Abstract
Background
The use of histamine-2 receptor antagonists (H2RA) in neonates is still debated because of possible risk of infection, necrotizing enterocolitis (NEC) and increased mortality.
Aim
To review whether the use of H2RA in neonates admitted to neonatal intensive care units (NICU) is associated with infection, NEC or mortality.
Materials and method
We performed a systematic search in PubMed, Web of Science and SCOPUS databases using the terms “histamine-2 receptor antagonists”, “infection”, “necrotizing enterocolitis”, “mortality”, “neonates” and related terms to identify studies published up to April 30, 2017. We included studies conducted in hospitalized neonates and exposed to H2RA. The primary outcomes were infection, NEC and mortality. We included reports of infections with clinical signs and positive culture, and NEC according to Bell stages (stage ≥II) based on standardised clinical and radiologic criteria. Among 1,144 studies identified, 10 fulfilled the selection criteria. Information extracted included study design, sample size and number of participants, along with the outcomes of interest. We conducted a meta-analysis of adjusted data and pooled estimates of infection, NEC and mortality are reported as odds ratios (OR) and 95% confidence intervals (95%CI).
Results
Ten studies were analysed. There were substantial associations between H2RA and infection (pooled OR: 2.09; 95%CI: 1.35–3.24; P = 0.001) and NEC (pooled OR: 2.81, 95%CI: 1.19–6.64; P = 0.02) but not with the mortality risk (pooled OR: 1.76; 95%CI: 0.50–6.16; P: 0.38).
Conclusion
Current evidence suggests that H2RA is associated with an increased risk of infection and NEC, but not with mortality in neonates admitted to NICU. The use of H2RA in neonates must be stringently considered when necessary.
Introduction
Histamine-2 receptor antagonists (H2RA) are often prescribed off-label to neonates admitted to neonatal intensive care units (NICU) [1] for prophylaxis or therapy of stress ulcers and gastroesophageal reflux disease (GERD). However, the safety and efficacy of H2RA in neonates is still debated [2]. This is due to gastric acid secretions being one of the main non-immune defenses against invading pathogens [3] and the sustained inhibition of gastric acid secretions alters the bacterial ecology favoring the gastric colonization of enteric bacteria and may facilitate microbial translocation across the gut barrier because of decreased neutrophil activity [4,5]. Studies have shown an increasing gastric pH within few minutes of H2RA administration [6,7], with effects on the H2 receptors activation and modelling of the immune responses, especially in the production of inflammatory cytokines [8–10].
A 2014 systematic review of clinical trials conducted in 1 to 15 years old children reported that H2RA were effective in reducing GERD signs and symptoms, but did not report adverse effects in a measurable manner, precluding a quantitative analysis on drug safety [11]. Other studies in neonates however have shown that H2RA may predispose to infections [3,12–14], necrotising enterocolitis (NEC) [14,15] and death [14,16], but there are no systematic analyses of this evidence.
We conducted a systematic review and meta-analysis to investigate whether the use of H2RA in neonates admitted to NICU is associated with an increased risk of infection, NEC and mortality.
Materials and methods
This study was conducted following the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) statement (S1 File) [17]. Institutional review board approval and informed consent were not required for this systematic review and meta-analysis. A study protocol was designed a priori and was registered in the PROSPERO database (registration number CRD42017060887).
Search strategy and selection criteria
We performed a systematic review using PubMed, Web of Science and SCOPUS databases to identify studies published up to April 30, 2017 without language restriction. Publications were identified using the terms “histamine-2 receptor antagonists”, “infection”, “necrotizing enterocolitis”, “mortality”, “neonates” and related terms. The full search strategy is described in the S1 Table. Two independent reviewers (MSF and RNSS) screened titles and abstracts for relevance and adequacy and disagreements were resolved by VSS and RQG. The manuscripts selected were read in full to confirm their eligibility and their reference lists were scanned to identify additional studies. We included studies conducted with neonates hospitalized in NICU and exposed to H2RA. We excluded studies in infants over 28 days old, those not containing original material or reporting data from ambulatory patients and studies including neonates with infections before initiating H2RA, congenital malformations or genetic syndromes, mothers with HIV, rubella, toxoplasmosis, cytomegalovirus or hepatitis B and C.
Outcomes
The primary outcomes were infection, NEC and mortality. We included reports of nosocomial infections with clinical signs and positive culture. NEC was classified according to Bell stages and included children with Bell stage ≥II [18].
Secondary outcomes included pneumonia, sepsis and urinary tract infections (UTI). For these outcomes, we consider studies that had defined a) pneumonia as the presence of clinical signs associated with positive culture or radiological findings with suggestive signals of pulmonary involvement by infectious agents (persistent infiltrate, consolidation and cavitation) and abnormal laboratory tests; b) sepsis as the presence of signs suggestive of infection associated with a positive blood culture, and c) UTI when there was a positive urine culture together with clinical findings.
We analyzed the mortality at any time during the follow-up period, as reported in the study included in the meta-analysis.
Data extraction and bias assessment
We used pre-formatted tables for data extraction, including author, publication year, country, study design, sample size, number of participants with infections, NEC or death by H2RA exposure. Not all studies reported the absolute numbers of the outcomes and frequencies were calculated from percentages. For articles not available in electronic databases or data unavailable in the articles included, we attempted to contact the authors to obtain relevant information. We had planned to extract data on the exposure time and dosage of H2RA to ascertain duration of exposure and a dose safety gradient; however, it was not possible to obtain the data for meta-analysis. The risk of bias for individual studies was assessed by two independent reviewers using the Newcastle-Ottawa Scale (NOS) [19] and disagreements were resolved by discussion.
Statistical analysis
We calculated the pooled odds ratio (OR) for the primary and secondary outcomes and used forest plots to present effect sizes with 95% confidence intervals (95%CI). Pooled unadjusted and adjusted estimates were calculated using Mantel-Haenszel and inverse variance methods, respectively. The meta-analysis was performed using random-effects model. Two-tailed P-values <0.05 were used to determine statistical significance. Statistical heterogeneity was assessed using the Cochran Q test [20] and quantified by the I2 index [21]. A subgroup analysis was performed according to the study design (cohort or case-control).
Leave-one-out sensitivity analysis was conducted by omitting one study at a time and examining the influence of each study on the pooled effect size [22]. Analyses were performed using Review Manager 5.3 (Cochrane IMS, Copenhagen, Denmark) and R-3.3.2 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
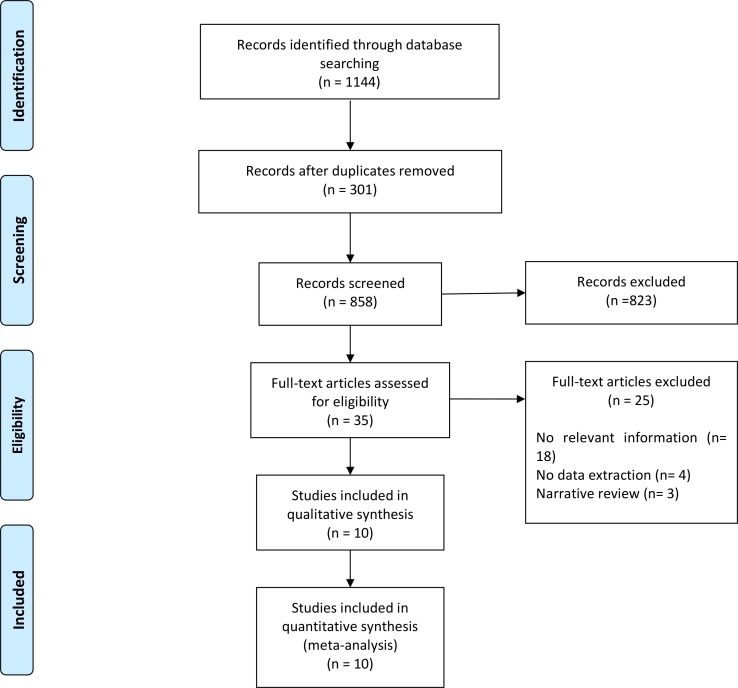
The literature search identified 1,144 records. After screening titles and abstracts, 35 full-text articles were assessed for eligibility and 10 were included (Fig 1). Table 1 summarizes the main characteristics of the 10 studies. Five studies used a case-control design [13,23–26] and five were cohorts [14,16,27–29]. No clinical trials were found. Nine studies focused on very-low birth weight babies [13,14,16,23,25–29] and one considered the whole preterm population (gestational age <37 weeks) [24]. Most studies reported only infection as an outcome [13,14,25,27–29], two reported only NEC [23,24] and four included the three main outcomes of infection, NEC and mortality [14,16,26,29].
Fig 1. Flowchart of studies for inclusion in the meta-analysis.
Table 1. Main characteristics of the studies analysed.
| Study | Country | Study design | Population | Subject characteristics | Risk factors used | Statistics strategy | Outcome |
|---|---|---|---|---|---|---|---|
| Rojas et al. (2005) [27] | Colombia | Cohort prospective | Very Low Birth Weight | Birth weight: <2000g. Gestational age: <35 weeks | Maternal factors: age, fever (>38°C), prenatal antibiotics, prenatal steroids, premature ruptured membranes, type of delivery (vaginal, elective caesarean section, emergency). Infant factors: birth weight, gestational age, gender, mechanical ventilation, oxygen, postnatal antibiotics, catheters (central and non-central), parenteral nutrition and gastric tube for enteral nutrition. | Univariate and Multivariate logistic regression | Infection |
| Guillet et al. (2006) [23] | USA | Case-control | Very Low Birth Weight | Birth weight: 401g-1500g. Gestational age: not available | Maternal factors: not available. Infant factors: birth weight, gender, race, site of birth and Apgar score. | Univariate and Multivariate logistic regression | Necrotizing enterocolitis |
| Bianconi et al. (2007) [13] | USA | Case-control | Very Low Birth Weight | Birth weight: not available. Gestational age: not available | Maternal factors: not available. Infant factors: birth weight, gestational age, gender, length of stay in NICU, duration of total parenteral nutrition, pharmaceutical substances used and duration for central vascular catheters. | Univariate analysis | Infection |
| Afjeh et al. (2012) [28] | Iran | Cohort retrospective | Very Low Birth Weight | Birth weight: <1500g. Gestational age: <37 weeks | Maternal factors: age, fever (>38°C), prenatal antibiotics, prenatal steroids, premature ruptured membranes, type of delivery (vaginal, elective caesarean section, emergency). Infant factors: birth weight, gestational age, gender, Apgar score, intubation at delivery room, duration of mechanical ventilation, duration of oxygen therapy, postnatal antibiotics, chest tube, catheters (central and non-central), parenteral nutrition and gastric tube for enteral nutrition. | Univariate and Multivariate logistic regression | Infection |
| Terrin et al. (2012) [14] | Italian | Cohort prospective | Very Low Birth Weight | Birth weight: 401-1500g. Gestational age: 24–32 weeks | Maternal factors: age, fever (>38°C), prenatal antibiotics, prenatal steroids, premature ruptured membranes, type of delivery (vaginal, elective or emergency caesarean section). Infant factors: birth weight, gestational age, gender, Apgar, duration of mechanical ventilation, oxygen therapy, postnatal antibiotics, catheters (central and non-central), parenteral nutrition and gastric tube for enteral nutrition. |
Univariate and Multivariate logistic regression | Infection, necrotizing enterocolitis and mortality |
| Bilali et al (2013) [24] | Greece | Case-control | Pre-term | Birth weight: not available. Gestational age: <37 weeks | Not available. | Univariate and Multivariate logistic regression | Necrotizing enterocolitis |
| Gupta et al. (2013) [25] | USA | Case-control | Very Low Birth Weight | Birth weight: <1500g Gestational age: <34 weeks |
Maternal factors: age, fever (oral temperature >38°C), antibiotics, steroids, caffeine use, premature ruptured membranes, chorioamnionitis, type of delivery. Infant factors: birth weight, gestational age, gender and formula feeding. |
Univariate analysis | Infection |
| Singh et al. (2016) [26] | Australia | Case-control | Very Low Birth Weight | Birth weight: < 1500g Gestational age: not available |
Maternal factors: not available. Infant factors: birth weight, gestational age, gender, Apgar, patent ductus arteriosus, mechanical ventilation, oxygen therapy, continuous positive airway pressure, postnatal antibiotics, catheters (central and non-central), parenteral nutrition, type of feeding, gastric tube for enteral nutrition and length of hospital stay. |
Univariate analysis | Infection, necrotizing enterocolitis and mortality |
| Romaine et al. (2016) [16] | USA | Cohort prospective | Very Low Birth Weight | Birth weight: <1500g. Gestational age (median): <32 weeks | Maternal factors: type of delivery (vaginal or caesarean section). Infant factors: birth weight, gestational age, gender, Apgar, mechanical ventilation, oxygen therapy, inotropic support and neutropenia. |
Univariate analysis | Infection, necrotizing enterocolitis and mortality |
| Santana et al. (2017) [29] | Brazil | Cohort retrospective | Very Low Birth Weight | Birth weight (median): <1500g;.Gestational age (median): <34 weeks | Maternal factors: age, fever (oral temperature >38°C), prenatal care, premature rupture of membranes, type of delivery (vaginal or caesarean section), hypertension, Diabetes mellitus, gestational diabetes. Infant factors: birth weight, gestational age, gender, Apgar, duration of mechanical ventilation, oxygen therapy, duration of catheters (central and non-central), duration of parenteral nutrition and duration of gastric tube for enteral nutrition. |
Univariate analysis | Infection, necrotizing enterocolitis and mortality |
The risk of bias assessments are summarized in Tables 2 and 3, respectively. Overall, cohort studies had a lower risk of bias than case-control studies. The use of different criteria across studies for the selection of comparison groups (not exposed to H2RA) may have introduced a high risk of bias, especially among case-control studies.
Table 2. Assessment of study quality and risk of bias from case-control studies.
| Study | Selection | Comparability | Exposure | |||||
|---|---|---|---|---|---|---|---|---|
| Adequate case definition | Representativeness of cases | Selection of controls | Definition of controls | Case and control are comparable | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | |
| Guillet et al., 2006 [23] | Yes | Yes | No | No | Yes | Yes | Yes | No |
| Bianconi et al., 2007 [13] | Yes | No | No | No | Yes | Yes | Yes | No |
| Bilali et al., 2013 [24] | Yes | Yes | No | Yes | Yes | Yes | Yes | No |
| Gupta et al., 2013 [25] | Yes | Yes | No | No | Yes | No | Yes | No |
| Singh et al., 2016 [26] | Yes | Yes | No | Yes | Yes | Yes | Yes | No |
Table 3. Assessment of study quality and risk of bias from cohort studies.
| Study | Selection | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposure cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Without outcome in the start | Cohorts are comparable | Assessment of outcome | Length of follow-up | Adequacy of follow-up | |
| Rojas et al., 2005 [27] | No | No | Yes | No | Yes | Yes | No | Yes |
| Afjeh et al., 2012 [28] | No | No | Yes | No | Yes | Yes | Yes | Yes |
| Terrin et al., 2012 [14] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Romaine et al., 2016 [16] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Santana et al., 2017 [29] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
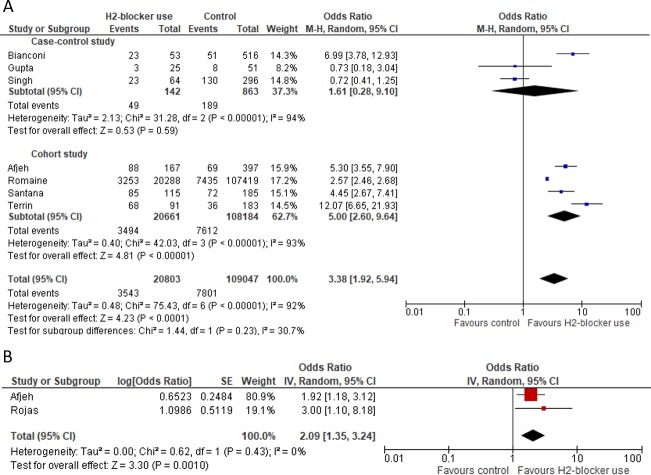
Seven studies involving 129,850 subjects were included in the pooled OR estimation for infection. Of these, 3,543 (17.0%) of 20,803 neonates receiving H2RA had infections compared to 7,801 (7.2%) of 109,047 not exposed to H2RA, resulting in a pooled OR of 3.38 (95%CI: 1.92–5.94; P <0.001) (Fig 2A). There was substantial between-study heterogeneity (I2: 92%; 95%CI: 86.2% - 95.4%) and the sub-group meta-analysis demonstrated cohort studies influenced substantially the pooled OR. Based on studies [27,28] that had adjusted values, the pooled OR for infection was 2.09 (95%CI: 1.35–3.24; P<0.001) and the between-study heterogeneity was 0% (Fig 2B).
Fig 2. Forest Plot for infection outcome.
A) unadjusted and B) adjusted pooled OR for infection.
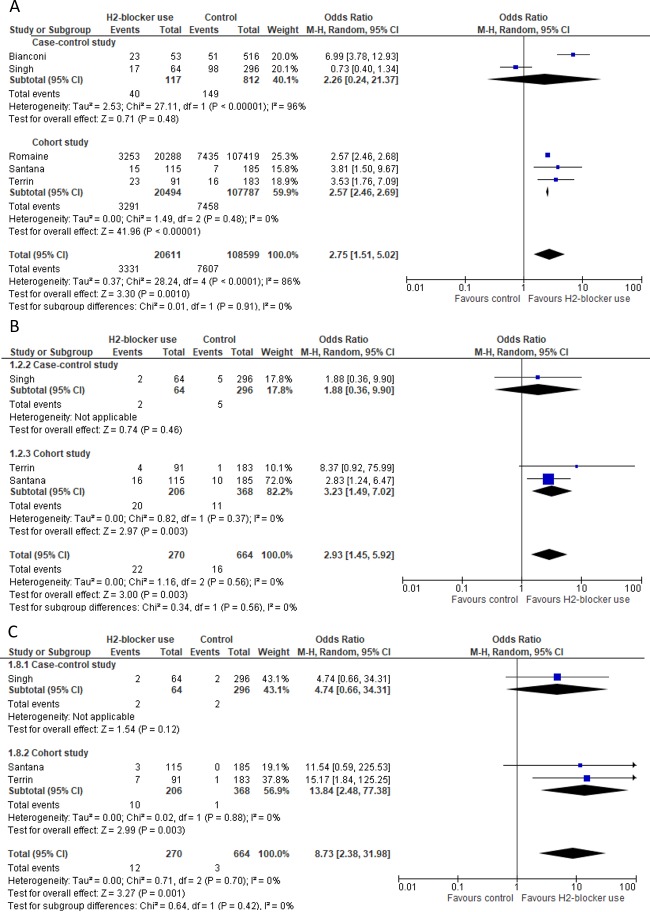
Some studies presented data for infection categories (Fig 3A–3C). Five studies had data for sepsis [13,14,16,26,29] and the pooled unadjusted OR was 2.75 (95%CI: 1.51–5.02; P: 0.001) (I2: 86%; 95%CI: 68.9%-93.6%). Subgroup analysis showed an association between sepsis and H2RA in cohort studies (OR: 2.57; 95%CI: 2.46–2.69; P <0.001) (I2: 0%; 95%CI: 0%-86.0%). Three studies reported pneumonia and urinary tract infections [14,26,29] with pooled ORs of 2.93 (95%CI: 1.45–5.92; P: 0.003) (I2: 0%; 95%CI: 0%-82.1%) and 8.73 (95%CI: 2.38–31.98; P: 0.001) (I2: 0%; 95%CI 0%-70.7%), respectively.
Fig 3. Forest Plot for sepsis, pneumonia and urinary tract infection.
A) Unadjusted pooled OR for sepsis, B) Unadjusted pooled OR for pneumonia and C) Unadjusted pooled OR for urinary tract infection.
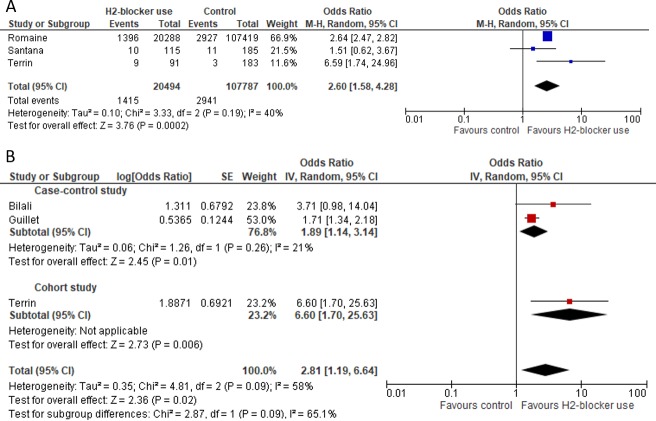
Unadjusted analyses from the three cohort studies evaluating NEC [14,16,29] indicated an association with H2RA (Fig 4A). Likewise, the meta-analysis of adjusted data [14,23,24] reported substantial association between NEC and H2RA (pooled OR: 2.81; 95%CI: 1.19–6.64; P: 0.02) (I2: 58%; 95% CI 0%-88.2%) (Fig 4B).
Fig 4. Forest Plot for necrotizing enterocolitis (NEC).
A) Unadjusted and B) Adjusted pooled OR for NEC.
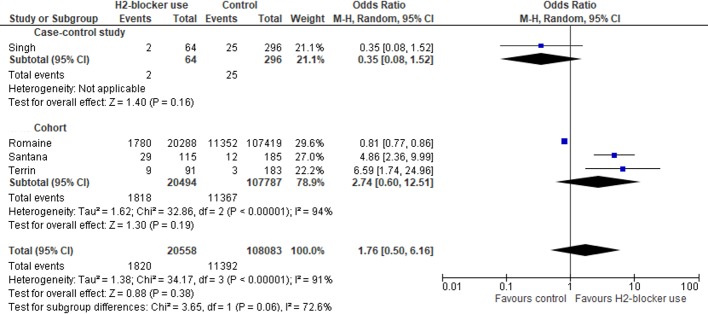
Fig 5 shows that the use of H2RA was not associated with mortality (pooled OR: 1.76; 95%CI: 0.50–6.16; P: 0.38) (I2: 83%; 95% CI: 80.6%-96.0%).
Fig 5. Forest Plot for mortality outcome.
Discussion
The frequent off-label use of H2RA in neonates steered the European Medicines Agency and the US Food and Drug Administration to encourage studies on their safety and agencies responsible for drug regulations increased their search for information on their adverse effects in paediatric populations [2,14]. These initiatives has resulted in an increased number of studies, providing an opportunity to further ascertain adverse effects. In this systematic review and meta-analysis, we found that the use of H2RA in neonates is associated with increased risk of infections and NEC, but not with mortality.
Gastric fluid is an important non-immune barrier against pathogens [3] and the sustained inhibition of gastric acid secretion increases the pH and modifies the gastric microbiota [4,5]. The effects of H2RA administration are not restricted to the gastric pH, since they also increase the production of pro-inflammatory cytokines and reduce immunological responses to infection [8–10].
The main reasons for the prescription of H2RA in NICUs are the management of GERD and the prophylaxis and treatment of stress ulcers, usually caused by other drugs [11]. However, neonates receiving H2RA are more likely to exhibit GERD-like symptoms, resulting in a false perception that GERD is persisting, leading in turn to an increase of drug dosage and treatment duration [7]. Several studies have reported an average of 18 days between H2RA administration and the occurrence of infection [14,30] and 19 days for the occurrence of NEC [23], although one study from Brazil reported that infections started 6 days after H2RA use [29].
Only two studies evaluated whether the H2RA dosage was associated with unfavourable outcomes, as neonates who developed infection or NEC had received higher doses than children without these outcomes, but these differences were not statistically significant [14,29]. It is also noteworthy that the studies had used a wide H2RA dose ranges, making it difficult to identify a safe and effective dose.
This meta-analysis also found an association between H2RA use and pneumonia, sepsis, and UTI. It is well established that the inhibition of gastric acid secretion alters the bacterial ecology favoring gastric colonization by enteric bacteria and may facilitate microbial translocation across the stomach barrier [4,5], which may contribute to the development of pneumonia and sepsis [31]. Although the increased risk of pneumonia and sepsis involves gastric colonization with gram-negative bacteria [31], the results of the studies included in this review do not support this assertion. Although Rojas et al. (2005) [27] and Terrin et al. (2012) [14] studies reported a higher prevalence of gram-negative microorganisms, et al. (2007) [13] reported the same proportion of gram-negative and positive microorganisms among neonates receiving and not receiving H2RA.
The prolonged use of mechanical ventilation, central and peripheral catheters, parenteral nutrition and other devices in NICU is also associated with an increased risk of infection [32–37]. Although some studies in this meta-analysis controlled for these factors [14,23,24,27–29], = , the regression model still identified an association with H2RA.
Gestational age and low birth weight also increase the risk of nosocomial infections and NEC[38]. However, after controlling for these factors, the use of H2RA was independently associated with infection and NEC.
Although there is not enough information to assess whether H2RA increase the length of hospitalization, two studies reported a potential increase in hospitalization time [14,29], and further studies should be encouraged to generate this information in neonates.
The combined analysis of case-control and cohort studies did not show an association between H2RA use and mortality. However, there was large heterogeneity between studies and a paucity of quality data to examine the effect of H2RA and mortality, which may have resulted in a type II error. Further studies are needed to generate sufficient data to examine this association.
Our findings should be interpreted with caution as the number of studies showing the adverse effects for H2RA use in neonates admitted to NICU is small. All studies included were observational and treatments were not randomised. While the meta-analysis of cohort studies showed an association between H2RA and increased risk of infection, NEC and death, these associations were not fully evident in the case-control studies. Some studies had poor quality which may increase the risk of bias. Moreover, it was not possible to perform a funnel plot analysis due to the small number of studies. Finally, it was not possible to identify safe dosage thresholds or usage time for H2RA in neonates due to the scarcity of data and the wide variation reported between and within the studies.
Despite these limitations, current available evidence shows an association between the use of H2RA, the risk of infections and NEC in neonates. Further safety studies including well defined patient groups are needed to increase the evidence for their safe use in neonates and to support the development of guidelines by regulatory agencies. In the meantime, the use of H2RAin neonates must be stringently considered, when necessary.
Supporting information
(DOCX)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Barney CK, Baer VL, Scoffield SH, Lambert DK, Cook M, Christensen RD. Lansoprazole, Ranitidine, and Metoclopramide. Adv Neonatal Care. 2009;9: 129–131. 10.1097/ANC.0b013e3181a88358 [DOI] [PubMed] [Google Scholar]
- 2.Terrin G, Canani RB, Passariello A, Caoci S, De Curtis M. Inhibitors of gastric acid secretion drugs increase neonatal morbidity and mortality. J Matern Neonatal Med. 2012;25: 77–79. 10.3109/14767058.2012.714975 [DOI] [PubMed] [Google Scholar]
- 3.Malcolm WF, Cotten CM. Metoclopramide, H 2 Blockers, and Proton Pump Inhibitors: Pharmacotherapy for Gastroesophageal Reflux in Neonates. Clin Perinatol. Elsevier Inc; 2012;39: 99–109. 10.1016/j.clp.2011.12.015 [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, et al. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med. 2006;203: 2907–17. 10.1084/jem.20061232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. British Journal of Pharmacology. 2013. pp. 38–45. 10.1111/bph.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana M, Tornaghi R, Petrillo M, Lora E, Bianchi Porro G, Principi N. Ranitidine treatment in newborn infants: effects on gastric acidity and serum prolactin levels. J Pediatr Gastroenterol Nutr. 1993;16: 406–11. Available: http://www.ncbi.nlm.nih.gov/pubmed/8315550 [PubMed] [Google Scholar]
- 7.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, a. J Pediatr Gastroenterol Nutr. 2009;49: 498–547. 10.1097/MPG.0b013e3181b7f563 [DOI] [PubMed] [Google Scholar]
- 8.Frei R, Ferstl R, Konieczna P, Ziegler M, Simon T, Rugeles TM, et al. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J Allergy Clin Immunol. 2013;132 10.1016/j.jaci.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 9.Jutel M, Akdis M, Akdis C a. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009;39: 1786–1800. 10.1111/j.1365-2222.2009.03374.x [DOI] [PubMed] [Google Scholar]
- 10.Takagaki K, Osawa S, Horio Y, Yamada T, Hamaya Y, Takayanagi Y, et al. Cytokine responses of intraepithelial lymphocytes are regulated by histamine H(2) receptor. J Gastroenterol. 2009;44: 285–296. 10.1007/s00535-009-0019-9 [DOI] [PubMed] [Google Scholar]
- 11.Van Der Pol R, Langendam M, Benninga M, Van Wijk M, Tabbers M. Efficacy and safety of histamine-2 receptor antagonists. JAMA Pediatr. 2014;168: 947–954. 10.1001/jamapediatrics.2014.1273 [DOI] [PubMed] [Google Scholar]
- 12.Canani RB, Terrin G. Gastric acidity inhibitors and the risk of intestinal infections. Curr Opin Gastroenterol. 2010;26: 31–35. 10.1097/MOG.0b013e328333d781 [DOI] [PubMed] [Google Scholar]
- 13.Bianconi S, Gudavalli M, Sutija VG, Lopez AL, Barillas-Arias L, Ron N. Ranitidine and late-onset sepsis in the neonatal intensive care unit. Journal of Perinatal Medicine. 2007. pp. 147–150. 10.1515/JPM.2007.017 [DOI] [PubMed] [Google Scholar]
- 14.Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, et al. Ranitidine is Associated With Infections, Necrotizing Enterocolitis, and Fatal Outcome in Newborns. Pediatrics. 2012;129: e40–e45. 10.1542/peds.2011-0796 [DOI] [PubMed] [Google Scholar]
- 15.Patole S. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis: a case of excessive collateral damage? Pediatrics. 2006;117: 531–532. 10.1542/peds.2005-2230 [DOI] [PubMed] [Google Scholar]
- 16.Romaine A, Ye D, Ao Z, Fang F, Johnson O, Blake T, et al. Safety of histamine-2 receptor blockers in hospitalized VLBW infants. Early Hum Dev. 2016;99: 27–30. 10.1016/j.earlhumdev.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin J a, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283: 2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 18.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978; 10.1097/00000658-197801000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 20.Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10: 101 10.2307/3001666 [DOI] [Google Scholar]
- 21.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21: 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 22.Sterne JAC, Egger M, Smith GD. Investigating and Dealing with Publication and Other Biases. Systematic Reviews in Health Care: Meta-Analysis in Context: Second Edition. 2008. pp. 189–208. 10.1002/9780470693926.ch11 [DOI] [Google Scholar]
- 23.Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117 10.1542/peds.2005-0664 [DOI] [PubMed] [Google Scholar]
- 24.Bilali A, Galanis P, Bartsocas C, Sparos L, Velonakis E. H2-blocker therapy and incidence of necrotizing enterocolitis in preterm infants: A case-control study. Pediatr Neonatol. 2013;54: 141–142. 10.1016/j.pedneo.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 25.Gupta RW, Tran L, Norori J, Ferris MJ, Eren AM, Taylor CM, et al. Histamine-2 receptor blockers alter the fecal microbiota in premature infants. J Pediatr Gastroenterol Nutr. 2013;56: 397–400. 10.1097/MPG.0b013e318282a8c2 [DOI] [PubMed] [Google Scholar]
- 26.Singh N, Dhayade A, Mohamed A-L, Chaudhari TV. Morbidity and Mortality in Preterm Infants following Antacid Use: A Retrospective Audit. Int J Pediatr. 2016;2016: 1–6. 10.1155/2016/9649162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojas MA, Efird MM, Lozano JM, Bose CL, Rojas MX, Rondón MA, et al. Risk factors for nosocomial infections in selected neonatal intensive care units in Colombia, South America. J Perinatol. 2005;25: 537–541. 10.1038/sj.jp.7211353 [DOI] [PubMed] [Google Scholar]
- 28.Afjeh SA, Sabzehei MK, Karimi A, Esmaili F. Ranitidin and nosocomial infection in very low birth weight infants. Arch Pediatr Infect Dis. 2013;1: 65–70. 10.5812/pedinfect.9051 [DOI] [Google Scholar]
- 29.Santana RNS, Santos VS, Ribeiro-Júnior RF, Freire MS, Menezes MAS, Cipolotti R, et al. Use of ranitidine is associated with infections in newborns hospitalized in a neonatal intensive care unit: A cohort study. BMC Infect Dis. BMC Infectious Diseases; 2017;17: 1–6. 10.1186/s12879-016-2122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palčevski G, Skočibušić N, Vlahović-Palčevski V. Unlicensed and off-label drug use in hospitalized children in Croatia: A cross-sectional survey. Eur J Clin Pharmacol. 2012;68: 1073–1077. 10.1007/s00228-012-1221-x [DOI] [PubMed] [Google Scholar]
- 31.Apte NM, Karnad DR, Medhekar TP, Tilve GH, Morye S, Bhave GG. Gastric colonization and pneumonia in intubated critically ill patients receiving stress ulcer prophylaxis: A randomized, controlled trial. Crit Care Med. 1992; 10.1097/00003246-199205000-00008 [DOI] [PubMed] [Google Scholar]
- 32.Bancalari E, Claure N. Weaning preterm infants from mechanical ventilation. Neonatology. 2008. pp. 197–202. 10.1159/000143722 [DOI] [PubMed] [Google Scholar]
- 33.Sant’Anna GM, Keszler M. Weaning infants from mechanical ventilation. Clinics in Perinatology. 2012. pp. 543–562. 10.1016/j.clp.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 34.Casner M, Hoesli SJ, Slaughter JC, Hill M, Weitkamp J-H. Incidence of catheter-related bloodstream infections in neonates following removal of peripherally inserted central venous catheters. Pediatr Crit Care Med. 2014;15: 42–8. 10.1097/PCC.0b013e31829f5feb [DOI] [PubMed] [Google Scholar]
- 35.Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream Infection, Venous Thrombosis, and Peripherally Inserted Central Catheters: Reappraising the Evidence. Am J Med. 2012;125: 733–741. 10.1016/j.amjmed.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 36.Uslu S, Ozdemir H, Comert S, Bolat F, Nuhoglu a. The effect of low-dose heparin on maintaining peripherally inserted percutaneous central venous catheters in neonates. J Perinatol. 2010;30: 794–799. 10.1038/jp.2010.46 [DOI] [PubMed] [Google Scholar]
- 37.Yildizdas D, Yapicioglu H, Levent Yilmaz H. Occurrence of ventilator-associated pneumonia in mechanically ventilated pediatric intensive care patients during stress ulcer prophylaxis with sucralfate, ranitidine, and omeprazole. J Crit Care. 2002;17: 240–245. 10.1053/jcrc.2002.36761 [DOI] [PubMed] [Google Scholar]
- 38.Bartels DB, Schwab F, Geffers C, Poets CF, Gastmeier P. Nosocomial infection in small for gestational age newborns with birth weight <1500 g: a multicentre analysis. Arch Dis Child Fetal Neonatal Ed. 2007;92: F449–53. 10.1136/adc.2006.114504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.