Abstract
Background
Postpartum depression (PPD) is a common mental health condition that can compromise the quality of life and functional capacity of mothers and cause health and developmental problems in children born to affected mothers.
Objectives
We set out to measure the prevalence of PPD comparing postpartum HIV-1 infected women with pre-pregnancy HIV care experience, newly diagnosed (in latest pregnancy) HIV-1 infected women and HIV negative women, and to identify predictors of major PPD among these women in a peri-urban clinic in South Africa.
Methods
We conducted a cross-sectional survey of 1151 adult (≥18 years) postpartum HIV-1 infected (690) and HIV negative (461) women who delivered up to 30 days before study enrolment, interviewed after their first post-natal visit (3–6 days post- delivery) at Midwife Obstetric Units in Gauteng, South Africa. PPD was categorised into no depression (CES-D 10 total score <5), low to medium depression (CES-D 10 total score ≥5 and <10) and major depressive symptoms (CES-D 10 total score≥10). We used ordered logistic regression to identify predictors of postpartum depression and report adjusted odds ratio (aOR) and 95% confidence intervals (CIs).
Results
Overall 288 (25.0%) women screened positive for postpartum depression, a total of 168 (14.6%) women had low to medium PPD and 120 (10.4%) had major PPD. A higher proportion of HIV negative women experienced PPD, 129/461 (28.0%) among HIV negative vs. 159/690 (23.0%) among HIV-1 infected. Among HIV positive women, there was no meaningful difference in PPD between newly HIV diagnosed and those diagnosed before the most recent pregnancy (aOR 1.3, 95% confidence interval (CI): 0.9–1.8). Predictors of PPD among HIV positive women were living with friends/in a house-share (aOR 0.5 for house-share vs. own home, 95% CI: 0.3–0.9), and attending antenatal care (ANC) for the most recent pregnancy (aOR 0.2 for ANC attendance vs. no ANC attendance, 95% CI: 0.0–0.5). Living with friends/in a house-share was also a predictor of PPD among HIV negative women (aOR 0.4 for house-share vs. own home, 95% CI: 0.2–0.8).
Conclusions and recommendations
Targeted symptom screening based on identified risk factors should be considered for postpartum women to increase PPD case-finding and referral to specialised social support services.
Background
Globally, maternal postpartum depression (PPD) is a major risk factor for non-obstetric postnatal maternal and child morbidity as well as mortality [1–3]. The proportion of postnatal women diagnosed with PPD varies widely from 10–15% in high-income countries to 30–60% in sub-Saharan Africa [4–7]. Untreated PPD can lead to chronic depression, disruptions of family and marital relationships, and can cause long-term health and developmental problems in children of affected women [5, 8]. Early diagnosis and treatment of PPD improves the prognosis for both mother and child [9, 10]. However, in high-income countries, almost 50% of PPD cases are undiagnosed, with only 30% of diagnosed cases receiving treatment [11–13]. In South Africa, an estimated 30–50% of women are likely to develop PPD [7, 14, 15], and although PPD-specific treatment estimates are unavailable, overall 75% of patients’ mental health problem, including depression, do not access treatment in South Africa [16, 17].
Infants are affected by their mother’s mental health problems as they depend on their mothers for their developmental and nutritional needs [18, 19]. Women with depressive symptoms report lower rates of breastfeeding compared to women without depressive symptoms (49% vs 61%) [19, 20]. A meta-analysis of studies from developing countries found that children of mothers with PPD were 50% more likely to be underweight or stunted [18]. Furthermore, depressed mothers are often disengaged from their infants which may lead to slower cognitive development, behavioural problems and long-term psychological difficulties [19, 21–24]. The quality of infant-mother relationships appears to predict behavioural problems and disruption of cognitive abilities in children [23]. Evidence also suggests that children of depressed mothers also experience higher mortality rates. A study conducted in Taiwan, which examined mortality of children up to age five years, found that children were at a 47% greater risk of death if their mother was depressed [25]. Similarly, a cohort study in Ghana reported a nearly three-fold increase in the risk of mortality by six months among infants born to women diagnosed with PPD [3].
Predictors of PPD in the South African context include relational factors such as marital status and partner’s financial/ moral support, an unplanned/unwelcome baby, infant health conditions as well as the mother’s educational attainment and employment situation [7, 14]. Additionally, personal or family history of depression, recent stressful life events, high childcare stress, low self-esteem and neuroticism are important factors [24, 26, 27]. However, the impact of exposure to HIV care either through the HIV diagnostic procedures and ARV treatment before pregnancy in mitigating the risk of PPD among HIV positive women is not well described.
In general, rates of PPD are high among women living with HIV, with PPD rates of above 40% in high HIV prevalence settings [5, 7, 15, 28, 29]. Many women learn about their HIV infection during, or shortly after a pregnancy which can adversely impact on their mental health and compromise their participation in antenatal care (ANC) including the prevention of mother-to-child transmission (PMTCT) programs and antiretroviral therapy adherence [6, 30, 31]. Therefore, screening, referral for diagnosis and treatment of PPD among postpartum HIV-1 infected women is vital for the attainment of the HIV care and treatment goals for both the mothers and their infants [32, 33].
It is unclear whether psychosocial support associated with HIV care/treatment positively impacts on the risk of PPD among HIV-1 infected with pre-pregnancy HIV care experience. In this study, we set out to measure the prevalence of PPD comparing HIV-1 infected women with pre-pregnancy HIV care experience, newly diagnosed (in latest pregnancy) HIV-1 infected women and HIV negative women, and to identify predictors of having depressive symptoms among these women.
Materials and methods
Study design and population
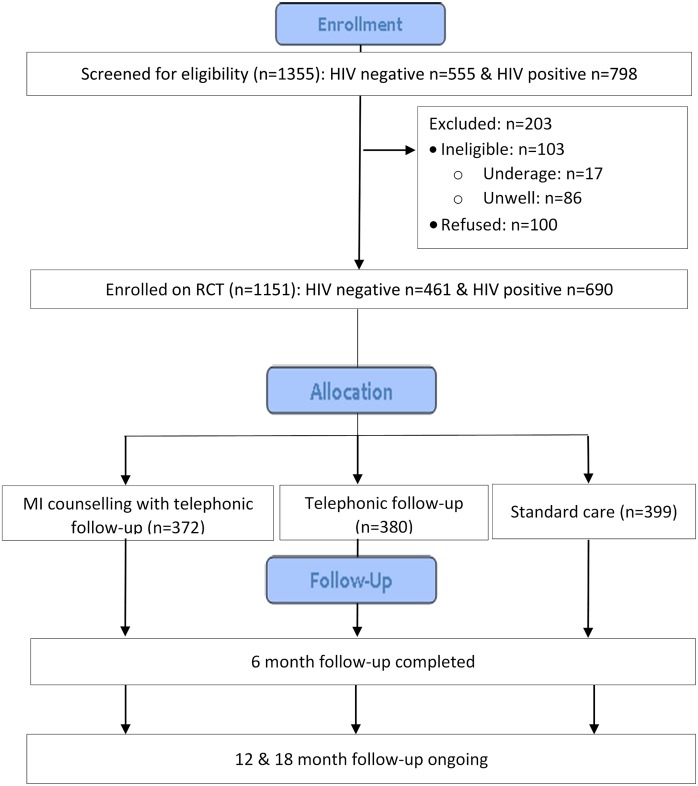
This data was collected as part of the baseline survey of a randomised controlled trial (RCT) (Pan African Clinical Trials Registry: PACTR201809886446171) among adult (≥18 years) women who gave birth up to 30 days before the date of enrolment at Midwife Obstetric Units (MOUs) based in Tshwane, Ekurhuleni and Johannesburg Metropolitan districts in the Gauteng Province, South Africa. New mothers were recruited consecutively via referrals from nurses at participating facilities and interviewed on the day of their first post-natal visit (scheduled three to six days after delivery). Study staff screened potential eligible women using study eligibility criteria, provided more information regarding the study, and obtained written informed consent using informed consent translated from English into Sotho and Zulu and administered in the participant’s preferred language (English, Sotho or Zulu). Study enrolment was conducted from October 2016 to January 2018. The sampling for the RCT was stratified by HIV status, and participants were randomised to either Active Tracing, Active Tracing with Motivational Interviewing (MI) counselling support tracing approaches or standard of care. Six month follow-up for the RCT has been completed, and the 12 and 18 month follow-up is ongoing (Fig 1). This analysis includes cross-sectional data collected using baseline questionnaire at study enrolment, which included 690 HIV-1 infected and 461 HIV-1 uninfected women (Fig 1). Participant demographic, socio-economic and contextual data were collected using an interviewer-administered structured questionnaire translated in English, Sotho and Zulu and administered in the participant’s preferred language (English, Sotho or Zulu).
Fig 1. CONSORT diagram of participant enrolment and follow-up in the parent study (RCT).
Analytical variables
PPD was measured using the CES-D 10 scale, a 10-question four-point scale (scores range 0 to 3) that measures general depressive symptoms experienced up to 7 days before the interview date [34–36]. The total score ranged from 0 to 30 with higher total scores reflecting greater frequency of depression (Cronbach’s alpha = 0.83). We created a variable for PPD categorised into no depression (CES-D 10 total score <5), low to medium depression (CES-D 10 total score ≥5 and <10) and major depressive symptoms (CES-D 10 total score≥10) [37, 38]. The CES-D 10 scale has been validated in South Africa and other low and middle-income countries (LMIC) and is used as a screening tool for PPD [35, 39–41].
Additional variables assessed included socio-demographic factors including age, highest education completed, marital status, employment status and work times (all-day, shift-work) and income-source. Perceived social support (PSS) was measured using a six-item scale in which participants indicated their overall level of satisfaction with available support given in each area [34]. Rating of overall satisfaction score for each item ranged from one to five. Total scores were categorised as either "high PSS” (score ≥26) or “medium PSS” (score ≤25) based on the distribution of scores in the sample. HIV knowledge was measured among HIV-positive women based on responses to 12 HIV knowledge questions, each with a possible score of 0 and 1 for incorrect and correct response respectively. Total knowledge scores were categorised as “Low” (score<11) or “Medium to high” (score ≥11) HIV knowledge. We also assessed factors related to ANC attendance, location and type of housing, whether the latest pregnancy was planned, new-born baby’s gender, number of child dependents (own and others'), support during pregnancy, and expected childcare support postpartum. Ethics approval for this study was obtained from the Wits Human Research Ethics Committee (Medical) (HREC No.M151041). All personal identifiers were removed from the final analytic data.
Statistical analysis
Descriptive analysis was used to summarise participant characteristics at study enrolment. Categorical variables were tabulated using frequencies and percentages. Continuous variables were described using medians and interquartile ranges (IQR) where appropriate. We used Ordinal logistic regression to identify predictors of postpartum depression and the associated 95% confidence intervals (95% CI). As we could only identify prediction and not causation, factors identified with a univariate p-value <0.1 and priori variables of importance and predictors were included in the multivariate model. Significance level was set at 0.05 for the multivariate model. The Likelihood ratio test was used to test for adherence to the proportional odds assumption. We report adjusted odds ratio (aOR) and 95% confidence intervals (CIs). Data analysis was conducted using STATA version 14 (StataCorp, College Station, TX).
Results
Socio-demographic factors
Table 1 shows demographic and contextual characteristics of study participants which included 1151 postpartum women of a median age of 29 years (IQR: 25–33) at study enrolment, of which over 80% had at least some secondary school education. A total of 461 (40.1%) were HIV negative, and 690 (59.9%) were HIV infected. Just over half (364/690 or 52.7%) of the HIV infected women were diagnosed with HIV before their latest pregnancy, and among these 152/364 (41.8%) had a prior (in-pregnancy) HIV diagnosis and had prior experience of the PMTCT program. A total of 249 (21.6%) women were married, with a higher marriage percentage among HIV negative mothers (26.9%) compared to HIV positive mothers (125/690 or 18.1%). Overall, 78.4% of women were in non-marital relationships, with 44.6% cohabiting with a partner. Over two-thirds of the women lived with a partner or spouse (64.6%), and 46.5% lived in their own home. Nearly two thirds (63.3%) of the mothers were unemployed, with 19.5% not seeking employment. Unemployment rates were similar across HIV status. However, unemployed HIV-infected infected women (47.0%) were more likely to be searching for work compared to HIV negative women (27.5%). The majority of women reported their spouse/ partner to be their primary source of income (56.2%), with a higher proportion of HIV negative mothers relying on spousal/ partner support than their HIV-infected counterparts (61.0% vs 53.2%). Over half of the women, 674 (58.6%), had high perceived social support.
Table 1. Participant characteristics (all women).
| HIV negative | Diagnosed during latest pregnancy | Pre-pregnancy HIV diagnosis | Total | |
|---|---|---|---|---|
| 461 (40.1) No. (%) |
326 (28.3) No. (%) |
364 (31.6) No. (%) |
1151 (100.0) No. (%) |
|
| Age (years) | ||||
| 18–25 | 182 (39.5) | 96 (29.4) | 53 (14.6) | 331 (28.8) |
| 26–30 | 147 (31.9) | 105 (32.2) | 90 (24.7) | 342 (29.7) |
| 31–35 | 92 (20.0) | 81 (24.8) | 122 (33.5) | 295 (25.6) |
| >35 | 40 (8.7) | 44 (13.5) | 99 (27.2) | 183 (15.9) |
| Highest level of education | ||||
| Tertiary level | 61 (13.2) | 50 (15.3) | 38 (10.4) | 149 (12.9) |
| Matric | 146 (31.7) | 86 (26.4) | 73 (20.1) | 305 (26.5) |
| High school | 234 (50.8) | 180 (55.2) | 229 (62.9) | 643 (55.9) |
| Primary school or less | 20 (4.3) | 10 (3.1) | 23 (6.3) | 53 (4.6) |
| Missing | - | - | 1 (0.3) | 1 (0.1) |
| Marital status | ||||
| Married | 124 (26.9) | 51 (15.6) | 74 (20.3) | 249 (21.6) |
| In a relationship (living together) | 178 (38.6) | 155 (47.5) | 179 (49.2) | 512 (44.5) |
| In a relationship (not living together) | 139 (30.2) | 103 (31.6) | 95 (26.1) | 337 (29.3) |
| Not in a relationship | 19 (4.1) | 16 (4.9) | 15 (4.1) | 50 (4.3) |
| Missing | 1 (0.2) | 1 (0.3) | 1 (0.3) | 3 (0.3) |
| Accommodation | ||||
| Own home | 173 (37.5) | 102 (31.3) | 144 (39.6) | 419 (36.4) |
| Family’s home | 153 (33.2) | 118 (36.2) | 113 (31.0) | 384 (33.4) |
| Friends or house-share | 134 (29.1) | 105 (32.2) | 107 (29.4) | 346 (30.1) |
| Missing | 1 (0.2) | 1 (0.3) | - | 2 (0.2) |
| Participant lives with | ||||
| With partner/spouse | 293 (63.6) | 202 (62.0) | 249 (68.4) | 744 (64.6) |
| Parents/relatives | 147 (31.9) | 101 (31.0) | 78 (21.4) | 326 (28.3) |
| Alone/with children | 19 (4.1) | 19 (5.8) | 32 (8.8) | 70 (6.1) |
| Missing | 2 (0.4) | 4 (1.2) | 5 (1.4) | 11 (1.0) |
| Location of primary house (when living in secondary house) | ||||
| current house | 168 (36.4) | 135 (41.4) | 153 (42) | 456 (39.6) |
| same province | 26 (5.6) | 24 (7.4) | 26 (7.1) | 76 (6.6) |
| Another province/rural-area | 120 (26.0) | 95 (29.1) | 112 (30.8) | 327 (28.4) |
| Another country | 147 (31.9) | 71 (21.8) | 72 (19.8) | 290 (25.2) |
| Missing | - | 1 (0.3) | 1 (0.3) | 2 (0.2) |
| Accommodation type | ||||
| House/Flat/Brick structure | 139 (30.2) | 106 (32.5) | 122 (33.5) | 367 (31.9) |
| House/room/flat in backyard | 220 (47.7) | 160 (49.1) | 172 (47.3) | 552 (48.0) |
| Informal dwelling/shack | 101 (21.9) | 60 (18.4) | 70 (19.2) | 231 (20.1) |
| Missing | 1 (0.2) | - | - | 1 (0.1) |
| Employment status | ||||
| Employed-work all day | 113 (24.5) | 95 (29.1) | 97 (26.6) | 305 (26.5) |
| Employed-shift work | 41 (8.9) | 36 (11.0) | 40 (11.0) | 117 (10.2) |
| Unemployed (not job hunting) | 127 (27.5) | 48 (14.7) | 50 (13.7) | 225 (19.5) |
| Unemployed (job hunting) | 180 (39.0) | 147 (45.1) | 177 (48.6) | 504 (43.8) |
| Primary source of income/ finances | ||||
| Paid job, salary or business | 108 (23.4) | 107 (32.8) | 101 (27.7) | 316 (27.5) |
| Government grant | 5 (1.1) | 20 (6.1) | 35 (9.6) | 60 (5.2) |
| Spouse/ partner | 280 (60.7) | 161 (49.4) | 206 (56.6) | 647 (56.2) |
| Parents/ relatives | 66 (14.3) | 36 (11.0) | 21 (5.8) | 123 (10.7) |
| Missing | 2 (0.4) | 2 (0.6) | 1 (0.3) | 5 (0.4) |
| Sex of new-born baby | ||||
| Male | 225 (48.8) | 169 (51.8) | 181 (49.7) | 575 (50.0) |
| Female | 236 (51.2) | 157 (48.2) | 183 (50.3) | 576 (50.0) |
| Number of other children of any age | ||||
| 0 children | 138 (29.9) | 60 (18.4) | 30 (8.2) | 228 (19.8) |
| =>1 children | 306 (66.4) | 259 (79.4) | 329 (90.4) | 894 (77.7) |
| Missing | 17 (3.7) | 7 (2.1) | 5 (1.4) | 29 (2.5) |
| Latest pregnancy planned? | ||||
| No | 209 (45.3) | 182 (55.8) | 195 (53.6) | 586 (50.9) |
| Yes | 252 (54.7) | 144 (44.2) | 169 (46.4) | 565 (49.1) |
| Baby father’s involvement in the pregnancy? | ||||
| Involved | 439 (95.2) | 305 (93.6) | 341 (93.7) | 1,085.0 (94.3) |
| Not involved | 22 (4.8) | 21 (6.4) | 23 (6.3) | 66 (5.7) |
| Perceived greatest supporter during the latest pregnancy | ||||
| Partner | 247 (53.6) | 189 (58.0) | 190 (52.2) | 626 (54.4) |
| Baby father(if not partner) | 99 (21.5) | 71 (21.8) | 98 (26.9) | 268 (23.3) |
| Family/friends/Other | 112 (24.3) | 65 (19.9) | 74 (20.3) | 251 (21.8) |
| Missing | 3 (0.7) | 1 (0.3) | 2 (0.5) | 6 (0.5) |
| Expected main childcare supporter | ||||
| Partner | 187 (40.6) | 142 (43.6) | 146 (40.1) | 475 (41.3) |
| Baby father(if not partner) | 88 (19.1) | 65 (19.9) | 97 (26.6) | 250 (21.7) |
| Family/friends/Other | 184 (39.9) | 119 (36.5) | 121 (33.2) | 424 (36.8) |
| Missing | 2 (0.4) | - | - | 2 (0.2) |
| ANC attendance | ||||
| No | 5 (1.1) | 4 (1.2) | 6 (1.6) | 15 (1.3) |
| Yes | 454 (98.5) | 322 (98.8) | 358 (98.4) | 1,134.0 (98.5) |
| Missing | 2 (0.4) | - | - | 2 (0.2) |
| HIV knowledge | ||||
| Low | - | 96 (29.4) | 115 (31.6) | 211 (30.6) |
| Medium to high | - | 227 (69.6) | 247 (67.9) | 474 (68.7) |
| Missing | - | 3 (0.9) | 2 (0.6) | 5 (0.7) |
| Perceived social support (PSS) | ||||
| High PSS | 298 (64.6) | 172 (52.8) | 204 (56.0) | 674 (58.6) |
| Medium PSS | 163 (35.4) | 154 (47.2) | 160 (44.0) | 477 (41.4) |
| Post-partum depression (PPD) | ||||
| No depression | 332 (72.0) | 257 (78.8) | 274 (75.3) | 863 (75.0) |
| Low to medium depression | 79 (17.1) | 43 (13.2) | 46 (12.6) | 168 (14.6) |
| Major depressive symptoms | 50 (10.9) | 26 (8.0) | 44 (12.1) | 120 (10.4) |
ANC: Antenatal care; HIV: human immunodeficiency virus; ART: antiretroviral treatment
Pregnancy history and social support
Only 19.8% of women were primiparous and for half of them (50.9%), the latest pregnancy was unplanned. The partner/baby’s father support during the latest pregnancy was generally high (75.5% HIV negative vs 79.6% of HIV-infected mothers). However, the expected partner/father support in childcare activities was higher (65.2% vs. 60.0% among HIV negative women) for HIV-infected women with lower expected family support for childcare (34.8%) compared to 39.9% among HIV negative women. Overall, ANC attendance was high with 98.5% having any ANC attendance during the latest the pregnancy. The majority of HIV-infected women had medium to high HIV knowledge (69.2%), 70% among women with pre-pregnancy diagnosis and 68% among newly diagnosed women. Perceived social support was higher among HIV negative women (64.6%) compared to HIV-infected women (54.4%).
Prevalence of PPD
Overall, 168/1151 (14.6%) of the sample had low to medium PPD and 120/1151 (10.4%) women screened positive for major PPD, 10.9% among HIV negative women and 10.1% among HIV positive women. Among HIV positive women, a higher proportion (12.1%) of those with a pre-pregnancy HIV diagnosis screened positive for major PPD compared to women with in-pregnancy HIV diagnosis (8.0%).
Predictors of PPD (multivariable analysis)
Table 2 shows crude and adjusted estimates from the ordinal logistic regression model with 95% CIs for experiencing low to medium PPD and major depression respectively.
Table 2. Predictors of postpartum depression by HIV status.
| PPD | HIV positive women (N = 461) |
HIV negative women (N = 690) |
||||
|---|---|---|---|---|---|---|
| Low to medium depression 168 (14.6) |
Major Depression 120 (10.4) |
Crude | Adjusted | Crude | Adjusted | |
| n (%) | n (%) | OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| HIV status | ||||||
| HIV negative | 79 (17.1) | 50 (10.8) | ||||
| HIV positive | 89 (25.8) | 70 (20.1) | ||||
| Timing of HIV diagnosis | ||||||
| HIV negative | 79 (17.1) | 50 (10.8) | ||||
| Diagnosed during latest pregnancy | 43 (13.2) | 26 (8) | 1 | |||
| Pre-pregnancy diagnosis | 46 (12.6) | 44 (12.1) | 1.3 (0.9–1.8) | |||
| Age (years) | ||||||
| 18–25 | 71 (21.5) | 28 (8.5) | 1 | 1 | 1 | |
| 26–30 | 41 (12) | 36 (10.5) | 0.7 (0.5–1.2) | 0.8 (0.5–1.2) | 0.8 (0.5–1.3) | |
| 31–35 | 36 (12.2) | 40 (13.6) | 0.8 (0.5–1.3) | 1.2 (0.7–2.0) | 1.2 (0.7–2.1) | |
| >35 | 20 (10.9) | 16 (8.7) | 0.7 (0.4–1.2) | 0.5 (0.2–1.1) | 0.5 (0.2–1.3) | |
| Highest level of education | ||||||
| Tertiary level | 21 (14.1) | 16 (10.7) | 1 | 1 | ||
| Matric | 52 (17) | 29 (9.5) | 0.8 (0.4–1.5) | 1.5 (0.7–2.9) | ||
| High school | 90 (14) | 65 (10.1) | 0.8 (0.5–1.4) | 1.2 (0.6–2.3) | ||
| Primary school or less | 5 (9.4) | 9 (17) | 0.7 (0.3–1.9) | 2.3 (0.8–6.6) | ||
| Marital status | ||||||
| Married | 29 (11.6) | 24 (9.6) | 1 | 1 | 1 | |
| In a relationship (living together) | 71 (13.9) | 52 (10.2) | 1.1 (0.7–1.8) | 1.3 (0.8–2.2) | 1.2 (0.7–2.1) | |
| In a relationship (not living together) | 60 (17.8) | 34 (10.1) | 1.4 (0.8–2.4) | 1.4 (0.8–2.4) | 1.1 (0.6–2.1) | |
| Not in a relationship | 8 (16) | 10 (20) | 1.6 (0.7–3.9) | 3.6 (1.4–9.7) | 2.3 (0.8–6.6) | |
| Accommodation | ||||||
| Own home | 56 (13.4) | 58 (13.8) | 1 | 1 | 1 | 1 |
| Family’s home | 69 (18) | 41 (10.7) | 1.1 (0.7–1.6) | 1.0 (0.5–1.7) | 0.9 (0.6–1.5) | 0.7 (0.5–1.2) |
| Friends or house-share | 42 (12.1) | 21 (6.1) | 0.5 (0.3–0.8) | 0.5 (0.3–0.9) | 0.7 (0.4–1.1) | 0.4 (0.2–0.8) |
| Participant lives with | ||||||
| With partner/spouse | 98 (13.2) | 73 (9.8) | 1 | 1 | 1 | |
| Parents/relatives | 61 (18.7) | 38 (11.7) | 1.6 (1.1–2.3) | 1.2 (0.7–2.2) | 1.2 (0.8–1.9) | |
| Alone/with children | 9 (12.9) | 7 (10) | 1.0 (0.5–2.1) | 1.1 (0.5–2.4) | 1.0 (0.4–2.8) | |
| Location of primary house (when living in secondary house) | ||||||
| current house | 74 (16.2) | 52 (11.4) | 1 | 1 | 1 | |
| same province | 10 (13.2) | 4 (5.3) | 0.6 (0.3–1.3) | 0.9 (0.4–2.1) | 0.6 (0.2–1.5) | |
| Another province/rural-area | 38 (11.6) | 35 (10.7) | 0.7 (0.4–1.0) | 0.9 (0.5–1.5) | 1.0 (0.6–1.6) | |
| Another country | 46 (15.9) | 29 (10) | 1.0 (0.6–1.5) | 1.4 (0.8–2.6) | 0.8 (0.5–1.3) | |
| Accommodation type | ||||||
| House/Flat/Brick structure | 67 (18.3) | 40 (10.9) | 1 | 1 | ||
| House/room/flat in backyard | 78 (14.1) | 61 (11.1) | 0.9 (0.6–1.3) | 0.8 (0.5–1.2) | ||
| Informal dwelling/shack | 23 (10) | 19 (8.2) | 0.7 (0.4–1.2) | 0.4 (0.2–0.7) | ||
| Employment status | ||||||
| Employed-work all day | 39 (12.8) | 26 (8.5) | 1 | 1 | 1 | |
| Employed-shift work | 14 (12) | 14 (12) | 1.5 (0.8–2.9) | 1.9 (0.9–3.6) | 0.8 (0.3–1.8) | |
| Unemployed (not job hunting) | 38 (16.9) | 22 (9.8) | 1.1 (0.6–2.1) | 0.7 (0.3–1.5) | 1.3 (0.7–2.2) | |
| Unemployed (job hunting) | 77 (15.3) | 58 (11.5) | 1.6 (1.0–2.5) | 1.0 (0.5–2.0) | 1.1 (0.6–1.8) | |
| Primary source of income/ finances | ||||||
| Paid job, salary or business | 38 (12) | 26 (8.2) | 1 | 1 | 1 | |
| Government grant | 5 (8.3) | 10 (16.7) | 1.5 (0.7–3.1) | 1.7 (0.7–4.1) | 2.2 (0.4–13.1) | |
| Spouse/ partner | 102 (15.8) | 66 (10.2) | 1.4 (0.9–2.2) | 1.8 (0.9–3.5) | 1.2 (0.7–2.0) | |
| Parents/ relatives | 22 (17.9) | 18 (14.6) | 2.1 (1.1–4.1) | 1.9 (0.7–4.7) | 1.5 (0.8–3.0) | |
| Sex of new-born baby | ||||||
| Male | 83 (14.4) | 57 (9.9) | 1 | 1 | 1 | |
| Female | 85 (14.8) | 63 (10.9) | 1.4 (1.0–1.9) | 1.4 (0.9–2.0) | 0.8 (0.5–1.2) | |
| Number of other children of any age | ||||||
| 0 children | 43 (18.9) | 21 (9.2) | 1 | 1 | ||
| =>1 children | 120 (13.4) | 99 (11.1) | 0.9 (0.5–1.5) | 0.9 (0.6–1.5) | ||
| Latest pregnancy planned? | ||||||
| No | 91 (15.5) | 69 (11.8) | 1 | 1 | 1 | |
| Yes | 77 (13.6) | 51 (9) | 0.7 (0.5–0.9) | 0.7 (0.5–1.0) | 0.9 (0.6–1.4) | |
| Baby father’s involvement in the pregnancy? | ||||||
| Involved | 160 (14.7) | 110 (10.1) | 1 | 1 | ||
| Not involved | 8 (12.1) | 10 (15.2) | 1.0 (0.5–2.1) | 1.7 (0.7–4.1) | ||
| Perceived greatest supporter during the latest pregnancy | ||||||
| Partner | 88 (14.1) | 56 (8.9) | 1 | 1 | ||
| Baby father(if not partner) | 39 (14.6) | 34 (12.7) | 1.3 (0.8–1.9) | 1.3 (0.8–2.2) | ||
| Family/friends/Other | 39 (15.5) | 29 (11.6) | 1.4 (0.9–2.2) | 1.1 (0.7–1.8) | ||
| Expected main childcare supporter | ||||||
| Partner | 61 (12.8) | 41 (8.6) | 1 | 1 | 1 | |
| Baby father(if not partner) | 36 (14.4) | 29 (11.6) | 1.1 (0.7–1.8) | 1.7 (1.0–2.9) | 1.6 (0.9–2.8) | |
| Family/friends/Other | 71 (16.7) | 50 (11.8) | 1.4 (0.9–2.0) | 1.5 (1.0–2.4) | 1.3 (0.8–2.3) | |
| ANC attendance | ||||||
| No | 5 (33.3) | 5 (33.3) | 1 | 1 | 1 | |
| Yes | 163 (14.4) | 115 (10.1) | 0.1 (0.0–0.4) | 0.2 (0.0–0.5) | 0.3 (0.1–1.5) | |
| HIV knowledge | ||||||
| Low | 28 (13.3) | 24 (11.4) | 1 | |||
| Medium to high | 61 (12.9) | 45 (9.5) | 0.9 (0.6–1.3) | |||
| Perceived social support (PSS) | ||||||
| High PSS | 97 (14.4) | 59 (8.8) | 1 | 1 | 1 | |
| Medium PSS | 71 (14.9) | 61 (12.8) | 1.3 (0.9–1.8) | 1.4 (1.0–2.2) | 1.3 (0.8–2.0) | |
OR: Odds ratio; aOR: Adjusted odds ratio; HIV: human immunodeficiency virus; ANC: Antenatal care
HIV-infected
Among HIV-infected women, there was no difference in odds of experiencing PPD by timing of HIV diagnosis in the univariate analysis (OR 1.3, 95%CI: 0.9–1.8). Living with friends or house-mates was associated with lower risk of experiencing PPD (aOR 0.5, 95%CI: 0.3–0.9), as well as having attended antenatal care during the latest pregnancy (aOR 0.2, 95% CI: 0.0–0.5).
HIV negative
Among HIV negative women living with friends or house-mates was associated with lower risk of experiencing PPD (aOR 0.4, 95% CI: 0.2–0.8).
On further analysis in a multivariable analysis including all women, newly diagnosed HIV-infected women were less likely to be depressed compared to HIV negative women (aOR 0.7, 95% CI: 0.5–0.99), while there was no difference in odds of experiencing depression between HIV negative women and those with pre-pregnancy HIV diagnosis.
Discussion
This is one of the largest studies assessing PPD in the sub-Sahara Africa setting, and one of the few that looks at differences in PPD-based HIV status and timing of HIV diagnosis. Results from our study show that a quarter of women had depressive tendencies after delivery, but only 10.9% of HIV negative and 10.1% of HIV positive women showed signs of major PPD.
The risk of major PPD in both our HIV positive and negative women is lower than previously reported PPD prevalence in low and middle-income countries, including South Africa, and is much closer to rates found in high-income countries [4–6]. Variations in PPD rates possibly emanate from screening tool preferences as well as the varying definitions for PPD across studies, with very few elaborating on the severity of the reported risk of PPD. A large prospective cohort study in the general postpartum population in Soweto, Johannesburg, reported PPD rates of 16.4% using a total score threshold of 20 on the Pitt Depression Questionnaire depressive symptoms [42]. Previous smaller studies in sub-Saharan Africa, which defined major PPD at total score thresholds ranging from 11–15 on the Edinburg Postnatal Depression Scale-10 (EPDS-10) [43], reported PPD prevalence of 33% in a mixed Zimbabwean cohort HIV-1 infected cohort [28], 45.1% was found among HIV-infected women in rural Mpumalanga (South Africa) [44].
However, our study consecutively enrolled postnatal women who delivered both at low-risk (MOU) and hospital facilities and the setting of enrolment (MOU) could have systematically excluded women with high-risk pregnancies who may also have greater risk of major PPD. There is also a possibility that symptoms were played down/ underreported due to the stigma associated with mental health disorders in many African cultures [45, 46]. Women may worry that their child caring capacity may be called into question and they may also be reluctant to take up treatment interventions involving prescription drugs while breastfeeding [10, 17, 47]. On average 50% of depressed women often go undiagnosed, with only 20% of those diagnosed seek treatment [11–13, 47].
We hypothesised that HIV-infected women with pre-pregnancy HIV diagnosis and hopefully some prior experience of HIV care would be more resilient and be at lower risk of major PPD than newly diagnosed HIV-infected women. However, we found no difference in major PPD by the timing of HIV diagnosis among HIV infected women, but found that women diagnosed with HIV during their latest pregnancy were less likely to experience PPD than HIV negative women. These results are contrary to results from two previous studies in Zambia and South Africa that found an increased risk of PPD among women who discovered their HIV-1 diagnosis during the last pregnancy [44, 48]. Similar to previous studies, we found no difference in PPD by HIV status [28, 33]. The impact of the PMTCT program and increased life expectancy of HIV-infected individuals may have lessened women’s concerns about the risk of HIV transmission to their infants as well as fears of premature death [49–51]. Addressing depression in HIV negative women remains a crucial HIV-preventive measure as untreated depression is associated with negative coping behaviours (unprotected sex, having multiple and concurrent sexual partners, use of illicit substances) that increase the risk for HIV [52].
Predictors of PPD identified among the combined sample of HIV-infected and negative mothers include living with friends or sharing a house with others, antenatal care attendance and timing of HIV diagnosis when compared to HIV negative women. In many settings including South Africa, older female family members experienced in child care are customarily tasked with providing childcare support and guidance to new mothers. Depending on the context, these family members may come and stay with the new mother in her own home, or the new mother may move to her family home to access this support. Women who live with friends or sharing with others may, therefore, have lower expectations of this type of support. Antenatal care engagement may expose women to services that may mitigate some of the pregnancy, labour and childcare related stressors that may contribute to the risk of PPD.
Perceived social support has been previously reported as a critical factor in mitigation of PPD [5, 28, 53, 54], but it wasn’t shown as important in our cohort of women which may need to be explored further to understand what factors could be helping them to cope positively with perinatal related stressors, as well as HIV among our HIV infected women.
The cross-sectional study design limits the interpretation of the study results, and causal associations cannot be inferred. Depressive symptoms were self-reported using a validated tool, but participant recall and social desirability bias cannot be excluded. Although the CES-D 10 scale is a screening tool and not a diagnostic interview, it has been shown to have high levels of sensitivity and specificity for postpartum depression [36]. The study was conducted in an urban setting with participants hailing from a mixture of formal and informal settlements, and the study results may not be generalizable to rural settings. Child outcomes were not measured which would have strengthened the results.
Conclusions
Our results show a lower prevalence of PPD than previously reported, with no difference noted by HIV status, possibly indicating increased normalisation of HIV disease among urban populations in South Africa. However, efforts to identify depressed mothers using targeted symptom screening based on risk factors, and linked to effective treatment interventions remain essential in improving postpartum mother and child outcomes.
Acknowledgments
We are grateful to all study participants for their contribution to this research.
Data Availability
The data used in this study is owned by the study site and National Department of Health (South Africa) and governed by the Human Research Ethics Committee (University of Witwatersrand, Johannesburg, South Africa). All relevant data is included in the paper, the full data are available from the Health Economics and Epidemiology Research Office for researchers who meet the criteria for access to confidential data and have approval from the owners of the data (information@heroza.org).
Funding Statement
This study has been made possible by the generous support of the American People and the President’s Emergency Plan for AIDS Relief (PEPFAR) through United States Agency for International Development (USAID) under the terms of Cooperative Agreements AID-674-A-12-00029 and 72067419CA00004 to the Health Economics and Epidemiology Research Office. Additional support for this study came from the Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) funding award 2016/353-ONO, and the South African National Research Foundation (NRF) funding award TTK 150708124222. The contents are the responsibility of the authors and do not necessarily reflect the views of PEPFAR, USAID, the United States, CIPHER or the NRF.
References
- 1.Stocky A, Lynch J. Acute psychiatric disturbance in pregnancy and the puerperium. Best Practice & Research Clinical Obstetrics & Gynaecology. 2000;14(1):73–87. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Depression: A Global Crisis. World Mental Health Day, October 10 2012. World Federation for Mental Health, Occoquan, Va, USA. 2012.
- 3.Weobong B, ten Asbroek AH, Soremekun S, Gram L, Amenga-Etego S, Danso S, et al. Association between probable postnatal depression and increased infant mortality and morbidity: findings from the DON population-based cohort study in rural Ghana. BMJ open. 2015;5(8):e006509 10.1136/bmjopen-2014-006509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madlala S, Kassier S. Antenatal and postpartum depression: effects on infant and young child health and feeding practices. South African Journal of Clinical Nutrition. 2017:1–7. [Google Scholar]
- 5.Peltzer K, Shikwane M. Prevalence of postnatal depression and associated factors among HIV-positive women in primary care in Nkangala district, South Africa. Southern African Journal of HIV Medicine. 2011;12(4):24–8. [Google Scholar]
- 6.Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;44(4):470–7. 10.1097/QAI.0b013e31802f1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokwena K, Shiba D. Prevalence of postnatal depression symptoms in a primary health care clinic in Pretoria, South Africa: management of health care services. African Journal for Physical Health Education, Recreation and Dance. 2014;20(Supplement 1):116–27. [Google Scholar]
- 8.Castle J. Early detection of postpartum depression: Screening in the first two to three days. J Lancaster Gen Hosp Winter. 2008;2009(3):4. [Google Scholar]
- 9.Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. The Cochrane Library. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Collaborating Centre for Mental Health, editor Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance: Updated edition2014: British Psychological Society. [PubMed]
- 11.Appleby L, Hirst E, Marshall S, Keeling F, Brind J, Butterworth T, et al. The treatment of postnatal depression by health visitors: impact of brief training on skills and clinical practice. Journal of Affective Disorders. 2003;77(3):261–6. [DOI] [PubMed] [Google Scholar]
- 12.Benvenuti P, Valoriani V, Vanni D. Prevention of postnatal depression. Clinical Neuropsychiatry. 2006;3(1):39–56. [Google Scholar]
- 13.England S, Ballard C, George S. Chronicity in postnatal depression. The European journal of psychiatry. 1994;8(2):93–6. [Google Scholar]
- 14.Stellenberg EL, Abrahams JM. Prevalence of and factors influencing postnatal depression in a rural community in South Africa. African journal of primary health care & family medicine. 2015;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochat TJ, Tomlinson M, Newell M-L, Stein A. Detection of antenatal depression in rural HIV-affected populations with short and ultrashort versions of the Edinburgh Postnatal Depression Scale (EPDS). Archives of women’s mental health. 2013;16(5):401–10. 10.1007/s00737-013-0353-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seedat S, Stein D, Herman A, Kessler R, Sonnega J, Heeringa S, et al. Twelve-month treatment of psychiatric disorders in the South African Stress and Health study (World Mental Health survey initiative). Social psychiatry and psychiatric epidemiology. 2008;43(11):889–97. 10.1007/s00127-008-0399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kathree T, Selohilwe OM, Bhana A, Petersen I. Perceptions of postnatal depression and health care needs in a South African sample: the “mental” in maternal health care. BMC women’s health. 2014;14(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bulletin of the World Health Organization. 2011;89(8):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wachs TD, Black MM, Engle PL. Maternal depression: a global threat to children’s health, development, and behavior and to human rights. Child Development Perspectives. 2009;3(1):51–9. [Google Scholar]
- 20.Woolhouse H, James J, Gartland D, McDonald E, Brown SJ. Maternal depressive symptoms at three months postpartum and breastfeeding rates at six months postpartum: Implications for primary care in a prospective cohort study of primiparous women in Australia. Women and Birth. 2016;29(4):381–7. 10.1016/j.wombi.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 21.Field T, Hernandez-Reif M, Diego M, Feijo L, Vera Y, Gil K, et al. Still-face and separation effects on depressed mother-infant interactions. Infant Mental Health Journal. 2007;28(3):314–23. 10.1002/imhj.20138 [DOI] [PubMed] [Google Scholar]
- 22.Weinberg MK, Tronick EZ, Beeghly M, Olson KL, Kernan H, Riley JM. Subsyndromal depressive symptoms and major depression in postpartum women. American Journal of Orthopsychiatry. 2001;71(1):87 [DOI] [PubMed] [Google Scholar]
- 23.Paris R, Bolton RE, Weinberg MK. Postpartum depression, suicidality, and mother-infant interactions. Archives of women’s mental health. 2009;12(5):309 10.1007/s00737-009-0105-2 [DOI] [PubMed] [Google Scholar]
- 24.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. General hospital psychiatry. 2004;26(4):289–95. 10.1016/j.genhosppsych.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Tsai S, Lin H. Increased mortality risk among offspring of mothers with postnatal depression: a nationwide population-based study in Taiwan. Psychological medicine. 2011;41(11):2287–96. 10.1017/S0033291711000584 [DOI] [PubMed] [Google Scholar]
- 26.Stewart DE, Robertson E, Dennis C-L, Grace SL, Wallington T. Postpartum depression: Literature review of risk factors and interventions. Toronto: University Health Network Women’s Health Program for Toronto Public Health; 2003. [Google Scholar]
- 27.Verreault N, Da Costa D, Marchand A, Ireland K, Dritsa M, Khalifé S. Rates and risk factors associated with depressive symptoms during pregnancy and with postpartum onset. Journal of psychosomatic obstetrics & gynecology. 2014;35(3):84–91. [DOI] [PubMed] [Google Scholar]
- 28.Chibanda D, Mangezi W, Tshimanga M, Woelk G, Rusakaniko S, Stranix-Chibanda L, et al. Postnatal depression by HIV status among women in Zimbabwe. Journal of Women’s Health. 2010;19(11):2071–7. 10.1089/jwh.2010.2012 [DOI] [PubMed] [Google Scholar]
- 29.Yator O, Mathai M, Vander Stoep A, Rao D, Kumar M. Risk factors for postpartum depression in women living with HIV attending prevention of mother-to-child transmission clinic at Kenyatta National Hospital, Nairobi. AIDS care. 2016;28(7):884–9. 10.1080/09540121.2016.1160026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner R, Honikman S. Maternal mental health and the first 1 000 days. SAMJ: South African Medical Journal. 2016;106(12):1164–7. [Google Scholar]
- 31.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. Jama. 2001;285(11):1466–74. [DOI] [PubMed] [Google Scholar]
- 32.Uwakwe R. Affective (depressive) morbidity in puerperal Nigerian women: validation of the Edinburgh Postnatal Depression Scale. Acta Psychiatrica Scandinavica. 2003;107(4):251–9. [DOI] [PubMed] [Google Scholar]
- 33.Rubin LH, Cook JA, Grey DD, Weber K, Wells C, Golub ET, et al. Perinatal depressive symptoms in HIV-infected versus HIV-uninfected women: a prospective study from preconception to postpartum. Journal of Women’s Health. 2011;20(9):1287–95. 10.1089/jwh.2010.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support: Practical and theoretical implications. Journal of social and personal relationships. 1987;4(4):497–510. [Google Scholar]
- 35.Baron EC, Davies T, Lund C. Validation of the 10-item centre for epidemiological studies depression scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC psychiatry. 2017;17(1):6 10.1186/s12888-016-1178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosack V, Shore ER. Screening for depression among pregnant and postpartum women. Journal of Community Health Nursing. 2006;23(1):37–47. 10.1207/s15327655jchn2301_4 [DOI] [PubMed] [Google Scholar]
- 37.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of. Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 38.Zhang W, O’Brien N, Forrest JI, Salters KA, Patterson TL, Montaner JS, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PloS one. 2012;7(7):e40793 10.1371/journal.pone.0040793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myer L, Smit J, Roux LL, Parker S, Stein DJ, Seedat S. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS patient care and STDs. 2008;22(2):147–58. 10.1089/apc.2007.0102 [DOI] [PubMed] [Google Scholar]
- 40.Chishinga N, Kinyanda E, Weiss HA, Patel V, Ayles H, Seedat S. Validation of brief screening tools for depressive and alcohol use disorders among TB and HIV patients in primary care in Zambia. BMC psychiatry. 2011;11(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natamba BK, Achan J, Arbach A, Oyok TO, Ghosh S, Mehta S, et al. Reliability and validity of the center for epidemiologic studies-depression scale in screening for depression among HIV-infected and -uninfected pregnant women attending antenatal services in northern Uganda: a cross-sectional study. BMC Psychiatry. 2014;14(1):303 10.1186/s12888-014-0303-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramchandani PG, Richter LM, Stein A, Norris SA. Predictors of postnatal depression in an urban South African cohort. Journal of affective disorders. 2009;113(3):279–84. 10.1016/j.jad.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 43.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. The British journal of psychiatry. 1987;150(6):782–6. [DOI] [PubMed] [Google Scholar]
- 44.Peltzer K, Rodriguez VJ, Lee TK, Jones D. Prevalence of prenatal and postpartum depression and associated factors among HIV-infected women in public primary care in rural South Africa: a longitudinal study. AIDS care. 2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hugo CJ, Boshoff DE, Traut A, Zungu-Dirwayi N, Stein DJ. Community attitudes toward and knowledge of mental illness in South Africa. Social psychiatry and psychiatric epidemiology. 2003;38(12):715–9. 10.1007/s00127-003-0695-3 [DOI] [PubMed] [Google Scholar]
- 46.Evagorou O, Arvaniti A, Samakouri M. Cross-cultural approach of postpartum depression: manifestation, practices applied, risk factors and therapeutic interventions. Psychiatric Quarterly. 2016;87(1):129–54. 10.1007/s11126-015-9367-1 [DOI] [PubMed] [Google Scholar]
- 47.Holt C, Milgrom J, Gemmill AW. Improving help-seeking for postnatal depression and anxiety: a cluster randomised controlled trial of motivational interviewing. Archives of Women’s Mental Health. 2017:1–11. [DOI] [PubMed] [Google Scholar]
- 48.Kwalombota M. The effect of pregnancy in HIV-infected women. AIDS care. 2002;14(3):431–3. 10.1080/09540120220123829 [DOI] [PubMed] [Google Scholar]
- 49.Barron P, Pillay Y, Doherty T, Sherman G, Jackson D, Bhardwaj S, et al. Eliminating mother-to-child HIV transmission in South Africa. Bulletin of the World Health Organization. 2013;91:70–4. 10.2471/BLT.12.106807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bor J, Herbst AJ, Newell M-L, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–5. 10.1126/science.1230413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joint United Nations Programme on HIV/AIDS. A progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2013.
- 52.Hutton HE, Lyketsos CG, Zenilman JM, Thompson RE, Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transmitted disease clinic. American Journal of Psychiatry. 2004;161(5):912–4. 10.1176/appi.ajp.161.5.912 [DOI] [PubMed] [Google Scholar]
- 53.Rahman A, Iqbal Z, Harrington R. Life events, social support and depression in childbirth: perspectives from a rural community in the developing world. Psychological medicine. 2003;33(7):1161–7. [DOI] [PubMed] [Google Scholar]
- 54.Taylor AL. Social Support: A Predictor of Postpartum Depression among HIV-positive and HIV-negative Women: Drexel University; 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study is owned by the study site and National Department of Health (South Africa) and governed by the Human Research Ethics Committee (University of Witwatersrand, Johannesburg, South Africa). All relevant data is included in the paper, the full data are available from the Health Economics and Epidemiology Research Office for researchers who meet the criteria for access to confidential data and have approval from the owners of the data (information@heroza.org).