Abstract
Objectives
Aim of this study was to assess the effect of a fermented milk product containing Bifidobacterium lactis CNCM I-2494 (FMP) on gastrointestinal (GI) symptoms and exhaled H2 and CH4 during a nutrient and lactulose challenge in patients with irritable bowel syndrome (IBS).
Methods
We included 125 patients with IBS (Rome III). Fasted subjects were served a 400ml liquid test meal containing 25g lactulose. The intensity of eight GI symptoms and the amount of exhaled H2 and CH4 were assessed before and during 4h after meal intake. The challenge was repeated after 14 days consumption of FMP or a control product in a double-blind, randomized, parallel design. The metabolic potential of fecal microbiota was profiled using 16S MiSeq analysis of samples obtained before and after the intervention.
Results
106 patients with IBS were randomized. No difference between FMP or control groups was found on GI symptoms or breath H2 and CH4 in the whole cohort. A post-hoc analysis in patients stratified according to their fasting H2 levels showed that in high H2 producers (fasting H2 level≥10ppm, n = 35), FMP consumption reduced fasting H2 levels (p = 0.003) and H2 production during the challenge (p = 0.002) and tended to decrease GI discomfort (p = 0.05) vs. control product. The Prevotella/Bacteroides metabolic potential at baseline was higher in high H2 producers (p<0.05) vs. low H2 producers and FMP consumption reduced this ratio (p<0.05) vs. control product.
Conclusions
The response to a fermented milk product containing Bifidobacterium lactis CNCM I-2494 (FMP) in patients with IBS seems to be associated with the metabolic potential of the gut microbiota.
Trial registration
ClinicalTrial.gov NCT01252550.
These results were presented as congress posters at Digestive Disease Week 2016 in San Diego, USA and United European Gastroenterology Week 2016 in Vienna, Austria.
Introduction
Gas-related symptoms such as bloating, abdominal distension and excessive flatulence are digestive complaints that can significantly affect well-being and quality of life [1–3]. Bloating and flatulence are the most common digestive symptoms reported by 15–20% of the general population in US and Europe [4, 5] and by up to 90% of patients with irritable bowel syndrome (IBS) [6]. Most patients suffering from functional gastrointestinal disorders (FGIDs) declare gas-related symptoms to be among their most bothersome symptoms [7]. No effective, safe and sustainable treatment for these symptoms is available today, partly since the physiology and pathophysiology of gas-related symptoms is complex and remains largely unknown [8, 9]. Notably, the relationship between objective markers of intestinal gas (volume, distribution, composition, frequency of evacuation) and perception of gas-related symptoms is still not clearly established [10].
Intestinal gas is produced predominantly in the colon, where unabsorbed meal residues are fermented by colonic bacteria [11, 12]. Within subjects, the volume and composition of intestinal gas production therefore vary in relation to the diet [13–15]. However, there is a great intra-, and even more so, inter-individual variability, as gas production in subjects maintained on a similar diet may differ substantially both in gas volume and composition [3, 16, 17]. This depends mainly on the composition and metabolic activity of the colonic microbiota [3]. A change in the composition of the diet, for example towards plant-based products rich in fermentable polysaccharides, can thus rapidly alter the composition and function of the gut microbiota [18, 19].
Different methods are available to assess intestinal gas production in vivo, most of them being exploratory (magnetic resonance imaging/MRI [10] or computed tomography/CT [16] and/or invasive (anal collection) [3]. The breath test is a non-invasive, standardized procedure allowing to measure gas production (H2, CH4) in end-expiratory breath samples, thereby providing an indirect assessment of intestinal gas production, as H2 and CH4 are solely produced by bacterial fermentation of undigested substrates, mainly in the colon [20]. Breath testing with measurement of H2 and CH4 after intake of different carbohydrates (e.g. lactose, fructose, glucose, lactulose) is used routinely in clinical practice to diagnose carbohydrate malabsorption, as well as small intestinal bacterial overgrowth [21]. Moreover, our group has recently developed a combined nutrient and lactulose challenge as a non-invasive test to study visceral sensitivity and to characterize symptom patterns and pathophysiology in IBS [22, 23].
Available scientific evidence based on several meta-analyses suggests that probiotics are beneficial in the management of FGIDs [24, 25]. Notably, specific probiotic strains belonging to Bifidobacterium have been shown to relieve the overall symptom burden in patients with FGID, and to reduce the perception of bloating and abdominal distension in patients with IBS [26]. Specifically, a fermented milk product containing Bifidobacterium lactis CNCM I-2494 and lactic acid bacteria (FMP) has been shown to improve well-being and digestive symptoms in women reporting minor digestive symptoms [27, 28] as well as improving bloating, digestive discomfort and reducing objectively measured abdominal distension in patients with IBS with predominant constipation (IBS-C) [29]. However, the mechanisms of action behind these clinical observations are still unclear.
The aims of the present exploratory study were i) to assess the effect of 14 days consumption of FMP on GI symptoms and exhaled H2 and CH4 during a combined nutrient and lactulose challenge test [22, 23] in patients with IBS and ii) to identify potential predictors of the intervention outcome such as intestinal gas production or pattern of symptoms at baseline.
Materials and methods
Study subjects
Adult patients aged between 18 and 65 years, fulfilling the Rome III criteria for IBS (all subtypes) [30] were prospectively included between May 2011 and August 2012 at a secondary/tertiary care outpatient clinic specialized in the management of FGIDs (Sahlgrenska University Hospital, Gothenburg, Sweden). The diagnosis was based on a typical clinical presentation and additional investigations if considered necessary by the gastroenterologist (MS, HT). Classification into IBS subtypes was done according to the Rome III criteria [30]. Exclusion criteria included the use of probiotics or antibiotics during the study or within one month before inclusion, severe psychiatric disease, other severe diseases, and a history of drug or alcohol abuse. All medications with known effects on the GI tract (proton pump inhibitors, laxatives, antidiarrheals, opioid analgesics, prokinetics, spasmolytics, antidepressants) were discontinued at least 48 hours before the challenge test. The study protocol was approved by the Regional Ethical Review Board in Gothenburg and all included subjects gave written informed consent. Part of the data from the subjects prior to the intervention has been presented in a recent publication [22]. However, the research question of the present publication is novel and the FMP intervention results have not been reported elsewhere.
Study design and products
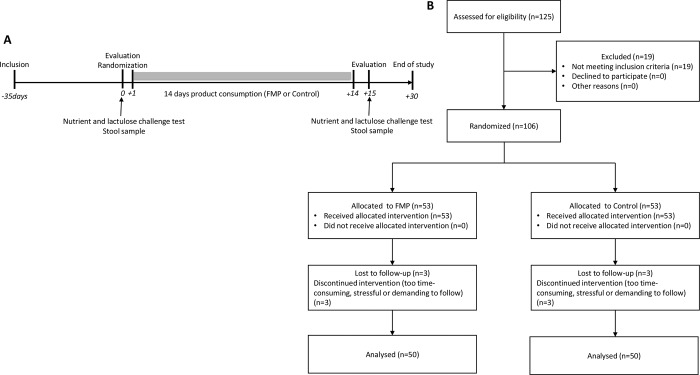
This study used a randomized, controlled, double-blind, parallel groups design (Fig 1A). At Visit 1 (inclusion), subjects deemed to be eligible for the study were included in a run-in period of up to 35 days designed to avoid any potential carry-over effect on gut microbiota from probiotic consumption prior to subject inclusion. At Visit 2 (baseline evaluation & randomization), subjects brought back a stool sample collected at home and performed a nutrient and lactulose challenge test in the clinic. The measured endpoints during the challenge were the intensity of eight meal-related GI symptoms, the overall level of digestive comfort and the amount of exhaled H2 and CH4 in breath (for details see supplementary material). Subjects were then randomized in a 1/1 ratio to consume 125g of either a fermented milk product (containing Bifidobacterium animalis subsp lactis CNCM I-2494, Lactobacillus bulgaricus CNCM I-1632 and CNCM I-1519, Streptococcus thermophilus CNCM I-1630 and Lactococcus lactis subsp lactis CNCM I-1631) or a control product (non-fermented milk product without bacterial strains and with similar lactose content) twice a day for 14 days. Both products were prepared at Danone Research facilities, Palaiseau, France, and shipped in blinded packaging with refrigeration to the study site at Sahlgrenska University Hospital, Gothenburg, Sweden. The randomization code was generated by an external CRO (Gothia Forum, Gothenburg, Sweden). Blinding was accomplished by ensuring that active and control products were of identical appearance, taste and texture. At Visit 3 (evaluation post 14 days product consumption period), subjects brought back a stool sample collected at home and performed a nutrient and lactulose challenge test. Compliance to the study product was measured by returning unused product to the study center. A follow-up visit (Visit 4) was performed 2 weeks after the end of the product consumption period. The randomization code was not to be broken until all assessments had been performed, all data had been entered into the database, and the database had been locked after a clean file procedure.
Fig 1.
A) Study design B) Flow chart demonstrating the number of patients in the different phases of the study.
Run-in period assessments
IBS-SSS questionnaire: the severity of IBS symptoms was determined with the validated IBS Severity Scoring System (IBS-SSS) questionnaire ranging from 0 (no symptoms) to 500 [31].
HAD questionnaire: general anxiety and depression were evaluated by the Hospital Anxiety and Depression scale (HAD) [32].
PHQ 15 questionnaire: the bothersomeness of 15 somatic symptoms was assessed by the Patient Health Questionnaire 15 (PHQ-15) [33].
Oroanal transit time test (OATT): OATT was assessed in subjects by ingestion of radiopaque rings [34].
Dietary habits: To determine their usual intake of energy and nutrients, as well as FODMAPs (Fermentable Oligo-, Di-, Monosaccharides and Polyols), all subjects completed a 4-day food diary during the run-in period, in which the quantities consumed were entered in grams or household measures. The intake of nutrients, including FODMAPs, was calculated using a dedicated software (Dietist XP, Kostdata.se, Stockholm, Sweden).
Fecal microbiota analysis
Fecal samples were collected before and at the end of the product consumption period from 62 IBS subjects. Fecal samples were processed in RNAlater solution (Ambion) as previously described [35]. Fecal total RNA was extracted using mechanical lysis (Fastprep FP120, ThermoSavant) followed by phenol/chloroform-based extraction as previously described [36] and analyzed by 16S sequencing on a MiSeq platform based on V3-V4 16S regions (see supporting information). The obtained data was analyzed using the open source software package Quantitative Insights Into Microbial Ecology (QIIME), v1.9 [37]. Representative sequences (i.e most abundant) for each Operational Taxonomical Units (OTUs) were taxonomically assigned using Silva database (version 119) (see supporting information). The fecal microbiota metabolic potential of a specific taxon was proportional to the number of 16S rRNA reads assigned to this taxon. The prevalence of Methanobacteriales in fecal samples was evaluated by quantitative PCR (qPCR) as described earlier [35].
Statistical analysis
Clinical parameters
For analysis of parameters during the run-in period (IBS-SSS, HAD, PHQ-15, OATT, dietary habits), a one-way ANOVA was performed with intervention group (FMP, control) as unique factor for continuous variable. For categorical variables, a chi-square test was performed. For analysis of planned study endpoints assessing the effect of 14 days consumption of a fermented milk product vs. control product on GI symptoms and exhaled H2 and CH4 during a combined nutrient and lactulose challenge test, an ANCOVA model was used with intervention group (FMP, control) as only factor. For post-hoc analyses aiming to identify potential predictors of the intervention outcome based on intestinal gas production or pattern of symptoms at baseline, analyses were performed on subsets based on demonstrated differences. For this, an ANCOVA model was used with multiple factors: intervention group (FMP, control), potential predictor (eg fasting H2 value T0 prior to intervention) and the interaction “intervention group*predictor”. Baseline values for study endpoints were taken as covariate. If the interaction “intervention group*predictor” was significant then least square means were computed. Benjamini-Hochberg multiplicity correction was applied for all tests. The sample size in this exploratory study was not based on a power calculation. Inferential statistical tests were performed with alpha risk level at 5%. Univariate and multivariate statistical analyses were performed using JMP v11 software (SAS Institute Inc., Cary, NC) and R (version 3.1.2).
Microbiota parameters
We first assessed the impact of intervention on gut microbiota in the whole cohort, followed by analysis on subgroups as defined by the post-hoc stratification. DESeq2, an approach specifically designed for RNA sequencing analysis and suitable for low number of subjects [38] was used for statistical analysis of microbiota parameters. Full statistical methods on microbiota parameters can be found in supporting information.
Results
Clinical characteristics at baseline
Clinical characteristics at baseline are demonstrated in Table 1. No differences were found for age, gender, Rome III subtype, BMI, IBS-SSS or PhQ-15 scores between groups randomized to FMP and control product, but the scores for HAD anxiety and depression were higher in patients randomized to receive the control product.
Table 1. Clinical characteristics of randomized subjects.
| Mean (SD) | FMP (n = 53) | Control (n = 53) | P value |
|---|---|---|---|
| age | 35.3 (11.5) | 35.7 (10.6) | NS |
| gender F/M (%) | 64.2 / 35.8 | 56.6 / 43.4 | NS |
| BMI | 23.4 (3.2) | 23.2 (4.0) | NS |
| IBS-SSS | 292.1 (110.3) | 268.3 (99.0) | NS |
| HAD anxiety | 6.9 (3.9) | 9.0 (4.7) | * |
| HAD depression | 4.0 (2.6) | 5.9 (3.5) | * |
| PHQ-15 | 12.6 (5.7) | 13.1 (4.5) | NS |
| IBS-C, n (%) | 10 (18.9%) | 10 (18.9%) | NS |
| IBS-D, n (%) | 17 (32.1%) | 16 (30.2%) | NS |
| IBS-M, n (%) | 21 (39.6%) | 16 (30.2%) | NS |
| IBS-U, n (%) | 5 (9.4%) | 11 (20.8%) | NS |
SD: Standard Deviation; BMI: body mass index; IBS-SSS: IBS Severity Scoring System; HAD: hospital anxiety and depression scale; PHQ-15: patient health questionnaire 15; IBS-C: irritable bowel syndrome with constipation; IBS-D: irritable bowel syndrome with diarrhea; IBS-M: irritable bowel syndrome with mixed pattern; IBS-U: irritable bowel syndrome unsubtyped; all questionnaire data are expressed as mean total scores; Statistical significance is determined by Oneway ANOVA. Benjamini Hochberg multiplicity correction was applied. NS for p value >0.05
* <0.05
Effect of intervention on planned study endpoints: GI symptoms, overall digestive comfort and exhaled H2 and CH4
Out of 125 subjects assessed for eligibility, a total of 106 patients were randomized and 100 patients completed the study (Fig 1B). 19 subjects did not meet the study inclusion criteria and 6 subjects (3 per group) discontinued the study. No significant difference between patients who received FMP or control product were found on GI symptoms, overall digestive comfort and exhaled H2 and CH4 following the 14 days intervention. All results on the planned study endpoints are presented in Table 2.
Table 2. Intervention results on planned study endpoints.
| Mean (SD) | FMP (n = 50) | Control (n = 50) | P value |
|---|---|---|---|
| Δ T0 H2 ppm | -3.50 (16.00) | 4.70 (15.50) | 0.23 |
| Δ T0 CH4 ppm | 1.00 (9.30) | -0.20 (5.00) | 0.51 |
| Δ H2 ppm | 0.57 (19.10) | 8.10 (25.14) | 0.23 |
| Δ CH4 ppm | 1.20 (10.39) | 0.24 (9.02) | 0.54 |
| Δ gas | -0.23 (3.11) | -0.65 (2.81) | 0.52 |
| Δ bloating | -0.42 (3.07) | -0.77 (2.88) | 0.54 |
| Δ discomfort | -0.87 (2.85) | -1.08 (2.83) | 0.54 |
| Δ distension | -0.54 (2.60) | -0.92 (2.86) | 0.54 |
| Δ nausea | -0.35 (3.36) | -0.22 (2.78) | 0.79 |
| Δ rumbling | -0.22 (2.81) | -0.88 (2.94) | 0.24 |
| Δ urgency | 0.01 (2.82) | -0.37 (2.60) | 0.39 |
| Δ pain | 0.01 (2.84) | -0.66 (2.34) | 0.23 |
| Δ comfort | -0.20 (2.60) | 0.70 (2.40) | 0.24 |
SD: Standard Deviation; FMP: fermented milk product (FMP); Control: non-fermented milk product; ppm: parts per million; H2: hydrogen; CH4: methane; T0: fasting value; all statistical analyses are covariance analyses of the 4h mean change (Δ) on measured endpoints from 1st to 2nd nutrient-lactulose challenge between the two study arms adjusted for 1st challenge values; all significance tests were two-sided and conducted at the 5% significance level; Benjamini Hochberg multiplicity correction was applied.
Post-hoc stratification of patients and effect of intervention
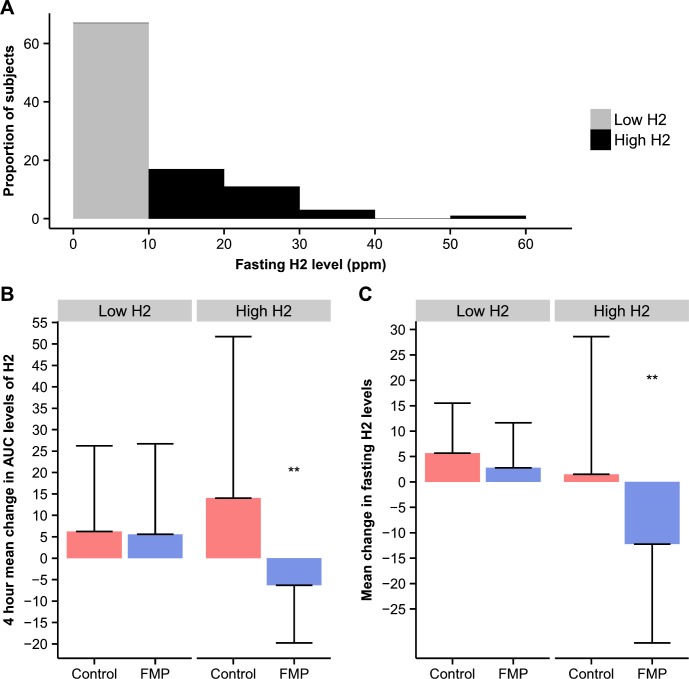
Most subjects (67%) had fasting H2 levels lower than 10ppm prior to intervention, with nearly half of the subjects (48%) having values between 0 and 2ppm (Fig 2A). A binary classification with an arbitrary cut-off at 10ppm H2 was performed prior to intervention, separating subjects into “low fasting H2 producers” (n = 67) and “high fasting H2 producers” (n = 33). No significant differences were found for age, gender, BMI, IBS subtype distribution, OATT, intake of energy or nutrients including FODMAPs, total scores for IBS-SSS, PHQ-15, HAD anxiety or HAD depression between high and low H2 producers. All data are summarized in Table 3. A post-hoc analysis on study endpoints (GI symptoms, overall digestive comfort and exhaled H2 and CH4) was conducted in patients stratified according to their fasting H2 levels (T0) prior to intervention. The FMP intervention in high H2 producers reduced the 4h mean AUC of H2 during the challenge vs. control (p = 0.002) (Fig 2B), but not CH4 (p = 0.31), and tended to decrease discomfort during the challenge vs. control (p = 0.05). The FMP intervention also reduced mean fasting levels (T0) of H2 vs. control (p = 0.004) in high H2 producers (Fig 2C). In contrast, no effect of the FMP intervention was seen on exhaled gas in low H2 producers, even if CH4 tended to be higher in the FMP group (p = 0.08). Regarding the evolution of GI symptoms during the challenge in low H2 producers, a difference was observed between the FMP and control groups for gas, discomfort, rumbling and pain, with a greater reduction of these symptoms in the control group. All post-hoc intervention results are compiled in Table 4.
Fig 2. Effect of intervention on breath hydrogen in IBS patients stratified according to fasting H2 levels.
A) Distribution of subjects in % according to H2 fasting value B) Effect of the intervention on the 4h mean change in AUC levels of H2 C) Effect of the intervention on the mean change in fasting H2 levels (T0); FMP: fermented milk product; Control: non-fermented milk product; High H2: fasting H2 level≥10ppm (n = 33); Low H2: fasting H2 level<10ppm (n = 67); ppm: parts per million; H2: hydrogen; T0: fasting value; statistical analyses for B) and C) are covariance analyses of the mean change (Δ) of the measured endpoints from 1st to 2nd nutrient-lactulose challenge between the two study arms adjusted for first challenge values; all significance tests were two-sided and conducted at the 5% significance level. Interaction was evaluated at 10% significance level; NS for p value >0.05, * <0.05, ** <0.01, *** <0.001.
Table 3. Clinical characteristics of fasting H2 based subgroups (post-hoc).
| Mean (SD) | Low H2 (n = 71) | High H2 (n = 35) | P value |
|---|---|---|---|
| age | 36.0 (10.5) | 34.5 (12.1) | 0.67 |
| gender F/M (%) | 64.8 / 35.2 | 51.4 / 48.6 | 0.19 |
| BMI | 23. 4 (3.9) | 23.1 (3.0) | 0.67 |
| IBS-SSS | 281.2 (100.0) | 278.1 (116.0) | 0.90 |
| HAD anxiety | 8.3 (4.5) | 7.4 (4.3) | 0.56 |
| HAD depression | 5.4 (3.4) | 4.1 (2.5) | 0.44 |
| PHQ-15 | 13.2 (4.9) | 12.2 (5.5) | 0.56 |
| IBS-C, n (%) | 15 (21.1) | 5 (14.3) | 0.83 |
| IBS-D, n (%) | 21 (29.6) | 12 (34.3) | |
| IBS-M, n (%) | 25 (35.2) | 12 (34.3) | |
| IBS-U, n (%) | 10 (14.1) | 6 (17.1) | |
| OATT (days) | 1.5 (1.2) | 1.3 (0.8) | 0.56 |
| Energy from diet (kcal) | 2198.7 (598.7) | 2037.0 (554.0) | 0.56 |
| %Carbohydrates | 44.2 (8.5) | 45.2 (8.3) | 0.67 |
| %Protein | 16.7(3.6) | 17.2 (4.4) | 0.67 |
| %Fat | 36.1 (6.8) | 34.4 (7.9) | 0.60 |
| FODMAPs (g) | 16.3 (10.0) | 14.1 (8.3) | 0.56 |
SD: Standard Deviation; High H2: fasting H2 level≥10ppm; Low H2: fasting H2 level<10ppm; BMI: body mass index; IBS-SSS: IBS Severity Scoring System; HAD: hospital anxiety and depression scale; PHQ-15: patient health questionnaire 15; IBS-C: irritable bowel syndrome with constipation; IBS-D: irritable bowel syndrome with diarrhea; IBS-M: irritable bowel syndrome with mixed pattern; IBS-U: irritable bowel syndrome unsubtyped; OATT: oroanal transit time; FODMAP: fermentable, oligo-, di-, mono-saccharides and polyols; all questionnaires are expressed as mean total scores; Statistical significance is determined by Oneway ANOVA for quantitative parameters. Multiple testing strategy consisted in Benjamini Hochberg adjustment for quantitative parameters and two-sided Chi2 test for qualitative parameters.
Table 4. Intervention results in fasting H2 based subgroups (post-hoc).
| Low H2 (n = 67) | High H2 (n = 33) | ||||||
|---|---|---|---|---|---|---|---|
| mean (SD) | Interaction P-value | FMP (n = 29) | Control (n = 38) | P value | FMP (n = 21) | Control (n = 12) | P value |
| Δ T0 H2 ppm | 0.06 | 2.8 (8.9) | 5.7 (9.9) | 0.31 | -12.2 (19.5) | 1.5 (27.1) | 0.004** |
| Δ T0 CH4 ppm | 0.36 | 3.2 (6.2) | 0.3 (4.5) | NA | -2.1 (11.9) | -1.5 (6.4) | NA |
| Δ H2 ppm | 0.01* | 5.6 (21.1) | 6.2 (20) | 0.79 | -6.3 (13.5) | 14 (37.7) | 0.002** |
| Δ CH4 ppm | 0.07 | 3.9 (7.0) | -0.2 (8.1) | NA | -2.5 (13.1) | 1.7 (11.8) | NA |
| Δ gas | 0.03* | 0.6 (2.4) | -1.0 (3.1) | 0.04* | -1.4 (3.7) | 0.4 (1.0) | 0.20 |
| Δ bloating | 0.18 | 0.0 (2.7) | -1.0 (3.0) | NA | -1.0 (3.5) | 0.1 (2.3) | NA |
| Δ discomfort | 0.005** | -0.1 (2.6) | -1.5 (2.9) | 0.03* | -1.9 (2.9) | 0.1 (2.2) | 0.05 |
| Δ distension | 0.20 | -0.1 (2.4) | -1.0 (3.2) | NA | -1.1 (2.8) | -0.5 (1.1) | NA |
| Δ nausea | 0.20 | -0.3 (2.9) | -0.6 (2.7) | NA | -0.5 (4.0) | 0.9 (3.0) | NA |
| Δ rumbling | 0.01* | 0.6 (2.9) | -1.2 (3.0) | 0.003** | -1.4 (2.3) | 0.1 (2.7) | 0.34 |
| Δ urgency | 0.10 | 0.4 (2.8) | -0.7 (2.6) | NA | -0.5 (2.9) | 0.6 (2.4) | NA |
| Δ pain | 0.02* | 0.3 (3.2) | -1.0 (2.2) | 0.004** | -0.4 (2.3) | 0.4 (2.5) | 0.43 |
| Δ comfort | 0.93 | 0.1 (2.1) | 0.9 (2.7) | NA | -0.7 (3.1) | 0.2 (1.4) | NA |
SD: Standard Deviation; FMP: fermented milk product (FMP); Control: non-fermented milk product; High H2: fasting H2 level≥10ppm; Low H2: fasting H2 level<10ppm; ppm: parts per million; H2: hydrogen; CH4: methane; N/A: not applicable; T0: fasting value; all statistical analyses are covariance analyses of the 4h mean change (Δ) on measured endpoints from 1st to 2nd nutrient-lactulose challenge between the two study arms adjusted for 1st challenge values; all significance tests were two-sided and conducted at the 5% significance level. Interaction was evaluated at 10% significance level
* <0.05
** <0.01
Effect of the intervention on gut microbiota metabolic potential
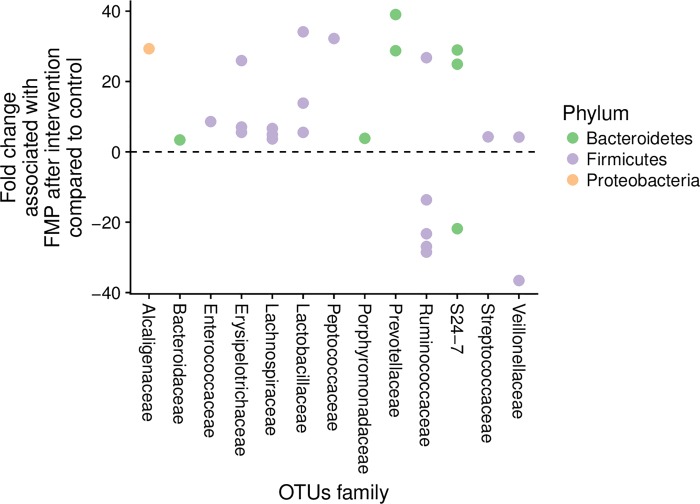
We examined the effect of the intervention on the metabolic potential of the gut microbiota. Six genera were identified as dominant and these included Bacteroides, Prevotella, Ruminococcaceae Incertae Sedis, Blautia, Faecalibacterium and Bifidobacterium. Together, the relative abundance of these six genera represented on average 40% of the microbiota metabolic potential in this study. None of these genera were different before as compared to after intervention (p>0.05). Next, we examined the response of the gut microbiota at a lower taxonomical level, i.e. OTUs. OTUs that were altered by FMP represented a median of 7.1% of 16S rRNA sequences. Fourteen (14) out of 21 OTUs that were up-regulated by FMP belonged to the Firmicutes phylum (Fig 3).
Fig 3. Impact of the intervention on the metabolic potential of the gut microbiota in subjects consuming FMP (n = 32) or control product (n = 30).
Effect of the intervention on microbial OTUs. Each dot corresponds to an OTU colored according to their taxonomical affiliation (Phylum).
Metabolic potential of gut microbiota in fasting H2-based subgroups and effect of the intervention
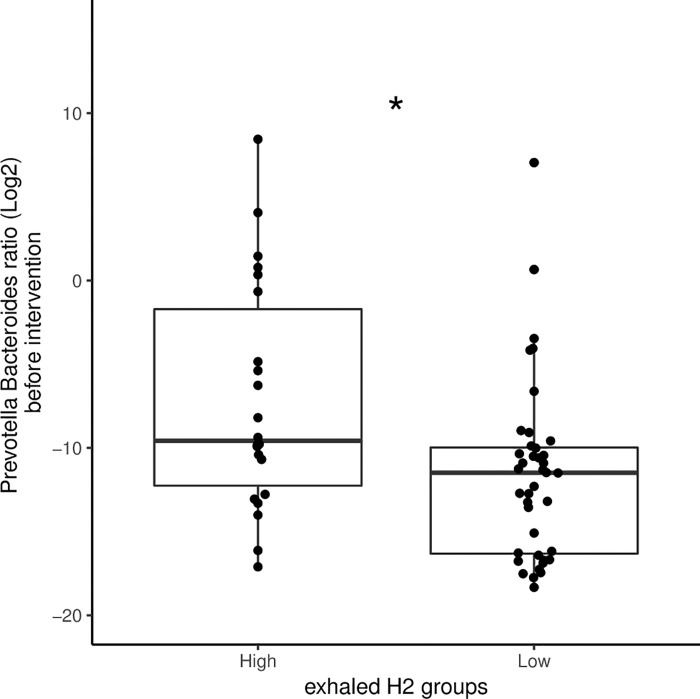
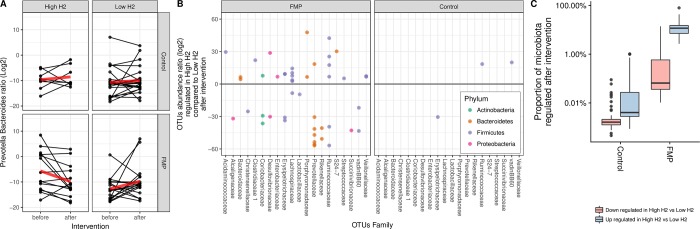
Next, we evaluated whether the dominant active microbiota prior to intervention, measured by 16S rRNA sequencing, differed at the genus level between high (n = 22) and low H2 producers (n = 40) for which fecal samples could be analyzed. From the six dominant genera, Prevotella and Bacteroides were significantly different between high and low H2 producers (p<0.05, FDR q-value <0.2). The ratio of Prevotella / Bacteroides was higher in high H2 compared to low H2 producers prior to intervention (p<0.05) (Fig 4). In addition, a targeted analysis on known H2-consuming bacteria was performed. The prevalence of Methanobacteriales, evaluated by quantitative PCR, abundance of Desulfovibrio (sulphate-reducing bacteria) and Blautia (acetogen) did not differ between H2-based subgroups (S1 Fig). Within the high H2 producers, there was a reduction in the Prevotella / Bacteroides ratio following 14 days consumption of the FMP (p<0.05), which was not observed after the control product (Fig 5A). DESeq2 analysis showed that 42 OTUs belonging to a number of families within Firmicutes, Bacteroidetes and Proteobacteria responded differently in high vs. low H2 producers after FMP, while only 3 OTUs were altered after the control product (Figs 5B and S2). Seven OTUs belonged to Prevotellaceae family and six of them were downregulated after FMP in high H2 producers vs. low H2 producers. In addition, the most dominant active OTUs on average, which belonged to Bacteroidaceae, was upregulated in high H2 producers after FMP vs. low H2 producers. The post-hoc stratification of patients according to fasting H2 levels showed that consumption of FMP significantly altered OTUs (p<0.05) representing on average 11.6% of the total 16S rRNA sequence vs. 7.1% in the whole cohort (Fig 5C). The prevalence of known H2-consuming bacteria (Methanobacteriales, Desulfovibrio, Blautia) did not change in any group after the intervention (S1 Fig).
Fig 4. Gut microbiota of IBS patients according to their fasting H2 levels.
Metabolic potential of the Prevotella/Bacteroides ratio in low (n = 40) and high (n = 22) H2 producers; Statistical significance was determined by Wilcoxon test; *P < 0.05.
Fig 5. Response of gut microbiota to intervention according to fasting H2 levels in IBS patients.
A) Metabolic potential of the Prevotella / Bacteroides ratio. Each line represents a subject before and after intervention. The red line represents the median evolution. B) Effect of the intervention (FMP vs. control) on microbial OTUs in high and low H2 producers. Each dot corresponds to an OTU with its associated family depicted on the x axis. OTUs are colored according to their taxonomical affiliation (Phylum). C) Proportion (showed in %) of whole microbiota metabolic potential regulated in high H2 versus low H2 producers upon intervention. High H2/control n = 8; High H2/FMP n = 14; Low H2/control n = 22; Low H2 FMP n = 18.
Discussion
In this exploratory study, we aimed i) to assess the effect of 14 days consumption of a FMP on GI symptoms and exhaled H2 and CH4 during a combined nutrient and lactulose challenge test in patients with IBS and ii) to identify potential predictors of the intervention outcome, such as intestinal gas production or pattern of symptoms at baseline.
No effect of the intervention was seen in the whole cohort. However, using post-hoc analyses, we demonstrated that a FMP intervention can reduce the production of intestinal gas in a subgroup of IBS patients. Hence, individuals exhibiting higher fasting production of hydrogen prior to the intervention benefited the most from the FMP. These subjects were characterized by a distinct gut microbiota activity level signature but did not differ otherwise from other patients regarding clinical parameters or dietary intake. These findings suggest that fasting breath H2 testing may be a potential predictor of FMP intervention outcome.
In the present trial, no difference between the FMP and the control intervention were found on study endpoints when considering the whole cohort. Patients with IBS form a highly heterogeneous population, with numerous subgroups identified based on severity and pattern of symptoms and the underlying pathophysiology [35, 39, 40]. This represents a true challenge when evaluating the efficacy of new treatments in this population. In this exploratory study, we chose to include all patients with IBS regardless of their symptom severity or predominant bowel habits. Our objective was to identify potential predictors of the intervention outcome within this heterogeneous cohort. This approach may help to recruit more specific and homogeneous cohorts of patients in future studies based on a surrogate marker of gut microbiota activity, thereby enhancing the chances of success.
Several randomized controlled trials have been conducted with probiotics in patients with FGID, and particularly in IBS [41–43]. To the best of our knowledge, none of these studies have shown an impact on intestinal gas production, even in subgroups of patients. We previously showed that the intensity of GI symptoms elicited by a nutrient and lactulose challenge in patients with IBS correlates poorly with levels of H2 and CH4 in breath [22, 23]. This was also the case in the present study. However, an interesting finding was a tendency towards reduction of discomfort during the challenge test following consumption of FMP in the subgroup of high H2 producing IBS patients. In these patients, perception of symptoms could in part be linked to objectively increased volumes of intestinal gas, and the FMP intervention would help to relieve the patients from their symptoms by decreasing the amount of gas. Previous attempts to use the lactulose hydrogen breath test (LHBT) as a predictor for dietary intervention outcomes in patients with FGID have failed [44]. However, in contrast to our study where the fasting H2 value was used to stratify patients prior to intervention, previous attempts used either AUC or threshold values for H2 following LHBT [44]. For a given individual, the reproducibility of the fasting H2 value over time is most certainly influenced by diet among other factors. A diet rich in fermentable carbohydrates leads to higher production of intestinal gas [3], but by standardizing conditions the day prior to the test, for example by providing a standard diet and controlling the duration of fasting, a better reproducibility in the measurements of fasting H2 might be achieved. A test-retest methodological study would be needed to validate this assumption.
There are several potential mechanisms of action for the FMP based on the post-hoc analyses on exhaled gas in our study. The first hypothesis on how consumption of FMP may reduce exhaled H2 is via modulation of the gut microbiota activity. The response of the gut microbiota to consumption of FMP has been reported in several clinical trials in healthy subjects and IBS patients [45, 46]. While these studies did not show an overall change of gut microbiota composition using 16S approach and metagenomics, McNulty et al. [45] reported that 7-week consumption of FMP in healthy women induced changes in the activity of some metabolic pathways related to carbohydrates and short chain fatty acids. In line with this, in the present study, we hypothesize that the differences observed in exhaled H2 between individuals could be related to the gut microbiota metabolic potential. While no difference could be observed in dominant genera, a higher metabolic potential of the Prevotella/Bacteroides ratio was observed in high H2 producers as compared to low H2 producers prior to the intervention. While the value of these findings is not yet fully understood, metagenomics analysis have shown that both genera exhibit different genetic capacity to produce hydrogenases [47]. FMP consumption also influenced the gut microbiota activity to a larger extent than the control product, especially in high H2 producers. In these patients, a higher number of OTUs were modulated by FMP vs. low H2 producers. However, the prevalence of Methanobacteriales able to consume H2, and abundance of genera amongst sulphate-reducing and acetogenic bacteria did not differ between low and high H2 producers at baseline or after the intervention. Lastly, we can hypothesize that the metabolism of the candidate probiotic strain B. animalis subsp lactis, previously shown to be active in the gut microbiota [45, 46], may reduce H2 production during the nutrient and lactulose challenge by metabolizing lactulose. Such metabolism would reduce the availability of lactulose to colonic bacteria that can degrade lactulose and produce gas [48]. However, FMP was not consumed close in time to the challenge test, as the participants arrived in the morning to the laboratory after an overnight fast.
We acknowledge that there are limitations with our study. First, the stratification between high and low H2 producers was not planned a priori in the study protocol and has been performed as a post-hoc analysis. Therefore, sample size in the FMP and control groups is unbalanced, with nearly twice as many individuals in the group who consumed FMP relative to the group who consumed the control product. Moreover, stratification of patients has been performed based on a single value, i.e. the fasting H2 value prior to the intervention. Reproducibility of this measurement, and therefore robustness of the ratio between high and low H2 producers needs to be assessed in an independent study. It should also be mentioned that 16S rRNA sequencing, although being a useful tool to assess the metabolic potential of gut microbiota, cannot provide information about functionality. To better understand the overall picture, techniques like metatranscriptomics would be needed. Finally, all patients were included at a secondary/tertiary referral center, which could prevent extrapolation of the results to an IBS population at the societal level. Therefore, the present findings need to be confirmed in a study where the subjects would be randomized according to their fasting H2 levels and performed with subjects from the general population.
In conclusion, the present findings indicate that 14 days consumption of a fermented milk product containing B. lactis CNCM I-2494 and lactic acid bacteria can reduce the production of intestinal gas in a subgroup of patients who are high H2 producers, both in fasting conditions and following a nutrient and lactulose challenge. Fasting H2 levels and Prevotella/Bacteroides ratio metabolic potential were associated with the outcome of the intervention. These findings need to be confirmed to assess whether the metabolic potential of gut microbiota can predict the response to a FMP in patients with IBS.
Supporting information
Methanobacteriales were detected in fecal DNA by qPCR A) at baseline in Low (n = 54) and High (n = 24) H2 producers and B) stratified by study group, before and after intervention. Blautia and Desulfovibrio 16S RNA-seq reads abundance were depicted into tertiles. Low, medium and high corresponded respectively to the first, second and third tertiles. Subject prevalence for each tertile was indicated at baseline in respectively C) E) and stratified by study group, before and after intervention respectively in D) F); High H2 control (n = 9), Low H2 control (n = 29), High H2 FMP (n = 15), Low H2 FMP (n = 25).
(TIFF)
Samples were compared between high H2 (n = 24) and low H2 (n = 54) producers after intervention.
(TIFF)
(DOCX)
(PDF)
(DOC)
Data Availability
The consent forms which were obtained by the study sponsor do no permit sharing any personal data, in conformity with the GDPR. We can share part of the data in anonymized and summarized form with group level data upon request, but any data that in any way may jeopardize anonymity of the subjects or contain sensitive information such as symptom and demographic information cannot be shared for ethical reasons, since this was not included in the ethical approval, and data sharing was not included in the patient information and therefore the subjects have not agreed to share their data with other research groups than the researchers directly involved in this study. Contact information for data requests: Regional Ethical Review Board in Gothenburg, Regionala etikprövningsnämnden, Box 401, 405 30 Göteborg, Sweden (email: registrator@etikprovning.se Phone: 010-475 08 00).
Funding Statement
This research was supported by the Swedish Medical Research Council (grants 13409, 21691 and 21692), the Marianne and Marcus Wallenberg Foundation, AFA Försäkring, the Faculty of Medicine, University of Gothenburg, as well as by Danone Nutricia Research.
References
- 1.Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, Characteristics, and Impact of Bloating Symptoms in Patients With Irritable Bowel Syndrome. Clinical Gastroenterology and Hepatology. 2009;7(1):68–72. 10.1016/j.cgh.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 2.Malagelada JR, Accarino A, Azpiroz F. Bloating and Abdominal Distension: Old Misconceptions and Current Knowledge. The American Journal Of Gastroenterology. 2017;112 (8)(Aug):1221–31. 10.1038/ajg.2017.129 [DOI] [PubMed] [Google Scholar]
- 3.Manichanh C, Eck A, Varela E, Roca J, Clemente JC, González A, et al. Anal gas evacuation and colonic microbiota in patients with flatulence: effect of diet. Gut. 2014;63(3):401 10.1136/gutjnl-2012-303013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. Abdominal Pain, Bloating, and Diarrheain the United States. Digestive Diseases and Sciences. 2000;45(6):1166–71. 10.1023/a:1005554103531 [DOI] [PubMed] [Google Scholar]
- 5.Tielemans MM, Jaspers Focks J, van Rossum LGM, Eikendal T, Jansen JBMJ, Laheij RJF, et al. Gastrointestinal Symptoms are Still Prevalent and Negatively Impact Health-Related Quality of Life: A Large Cross-Sectional Population Based Study in The Netherlands. PLoS ONE. 2013;8(7):e69876 10.1371/journal.pone.0069876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96(12):3341–7. 10.1111/j.1572-0241.2001.05336.x [DOI] [PubMed] [Google Scholar]
- 7.Agrawal A, Whorwell PJ. Review article: abdominal bloating and distension in functional gastrointestinal disorders-epidemiology and exploration of possible mechanisms. Alimentary Pharmacology & Therapeutics. 2008;27(1):2–10. 10.1111/j.1365-2036.2007.03549.x [DOI] [PubMed] [Google Scholar]
- 8.Azpiroz F. Intestinal gas dynamics: mechanisms and clinical relevance. Gut. 2005;54(7):893–5. 10.1136/gut.2004.048868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azpiroz F, Malagelada JR. Abdominal Bloating. Gastroenterology. 2005;129(3):1060–78. 10.1053/j.gastro.2005.06.062 [DOI] [PubMed] [Google Scholar]
- 10.Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, et al. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology. 2017;152(1):124–33.e2. 10.1053/j.gastro.2016.09.062 [DOI] [PubMed] [Google Scholar]
- 11.Levitt MD, Hirsh P, Fetzer CA, Sheahan M, Levine AS. H2 excretion after ingestion of complex carbohydrates. Gastroenterology. 1987;92(2):383–9. 10.5555/uri pii:0016508587901326. [DOI] [PubMed] [Google Scholar]
- 12.Flourié B, Leblond A, Florent C, Rautureau M, Bisalli A, Rambaud JC. Starch malabsorption and breath gas excretion in healthy humans consuming low- and high-starch diets. Gastroenterology. 1988;95(2):356–63. 10.1016/0016-5085(88)90491-X. [DOI] [PubMed] [Google Scholar]
- 13.Steggerda FR. Gastrointestinal gas following food consumption. Annals of the New York Academy of Sciences. 1968;150(1):57–66. 10.1111/j.1749-6632.1968.tb19031.x [DOI] [PubMed] [Google Scholar]
- 14.Steggerda FR, Dimmick JF. Effects of Bean Diets on Concentration of Carbon Dioxide in Flatus. The American Journal of Clinical Nutrition. 1966;19(2):120–4. 10.1093/ajcn/19.2.120 [DOI] [PubMed] [Google Scholar]
- 15.Stone-Dorshow T, Levitt MD. Gaseous response to ingestion of a poorly absorbed fructo-oligosaccharide sweetener. The American Journal of Clinical Nutrition. 1987;46(1):61–5. 10.1093/ajcn/46.1.61 [DOI] [PubMed] [Google Scholar]
- 16.Bendezú RA, Barba E, Burri E, Cisternas D, Malagelada C, Segui S, et al. Intestinal gas content and distribution in health and in patients with functional gut symptoms. Neurogastroenterology & Motility. 2015;27(9):1249–57. 10.1111/nmo.12618 [DOI] [PubMed] [Google Scholar]
- 17.Tomlin J, Lowis C, Read NW. Investigation of normal flatus production in healthy volunteers. Gut. 1991;32(6):665–9. PMC1378885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. Epub 2013/12/11. 10.1038/nature12820 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooda S, Boler BMV, Serao MCR, Brulc JM, Staeger MA, Boileau TW, et al. 454 Pyrosequencing Reveals a Shift in Fecal Microbiota of Healthy Adult Men Consuming Polydextrose or Soluble Corn Fiber. The Journal of Nutrition. 2012;142(7):1259–65. 10.3945/jn.112.158766 [DOI] [PubMed] [Google Scholar]
- 20.Levitt MD. Production and Excretion of Hydrogen Gas in Man. New England Journal of Medicine. 1969;281(3):122–7. 10.1056/NEJM196907172810303 . [DOI] [PubMed] [Google Scholar]
- 21.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55(3):297–303. 10.1136/gut.2005.075127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Nevé B, Brazeilles R, Derrien M, Tap J, Guyonnet D, Ohman L, et al. Lactulose Challenge Determines Visceral Sensitivity and Severity of Symptoms in Patients With Irritable Bowel Syndrome. Clinical Gastroenterology and Hepatology; 2/14/2016: Elsevier; 2016. p. 226–33. 10.1016/j.cgh.2015.09.039 [DOI] [PubMed] [Google Scholar]
- 23.Le Nevé B, Posserud I, Bohn L, Guyonnet D, Rondeau P, Tillisch K, et al. A Combined Nutrient and Lactulose Challenge Test Allows Symptom-Based Clustering of Patients With Irritable Bowel Syndrome. Am J Gastroenterol. 2013;108(5):786–95. Functional GI Disorders. 10.1038/ajg.2013.75 [DOI] [PubMed] [Google Scholar]
- 24.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325–32. 10.1136/gut.2008.167270 [DOI] [PubMed] [Google Scholar]
- 25.Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. A Meta-analysis Of Randomised Controlled Trials On The Effect Of Probiotics On Functional Constipation In Adults. Gut. 2014;63(Suppl 1):A196–A7. [DOI] [PubMed] [Google Scholar]
- 26.Hungin APS, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice–an evidence-based international guide. Alimentary Pharmacology & Therapeutics. 2013;38(8):864–86. 10.1111/apt.12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyonnet D, Schlumberger A, Mhamdi L, Jakob S, Chassany O. Fermented milk containing Bifidobacterium lactis DN-173 010 improves gastrointestinal well-being and digestive symptoms in women reporting minor digestive symptoms: a randomised, double-blind, parallel, controlled study. British Journal of Nutrition. 2009;102(11):1654–62. 10.1017/S0007114509990882 [DOI] [PubMed] [Google Scholar]
- 28.Marteau P, Guyonnet D, Lafaye de Micheaux P, Gelu S. A randomized, double-blind, controlled study and pooled analysis of two identical trials of fermented milk containing probiotic Bifidobacterium lactis CNCM I-2494 in healthy women reporting minor digestive symptoms. Neurogastroenterology & Motility. 2013;25(4):331–e252. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Alimentary Pharmacology & Therapeutics. 2009;29(1):104–14. 10.1111/j.1365-2036.2008.03853.x [DOI] [PubMed] [Google Scholar]
- 30.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional Bowel Disorders. Gastroenterology. 2006;130(5):1480–91. 10.1053/j.gastro.2005.11.061 [DOI] [PubMed] [Google Scholar]
- 31.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary Pharmacology & Therapeutics. 1997;11(2):395–402. 10.1046/j.1365-2036.1997.142318000.x [DOI] [PubMed] [Google Scholar]
- 32.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 33.Nordin STEV, Palmquist EVA, Nordin MARI. Psychometric evaluation and normative data for a Swedish version of the Patient Health Questionnaire 15-Item Somatic Symptom Severity Scale. Scandinavian Journal of Psychology. 2013;54(2):112–7. 10.1111/sjop.12029 [DOI] [PubMed] [Google Scholar]
- 34.Törnblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simrén M. Colonic Transit Time and IBS Symptoms: What's the Link? The American Journal Of Gastroenterology. 2012;107(May):754–60. 10.1038/ajg.2012.5 [DOI] [PubMed] [Google Scholar]
- 35.Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. 2017;152(1):111–23.e8. 10.1053/j.gastro.2016.09.049 [DOI] [PubMed] [Google Scholar]
- 36.Matsuda K, Tsuji H, Asahara T, Matsumoto K, Takada T, Nomoto K. Establishment of an Analytical System for the Human Fecal Microbiota, Based on Reverse Transcription-Quantitative PCR Targeting of Multicopy rRNA Molecules. Applied and Environmental Microbiology. 2009;75(7):1961–9. 10.1128/AEM.01843-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335 10.1038/nmeth.f.303 https://www.nature.com/articles/nmeth.f.303#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss S, Xu ZZ, Peddada S, Amir A, Bittinger K, Gonzalez A, et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome. 2017;5:27 10.1186/s40168-017-0237-y PMC5335496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennet SMP, Polster A, Törnblom H, Isaksson S, Capronnier S, Tessier A, et al. Global Cytokine Profiles and Association With Clinical Characteristics in Patients With Irritable Bowel Syndrome. The American Journal Of Gastroenterology. 2016;111(August):1165–76. 10.1038/ajg.2016.223 [DOI] [PubMed] [Google Scholar]
- 40.Martínez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C, et al. The Jejunum of Diarrhea-Predominant Irritable Bowel Syndrome Shows Molecular Alterations in the Tight Junction Signaling Pathway That Are Associated With Mucosal Pathobiology and Clinical Manifestations. The American Journal Of Gastroenterology. 2012;107(May):736–46. 10.1038/ajg.2011.472 https://www.nature.com/articles/ajg2011472#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 41.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, et al. Efficacy of an Encapsulated Probiotic Bifidobacterium infantis 35624 in Women with Irritable Bowel Syndrome. Am J Gastroenterol. 2006;101(7):1581–90. 10.1111/j.1572-0241.2006.00734.x [DOI] [PubMed] [Google Scholar]
- 42.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterology & Motility. 2005;17(5):687–96. 10.1111/j.1365-2982.2005.00695.x [DOI] [PubMed] [Google Scholar]
- 43.O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–51. 10.1053/j.gastro.2004.11.050 [DOI] [PubMed] [Google Scholar]
- 44.Yao CK, Barrett JS, Philpott H, Chung ART, van Langenberg D, Garg M, et al. Poor predictive value of breath hydrogen response for probiotic effects in IBS. Journal of Gastroenterology and Hepatology. 2015;30(12):1731–9. 10.1111/jgh.13015 [DOI] [PubMed] [Google Scholar]
- 45.McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Science Translational Medicine. 2011;3(106):106ra–ra. 10.1126/scitranslmed.3002701 PMC3303609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veiga P, Pons N, Agrawal A, Oozeer R, Guyonnet D, Brazeilles R, et al. Changes of the human gut microbiome induced by a fermented milk product. Scientific Reports. 2014;4:6328 10.1038/srep06328 http://www.nature.com/articles/srep06328#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maier L, Vyas R, Cordova Carmen D, Lindsay H, Schmidt Thomas Sebastian B, Brugiroux S, et al. Microbiota-Derived Hydrogen Fuels Salmonella Typhimurium Invasion of the Gut Ecosystem. Cell Host & Microbe. 2013;14(6):641–51. 10.1016/j.chom.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 48.Sahota SS, Bramley PM, Menzies IS. The Fermentation of Lactulose by Colonic Bacteria. Microbiology. 1982;128(2):319–25. 10.1099/00221287-128-2-319 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methanobacteriales were detected in fecal DNA by qPCR A) at baseline in Low (n = 54) and High (n = 24) H2 producers and B) stratified by study group, before and after intervention. Blautia and Desulfovibrio 16S RNA-seq reads abundance were depicted into tertiles. Low, medium and high corresponded respectively to the first, second and third tertiles. Subject prevalence for each tertile was indicated at baseline in respectively C) E) and stratified by study group, before and after intervention respectively in D) F); High H2 control (n = 9), Low H2 control (n = 29), High H2 FMP (n = 15), Low H2 FMP (n = 25).
(TIFF)
Samples were compared between high H2 (n = 24) and low H2 (n = 54) producers after intervention.
(TIFF)
(DOCX)
(PDF)
(DOC)
Data Availability Statement
The consent forms which were obtained by the study sponsor do no permit sharing any personal data, in conformity with the GDPR. We can share part of the data in anonymized and summarized form with group level data upon request, but any data that in any way may jeopardize anonymity of the subjects or contain sensitive information such as symptom and demographic information cannot be shared for ethical reasons, since this was not included in the ethical approval, and data sharing was not included in the patient information and therefore the subjects have not agreed to share their data with other research groups than the researchers directly involved in this study. Contact information for data requests: Regional Ethical Review Board in Gothenburg, Regionala etikprövningsnämnden, Box 401, 405 30 Göteborg, Sweden (email: registrator@etikprovning.se Phone: 010-475 08 00).