activity patterns explain method-related differences in mammalian species richness estimates. J Mammal. 2019;100(1):192–201. [Google Scholar]
- 83.Coe MJ, Cumming DH, Philipson J. Biomass and productivity of large African herbivores in relation to rainfall and primary production. Oecologia. 1976;22:341–54. 10.1007/BF00345312 [DOI] [PubMed] [Google Scholar]
- 84.Carbone C, Gittleman JL. A common rule for the scaling of carnivore density. Science (80-). 2002;295(5563):2273–6. [DOI] [PubMed] [Google Scholar]
- 85.Smith LE. Wildlife resources of the hadza. In: Human Behavior and Evolution Society; Berlin; 2004. [Google Scholar]
- 86.Plumptre AJ. Monitoring mammal populations with line transect techniques in African forests. J Appl Ecol. 2000;37:356–68. [Google Scholar]
- 87.Barnes RFW. How reliable are dung counts for estimating elephant numbers? Afr J Ecol. 2001;39:1–9. [Google Scholar]
- 88.Viquerat SMA, Serge BK, Kiffner C, Waltert M. A comparison of regression based estimates of dung decay in two African forest duiker species (Cephalophus monticola, C. ogilby). Ecotropica. 2013;19:33–8. [Google Scholar]
- 89.Viquerat SMA, Bobo KS, Müller M, Kiffner C, Waltert M. Estimating forest duiker (Cephalophinae) density in Korup national park: a Case study on the performance of three line transect methods. South African J Wildl Res. 2012;42:1–10. [Google Scholar]
- 90.van Vliet N, Nasi R, Lumaret J. Factors influencing duiker dung decay in north-east Gabon: are dung beetles hiding duikers? Afr J Ecol. 2009;47:40–7. [Google Scholar]
- 91.Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68(4):619–40. [Google Scholar]
- 92.Marques FFC, Buckland ST, Goffin D, Dixon CE, Borchers DL, Mayle BA, et al. Estimating deer abundance from line transect surveys of dung: sika deer in southern Scotland. J Appl Ecol. 2001;38(2):349–63. [Google Scholar]
- 93.Fynn RWS, Augustine DJ, Peel MJS, de Garine-Wichatitsky M. Strategic management of livestock to improve biodiversity conservation in African savannahs: a conceptual basis for wildlife-livestock coexistence. J Appl Ecol. 2016;53(2):388–97. [Google Scholar]
- 94.Caro T. Guidelines for wildlife monitoring: savannah herbivores. Trop Conserv Sci. 2016;9(1):1–15. [Google Scholar]
- 95.Kingdon J. The Kingdon Field Guide to African Mammals. San Diego: Academic Press; 1997. [Google Scholar]
- 96.Githiru M, Des K. The forgotten Grevy’s zebra Equus grevyi population along the Kasigau Corridor ranches, SE Kenya: recent records and conservation issues. Afr J Ecol. 2017;55(4):554–63. [Google Scholar]
- 97.Bello C, Galetti M, Pizo MA, Magnago LFS, Rocha MF, Lima RAF, et al. Defaunation affects carbon storage in tropical forests. Sci Adv. 2015;1(11):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panfil SN, Harvey CA. REDD+ and biodiversity conservation: a review of the biodiversity goals, monitoring methods, and impacts of 80 REDD+ Projects. Conserv Lett. 2016;9(2):143–50. [Google Scholar]
- 99.Bryan BA, Runting RK, Capon T, Perring MP, Cunningham SC, Kragt ME, et al. Designer policy for carbon and biodiversity co-benefits under global change. Nat Clim Chang. 2016;6:301–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Reported parameters for each model include number of encounters within truncation distance (n), global detection probability (Pa) and associated 95% confidence intervals (Pa-lower—Pa-upper), estimated strip width, and corresponding chi-squared goodness of fit (GOF)–p-value.
(DOCX)
Densities based on sightings were indicated by “S” following the species name, other densities indicate sign densities. Columns with the letter “L” following the species name indicate the lower 95% confidence interval for the density estimate, columns with the letter “U” following the species name, indicate the upper 95%-confidence intervals of the density estimates.
(DOCX)
Population growth rates were estimated from 2015 to 2018 for all six strata [three REDD+ strata (W+, G+, and S+) three control land-use strata (W, P, and SW)] using generalized linear models with log-link. Empty cells denote that the species were not detected in this stratum. Regression coefficients for species highlighted in grey were not utilized for comparisons across land-use strata (main manuscript Fig 6) because those species were either livestock species, or had only one estimate in time that exceeded 0 signs or individuals per km2. For Kirk’s dik-dik we used the sign density, because signs were observed in all strata.
(DOCX)
Histograms (blue bars) represent sighting frequency and the red line is the fitted detection function.
(TIFF)
Histograms (blue bars) represent sighting frequency and the red line is the fitted detection function.
(TIFF)
Data Availability Statement
The underlying data have been made available as supporting documents.