Abstract
Our aim in this report was to describe the characteristics of the first clinical isolate of Escherichia coli (EC-PAG-733) harboring the mcr-1 gene found in Mexico. This isolate was obtained from a fecal sample from a young child with an oncological condition. We obtained the whole-genome sequence using next-generation sequencing and analyzed the sequence by bioinformatics tools. EC-PAG-733 was resistant to third- and fourth-generation cephalosporins and was susceptible to all carbapenems and amikacin; it was also resistant to ciprofloxacin, levofloxacin, gentamicin and colistin at a minimum inhibitory concentration (MIC) of 4 μg/mL. This isolate was classified as O11:H25-ST457. EC-PAG-733 harbored an ESBL type CTX-M-55 as well as several virulence factors that have been associated with Enteroaggregative Escherichia coli (EAEC). The mcr-1 gene was located within an IncI2 plasmid. The results of this whole genome shotgun project were deposited in DDBJ/ENA/GenBank under the accession number QKXE00000000.
Introduction
Since its discovery, the resistance to polymyxins that is mediated by the mcr-1 gene has been considered to be an emerging resistance mechanism of global concern [1]. One of the main reasons for this alarm is that polymyxins are one of the last treatment options for infections caused by carbapenem-resistant enterobacteria, and the underlying mechanism of this resistance is transmitted by plasmids [2,3].
The use of colistin for growth promotion and for prophylaxis in farm animals has especially contributed to the emergence of colistin resistance; furthermore, its use for the treatment of infections due to carbapenem-resistant bacilli, such as Pseudomonas aeruginosa, Acinetobacter baumannii and Enterobacteriaceae, has been increasing [2]. In Latin America, the rate of colistin resistance resulting from this group of bacteria is estimated to be 1.5% [4]. In Mexico, the frequency of resistance to colistin in Gram negative bacilli is unknown.
Escherichia coli strains containing the mcr-1 gene have been isolated from animal food products, humans and other animal sources around the world, including in many Latin America countries [5–8]. There are several genetic variants of the mcr gene that could be carried on plasmids or integrated into chromosomes that are mainly distributed within members of Enterobacteriaceae [6]. In Mexico, only one study has reported the identification of an E coli isolate containing the mcr-1 gene, which was obtained from a pig stool sample [9].
In 2017, we established a surveillance program in our hospital to detect emerging resistance mechanisms in Gram negative bacilli, which allowed us to identify an isolate of E. coli containing the mcr-1 gene in a pediatric patient. The aim of this report was to characterize a clinical isolate of E. coli (EC-PAG-733) that contains the mcr-1 gene and to describe the epidemiological features of the patient from whom this strain was obtained.
Materials and methods
Ethics statement
This study was approved by the biosecurity, ethics, and research committees of the INP (IRB:00008064 and IRB:00008065) Number 2018/017. The Review Board exempted this study from the need to request informed consent because the isolate described in the study was obtained by routine procedures and did not affect the patient. There is no identifying information in this document or in any supporting information file that could compromise patient confidentiality or participant privacy.
Microbiological procedures
An isolate of E. coli known as EC-PAG-733 was obtained from a fecal sample from a pediatric patient in 2017. We obtained the identification, and susceptibility profile using the Phoenix Automated Microbiology System (Becton Dickinson, USA). We determined the MIC of colistin by broth microdilution method. The results were interpreted according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI). The detection of extended-spectrum beta-lactamases (ESBL) was performed using a double-disk diffusion test [10].
Molecular procedures
We obtained the total DNA using a QIAamp DNA Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. As part of the general procedures used for our surveillance program, we first amplified blaCTX-M-1, blaCTX-M-2, blaCTX-M-9, blaSHV, blaTEM, blaLAT, and blaDHA genes by PCR [11] with a GenAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) and AmpliTaq Gold 360 MasterMix (Applied Biosystems). In addition, we searched several fluoroquinolone resistance genes, such as aac(6´)-Ib-cr, qnrA, qnrB and qnrS [12,13]. We amplified the mcr-1 gene using previously reported primers [1]. The obtained fragment was sequenced using an ABI Prism 310 analyzer. The sequence was analyzed with the blastn algorithm [14,15]. To obtain the phylogroup of EC-PAG-733, we used the method described by Clermont et al. [16]. Multilocus sequence typing (MLST) was performed by the amplification of seven housekeeping genes [17]. The sequences were analyzed using the program and database available at http://enterobase.warwick.ac.uk/species/ecoli/allele_st_search [18].
Subsequently, we obtained the whole genome sequence with the Ion Torrent S5 XL sequencer (Thermo Fisher Scientific, Waltham, MA, USA). The genome was assembled and annotated with the Rapid Annotation by Subsystem Technology (RAST) server and analyzed using bioinformatics tools to identify any virulence genes, acquired antimicrobial resistance genes, the serotype; to type the plasmids, to confirm the MLST results and to determinate the probability to be a human pathogen [19–25]. We analyzed the plasmid that contained the mcr-1 gene with blastn and MAUVE 2.4.0 [14, 15, 26].
Results
The EC-PAG-733 isolate was obtained from a pediatric patient diagnosed with pre-B acute lymphoblastic leukemia (L1). The patient had been admitted several times due to febrile neutropenia that required antimicrobial treatments prior to the isolation of EC-PAG-733. The patient required HSCT to treat her hematological disorder.
The first admission for HSCT required a total of 32 days of hospital stay; the patient received an umbilical cord transplant, which resulted in the loss of the graft; the patient had septic shock that was treated with cefepime for 14 days. During the second admission (35 days), the child received a new allogeneic transplant from a nonrelated donor; the graft was successful 10 days after the transplant. The patient presented with febrile neutropenia and was treated with meropenem for 14 days.
During the third admission (60 days), the patient presented with febrile neutropenia, a systemic adenoviral infection, diarrhea that was caused by Clostridium difficile and lymphoproliferative disease due to Epstein Barr virus. During this hospitalization, the patient received antimicrobial treatments with ceftriaxone (10 days), cefepime (3 days), and meropenem (5 days), but not polymyxins.
On day 34 after the third admission, the child presented with noninvasive diarrhea, which was self-limiting after 7 days. No pathogen was identified by a molecular gastrointestinal panel (xTAG Gastrointestinal Pathogen Panel, Luminex Molecular Diagnostics, Toronto, Canada). A stool culture was performed, and the unique isolate was identified as an E. coli strain (EC-PAG-733).
The EC-PAG-733 isolate was susceptible to all carbapenems and amikacin, and it was resistant to third and fourth generation cephalosporins (the phenotypic ESBL test was positive), fluoroquinolones, and gentamicin; the MIC of colistin was 4 μg/mL, so this isolate was classified as a non-wild-type strain (Table 1).
Table 1. Susceptibility profile of EC-PAG-733.
| Agent | AMP | SAM | CFZ | FOX | CAZ | FEP | PTZ | MEM | IPM | ETP | AMK | CIP | LEV | GEN | SXT | CL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC* | >16 | >16/8 | >8 | ≤8 | >16 | >16 | >64/4 | <1 | <1 | <1 | ≤8 | >2 | ≥4 | ≥8 | >2/38 | 4 |
*In μg/mL. AMP: ampicillin; SAM: ampicillin-sulbactam; CFZ: cefazoline; FOX: cefoxitin; CAZ: ceftazidime; FEP: cefepime; PTZ: piperacillin/tazobactam; MEM: meropenem; IPM: imipenem; ETP: ertapenem; CIP: ciprofloxacin; LEV: levofloxacin; AMK: amikacin; GEN: gentamicin; SXT: trimethoprim-sulfamethoxazole; CL: colistin.
Of all the genes that we searched by PCR, we only detected the blaCTX-M-1 group and mcr-1. Based on the MLST results, we concluded that EC-PAG-733 was a ST457 clone, which was confirmed by the analysis of the complete genome. The isolate was classified as a member of the F phylogroup, and its serotype was O11:H25 and contained fimH145. Furthermore, the isolate was predicted as a human pathogen (probability: 0.931; number of matches with pathogenic families: 533).
EC-PAG-733 (S1 Supporting Information) contained several genes that conferred resistance to aminoglycosides (aac(3)-IIa and aadA1), beta-lactams (blaCTX-M-55), chloramphenicol (floR), sulfonamides (sul3), trimethoprim (dfrA14), tetracyclines (tetA), and colistin (mcr-1). Furthermore, we identified mutations in gyrA (S83L, D87) and parC (S80I) that are associated with resistance to fluoroquinolones.
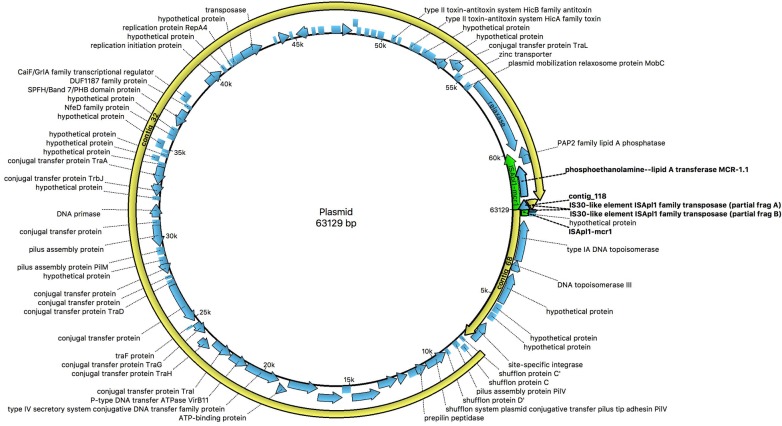
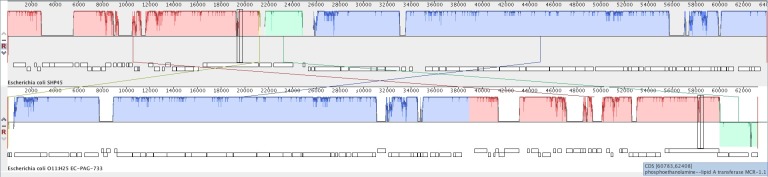
Using plasmid finder software, we identified the mcr-1 gene, which was encoded in a plasmid that belongs to IncI2 within the EC-PAG-733 genome. Within the sequence that was submitted to and annotated by the NCBI, we observed that three contigs, 118, 32 and 68, that comprised practically the entire plasmid (S2 Supporting Information). The plasmid was 63,129 bp in length; the ISApl1 insertion sequence was located upstream of the mcr-1 gene (Fig 1 and S3 Supporting Information). We found that the plasmid exhibited high identity with other plasmids that contain the mcr-1 gene, including pMCR-H9, p14EC033a, plasmid A, and pHNSHP45, based on plasmid alignment (S4 Supporting Information). We compared the pEC-PAG-733 genome with the sequence of the first plasmid that was reported to harbor the mcr-1 gene (Fig 2).
Fig 1. The circular map of the pEC-PAG-733 genome (Ugene).
The location of the mcr-1 gene is shown.
Fig 2. Alignment of pEC-PAG-733 and pHNSHP45 (accession KP347127.1) produced using MAUVE software.
The isolate harbored the virulence-related genes eilA (HilA family transcriptional regulator), iss (increased serum survival), air (Enteroaggregative immunoglobulin repeat protein), cma (colicin M) and lpfA (long polar fimbriae).
We deposited the sequence that was obtained from the whole genome shotgun project in DDBJ/ENA/GenBank under the accession number QKXE00000000 (EC-PAG-733; Supporting Information).
Discussion
There is a paucity of data regarding the presence of E. coli containing the mcr-1 gene in pediatric patients, either in carriers or those with clinical infections [27,28]. We described the epidemiological characteristics of the patient from whom EC-PAG-733 was isolated. The presence of E. coli containing mcr-1 both in infected patients and in healthy carriers has been associated with several risk factors, including male gender, the presence of immunosuppression, previous hospital stays and the use of antibiotics, especially carbapenems and fluoroquinolones [29, 30]. The patient was subject to multiple well-known risk factors, such as previous carbapenem treatment and the presence of underlying diseases that resulted in immunosuppression (leukemia and HSCT). The patient did not receive colistin treatment during any of the hospital stays; however, strains of E. coli containing mcr-1 that were isolated from immunosuppressed patients who did not receive colistin treatment have also been reported [8].
One very relevant epidemiological factor was the fact that this patient had close contact with relatives dedicated to raising pigs, which could be the source of this pathogen; living near a farm has been found to be a risk factor that increases the probability of colonization for healthy individuals [30].
We observed a clear correlation between the resistance phenotype and genotype. We did not find carbapenem resistance. The CTX-M-55 beta-lactamase confers resistance to all beta-lactams except carbapenems, which explains the resistance of EC-PAG-733 to cefazoline, cefoxitin, ceftazidime, and cefepime. The gentamicin resistance could be explained by the presence of the aac(3)-IIa and aadA1 genes. In addition, in the genome of EC-PAG-733, we found mutations in the gyrA and parC genes that have been closely associated with resistance to fluoroquinolones [31], which explained the MICs of ciprofloxacin and levofloxacin of ≥2 and ≥4 μg/mL, respectively.
EC-PAG-733 belongs to the F phylogroup, which is closely related to the B2 phylogroup; some clonal complexes (CC) derived from the F phylogroup are considered emerging lineages that are associated with resistance and mcr-1, such as the epidemic strains of the ST648 complex [32,33]. This finding indicates that EC-PAG-733 could be considered as a pathogen; in addition, other clones of E. coli, such as ST457, could also contribute to the dissemination of the mcr-1 gene.
Several clones have been associated with the spread of the mcr-1 gene, which suggests that horizontal transmission is the mechanism that has allowed for its worldwide dissemination [34]. In Latin American countries, clinical isolates of E. coli with the mcr-1 gene have been reported in many countries, including Brazil [8,35], Venezuela [36], Argentina [37], and Ecuador [7]; these isolates belong to several different STs, which indicates that the dissemination of the mcr-1 gene has been the result of several clonal origins in this region of the world. None of these studies reported the presence of the ST457 clone. In Mexico, there was only one previous report of E. coli containing the mcr-1 gene, which was an E. coli ST44 strain that was isolated from a stool sample from a pig. This isolate and EC-PAG-733 shared several resistance genes, including CTX-M-55, aac(3)-IIa, sul3, tetA, dfrA14, and mutations in gyrA and parC [9].
E. coli ST457 containing the mcr-1 gene has been isolated from carriers, patients and animals in other parts of the world. Some of these reports have indicated that the mcr-1 gene coexists with other acquired resistance mechanisms, as was found in EC-PAG-733, which harbored both mcr-1 and CTX-M-55. In Taiwan, in 2014, an E. coli ST457 isolate from a blood culture was reported that harbored the CTX-M-1 group ESBL and mcr-1 genes [38]. Other groups and families of beta-lactamases have been found in E. coli ST457 with the mcr-1 gene, as was described for an isolate obtained from a bile culture with carbapenem resistance, but no carbapenemase production, and also carrying CTX-M-14 (CTX-M-9 group), CMY-2 and TEM-1 [39].
Other study has documented four isolates of ST457 E. coli, which were found in cattle with mastitis, that harbored CTX-M-27; these isolates had some molecular characteristics in common with EC-PAG-733, such as the presence of aac(3)-II and dfrA14, which are related to aminoglycoside and trimethoprim resistance, respectively [40]; in addition, ST457 clone strains without resistance genes have been isolated from healthy Korean carriers [41].
E. coli isolates containing the mcr-1 and carbapenemase genes have been described mainly with enzymes NDM, KPC and OXA-48 families [42]. EC-PAG-733 was not carbapenem resistant. In E. coli ST457, in particular, the coexistence of mcr-1 with these enzymes has not been reported. However, the appearance of a mcr-1 gene-containing isolate in our hospital resulted in an epidemiological alert because we had recently reported six cases of infection produced by carbapenemase-producing enterobacteria [43]. The horizontal transfer of the mcr-1 gene into carbapenem-resistant Enterobacteriaceae would severely limit antimicrobial treatment options, so it is important to strengthen our surveillance of bacteria exhibiting this type of resistance mechanism. In addition, the mcr-1 gene has also been associated with the high-risk clone ST131. E. coli ST131 strains containing mcr-1 have been isolated from several types of samples, including urine, blood, abscesses and peritoneal fluid [8,38,41,44]. In our hospital, > 40% of ESBL-producing E. coli isolates were ST131 (unpublished data).
We located the mcr-1 gene into an IncI2 plasmid; it belonged to the same incompatibility group of pHNSHP45 as described by Liu et al. in the first report describing mcr-1 in China [1]. There is evidence that as many as 34% of the plasmids that possess the mcr-1 gene belong to IncI2 group. This group has been described frequently in Asia; moreover, IncI2 plasmids containing mcr-1 have also been found in other countries, including the USA [3, 44,45]. Furthermore, within a single animal isolate that was obtained in Mexico, the mcr-1 gene was located in an Incp0111 plasmid [9]. These findings indicate that could be several clones and plasmids that participate in the dissemination of the mcr-1 gene.
EC-PAG-733 belongs to the O11 serogroup, which has been associated with diarrheagenic E. coli, extraintestinal pathogenic E. coli (ExPEC), and avian pathogenic E. coli (APEC) [46,47]. It also contains several virulence factors and belongs to the F phylogroup. We could not establish that the diarrhea of the patient was caused by EC-PAG-733, since it did not correspond to a classic diarrheagenic E. coli type; however, this strain was the unique isolate identified from the stool culture, and we did not detect any other pathogens in the gastrointestinal panel. Moreover, bioinformatic analysis indicated that this isolate had characteristics that would allow it to be a human pathogen.
The virulence factors in clinical isolates containing mcr-1 have not been widely examined [48,49]. Therefore, it was of great interest to us to describe the virulence genes in EC-PAG-733. The isolate was found to contain the eilA gene, which encodes a homolog of the transcriptional regulator HilA and may contribute to the virulence of EAC [50]. This regulator has also been found in EAEC isolates from carriers and patients with diarrhea; the frequency of this gene in these groups was not significant [51].
EC-PAG-733 was also found to harbor the iss gene, which is involved in resistance to the avian complement system [52]. This gene was found in 47% of E. coli isolates that produced CTX-M-1 [11]. In ExPEC isolates obtained from blood cultures, this gene was detected at a lower frequency (9.3%) [53]; within a collection of 85 uropathogenic E. coli isolates that were obtained from patients with urinary tract infections, the iss gene was detected at a 20% frequency; these isolates were classified as belonging to either the A, B2 or D phylogroups [54]. Additionally, the iss gene has been found in Shiga toxin-producing E. coli that were isolated from patients with and without bloody diarrhea and was more frequently found in isolates that did not produce bloody diarrhea; these results were statistically significant [55]. This gene has also been associated with APEC [46].
In EAEC, the air gene is regulated by HilA. This gene was identified at a frequency of 39.5% in isolates from this pathogroup. In HEp-2 cells, Air was shown to contribute to adherence [50], but did not participate in colonization within a mouse model [56]; however, when the frequency of this gene in EAEC isolates obtained from carriers and patients with diarrhea was compared, a significant difference was observed [51].
The cma gene, which encodes colicin M, was found in EC-PAG-733. This virulence factor was also detected in an E. coli ST3204 strain that coproduced NDM-16 and MCR-1 [49]. Colicin M, which is a toxin that is secreted by the bacteria to kill other bacteria of the same species or related species [57], was detected in EC-PAG-733.
In addition, EC-PAG-733 was found to harbor the lpfA gene, which encodes a fimbrial protein that is associated with the invasion of epithelial tissue. This gene has been found in several clones of E. coli as well as Shiga toxin-producing E. coli isolated from children. Additionally, the lpfA gene was found more frequently in samples of nonbloody diarrhea than in samples of bloody diarrhea [55]. The lpfA gene has also been described as a virulence factor within a hybrid enteroagreggative E. coli O104:H4 that had acquired the Shiga-toxin 2 gene [58].We proposed that the coexistence of virulence factors, such as eilA, iss, air, cma and lpfA, in EC-PAG-733 could enhance its role as a pathogen.
Conclusions
To the best of our knowledge, this is the first study that has reported the characteristics of a clinical isolate of E. coli harboring the mcr-1 gene that was obtained from a Mexican pediatric patient. The patient had multiple risk factors for being a carrier or suffering from infectious disease caused by multidrug-resistant microorganisms, such as a prolonged hospital stay, previous treatment with antibiotics and immunosuppression.
The frequency of community-acquired infections caused by bacteria harboring mcr-1 in our country is almost completely unknown because first and second level care hospitals do not have the diagnostic tools required to accurately determine resistance to polymyxins or the genetic characteristics of isolates.
In our hospital, we consider it is essential to increase the surveillance of emerging mechanisms of resistance, such as mcr-1, in a way that involves all healthcare professionals who participate in the diagnosis, treatment, control and prevention of infectious diseases.
Supporting information
(RAR)
(FAS)
(TXT)
(RAR)
Data Availability
All relevant data are within the manuscript; The Whole Genome Shotgun project of EC-PAG-733 was deposited at DDBJ/ENA/GenBank under the accession QKXE00000000.
Funding Statement
Funded by AAA CONSEJO NACIONAL DE CIENCIA Y TECNOLOGIA FOSSIS-2017-1-289537 https://www.conacyt.gob.mx/. AAA,JMV, ADCR Recurso Fiscal Instituto Nacional de Pediatria 2017 Number 2018/017 https://www.pediatria.gob.mx/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis.2016;16:161–8. 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 2.Karaiskos I, Antoniadou A, Giamarellou H. Combination therapy for extensively-drug resistant gram-negative bacteria. Expert Rev Anti Infect Ther. 2017;15:1123–40. 10.1080/14787210.2017.1410434 [DOI] [PubMed] [Google Scholar]
- 3.Matamoros S, van Hattem JM, Arcilla MS, Willemse N, Melles DC, Penders J, et al. Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci Rep. 2017;7:15364 10.1038/s41598-017-15539-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford PA, Kazmierczak KM, Biedenbach DJ, Wise MG, Hackel M, Sahm DF. Correlation of β-lactamase production and colistin resistance among enterobacteriaceae isolates from a global surveillance program.antimicrob Agents Chemother. 2015;60:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quiroga C, Nastro M, Di Conza J. Current scenario of plasmid-mediated colistin resistance in Latin America. Rev Argent Microbiol. 2018; pii: S0325–7541 [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Zhang H, Liu YH, Feng Y. Towards Understanding MCR-like colistin resistance. Trends Microbiol. 2018;26:794–808. 10.1016/j.tim.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 7.Ortega-Paredes D, Barba P, Zurita J. Colistin-resistant Escherichia coli clinical isolate harbouring the mcr-1 gene in Ecuador. Epidemiol Infect. 2016; 144:2967–70. 10.1017/S0950268816001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira FA, Zaccariotto TR, Piveta C, Hofling CC, Resende MR, Levy CE, et al. , MCR-1-positive colistin-resistant Escherichia coli in immunocompromised hospitalised patients.Int J Antimicrob Agents. 2018. pii: S0924–8579. [DOI] [PubMed] [Google Scholar]
- 9.Garza-Ramos U, Tamayo-Legorreta E, Arellano-Quintanilla DM, Rodriguez-Medina N, Silva-Sanchez J, Catalan-Najera, et al. , Draft genome sequence of a multidrug- and colistin-resistant mcr-1-Producing Escherichia coli isolate from a swine farm in Mexico. Genome Announc. 20186;pii: e00102–18. 10.1128/genomeA.00102-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CLSI. Performance Standards for Antimicrobial Susceptibility Testing 28th edi CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute;2018. [Google Scholar]
- 11.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65:490–5. 10.1093/jac/dkp498 [DOI] [PubMed] [Google Scholar]
- 12.Cattoir V, Poirel L, Rotimi V, Soussy C-J, Nordmann P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J Antimicrob Chemother. 2007;60:394–7. 10.1093/jac/dkm204 [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Martínez JM, Machuca J, Cano ME, Calvo J, Martínez-Martínez L, Pascual A. Plasmid-mediated quinolone resistance: Two decades on. Drug Resist Updat Rev Comment Antimicrob Anticancer Chemother. 2016;29:13–29. [DOI] [PubMed] [Google Scholar]
- 14.Morgulis A, Coulouris G, Raytselis Y, Madden TL, Agarwala R, Schäffer AA. Database indexing for production MegaBLAST searches. Bioinforma Oxf Engl. 2008;24:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol J Comput Mol Cell Biol. 2000;7:203–14. [DOI] [PubMed] [Google Scholar]
- 16.Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5:58–65. 10.1111/1758-2229.12019 [DOI] [PubMed] [Google Scholar]
- 17.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006; 60:1136–51. 10.1111/j.1365-2958.2006.05172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alikhan N-F, Zhou Z, Sergeant MJ, Achtman M. A genomic overview of the population structure of Salmonella. PLoS Genet. 2018; 14:e1007261 10.1371/journal.pgen.1007261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–4. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol. 2014;52:1501–10. 10.1128/JCM.03617-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joensen KG, Tetzschner AMM, Iguchi A, Aarestrup FM, Scheutz F. Rapid and easy In silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J Clin Microbiol. 2015;53:2410–26. 10.1128/JCM.00008-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–903. 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosentino S, Voldby Larsen M, Møller Aarestrup F, Lund O. PathogenFinder—distinguishing friend from foe using bacterial whole genome sequence data. Plos One. 2013;8:e77302 10.1371/journal.pone.0077302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clausen PTLC, Zankari E, Aarestrup FM, Lund O. Benchmarking of methods for identification of antimicrobial resistance genes in bacterial whole genome data. J Antimicrob Chemother. 2016; 71:2484–8. 10.1093/jac/dkw184 [DOI] [PubMed] [Google Scholar]
- 25.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol.2012; 50:1355–61. 10.1128/JCM.06094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14(7):1394–403. 10.1101/gr.2289704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y-Y, Wang Y-L, Sun Q-L, Huang Z-X, Wang H-Y, Zhang R, et al. Colistin resistance gene mcr-1 in gut flora of children. Int J Antimicrob Agents. 2017;50:593–7. 10.1016/j.ijantimicag.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 28.Birgy A, Madhi F, Hogan J, Doit C, Gaschignard J, Caseris M, et al. CTX-M-55-, MCR-1-, and FosA-producing multidrug-resistant Escherichia coli infection in a child in France. Antimicrob Agents Chemother. 2018;62: pii e00127–18. 10.1128/AAC.00127-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wangchinda W, Pati N, Maknakhon N, Seenama C, Tiengrim S, Thamlikitkul V. Collateral damage of using colistin in hospitalized patients on emergence of colistin-resistant Escherichia coli and Klebsiella pneumoniae colonization and infection. Antimicrob Resist Infect Control. 2018;7:84 10.1186/s13756-018-0375-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Tian G-B, Zhang R, Shen Y, Tyrrell JM, Huang X, et al. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: an epidemiological and clinical study. Lancet Infect Dis. 2017;17:390–9. 10.1016/S1473-3099(16)30527-8 [DOI] [PubMed] [Google Scholar]
- 31.Hooper DC, Jacoby GA. Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harb Perspect Med. 2016; 6 pii: a025320 10.1101/cshperspect.a025320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Seward CH, Wu Z, Ye H, Feng Y. Genomic insights into the ESBL and MCR-1-producing ST648 Escherichia coli with multi-drug resistance. Sci Bull. 2016; 61:875–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson JR, Johnston BD, Gordon DM. Rapid and specific detection of the Escherichia coli Sequence type 648 complex within phylogroup F. J Clin Microbiol. 2017;55:1116–1121. 10.1128/JCM.01949-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Li Z, Lin J, Wang X, Deng X, Feng Y. Complex dissemination of the diversified mcr-1-harbouring plasmids in Escherichia coli of different sequence types. Oncotarget. 2016;7:82112–22. 10.18632/oncotarget.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonini MA, Soares Jardim Lacerda Batista J, Bueno Freitas L, Borghi M, Santos Almeida M, Cruz Spano L, et al. Carbapenem-susceptible Escherichia coli ST3901 carrying mcr-1 and blaCTX-M genes isolated from a diabetic foot infection in Brazil. J Glob Antimicrob Resist. 2018;13:209–10. 10.1016/j.jgar.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 36.Delgado-Blas JF, Ovejero CM, Abadia-Patiño L, Gonzalez-Zorn B. Coexistence of mcr-1 and blaNDM-1 in Escherichia coli from Venezuela. Antimicrob Agents Chemother. 2016;60:6356–8. 10.1128/AAC.01319-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tijet N, Faccone D, Rapoport M, Seah C, Pasterán F, Ceriana P, et al. Molecular characteristics of mcr-1-carrying plasmids and new mcr-1 variant recovered from polyclonal clinical Escherichia coli from Argentina and Canada. PloS One. 2017;12:e0180347 10.1371/journal.pone.0180347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo SC, Huang WC, Wang HY, Shiau YR, Cheng MF, Lauderdale TL. Colistin resistance gene mcr-1 in Escherichia coli isolates from humans and retail meats, Taiwan. J Antimicrob Chemother. 2016;71:2327–9. 10.1093/jac/dkw122 [DOI] [PubMed] [Google Scholar]
- 39.Yu H, Qu F, Shan B, Huang B, Jia W, Chen C, et al. Detection of the mcr-1 Colistin resistance gene in carbapenem-resistant Enterobacteriaceae from different hospitals in China. Antimicrob Agents Chemother. 2016; 60:5033–5. 10.1128/AAC.00440-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki S, Ohnishi M, Kawanishi M, Akiba M, Kuroda M. Investigation of a plasmid genome database for colistin-resistance gene mcr-1. Lancet Infect Dis. 2016;16:284–5. 10.1016/S1473-3099(16)00008-6 [DOI] [PubMed] [Google Scholar]
- 41.Joo EJ, Kim SJ, Baek M, Choi Y, Seo J, Yeom JS, et al. Fecal carriage of antimicrobial-resistant Enterobacteriaceae in healthy Korean adults. J Microbiol Biotechnol. 2018;28:1178–84. 10.4014/jmb.1801.12060 [DOI] [PubMed] [Google Scholar]
- 42.Rhouma M, Letellier A. Extended-spectrum β-lactamases, carbapenemases and the mcr-1 gene: is there a historical link? Int J Antimicrob Agents. 2017;49:269–71. 10.1016/j.ijantimicag.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 43.Aquino-Andrade A, Merida-Vieyra J, Arias de la Garza E, Arzate-Barbosa P, De Colsa Ranero A. Carbapenemase-producing Enterobacteriaceae in Mexico: report of seven non-clonal cases in a pediatric hospital. BMC Microbiol. 2018;18:38 10.1186/s12866-018-1166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortiz de la Tabla V, Ortega A, Buñuel F, Pérez-Vázquez M, Marcos B, Oteo J. Detection of the high-risk clone ST131 of Escherichia coli carrying the colistin resistance gene mcr-1 and causing acute peritonitis. Int J Antimicrob Agents. 2017;49:115–6. 10.1016/j.ijantimicag.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 45.Meinersmann RJ, Ladely SR, Plumblee JR, Cook KL, Thacker E. Prevalence of mcr-1 in the cecal contents of food animals in the United States. Antimicrob Agents Chemother. 2017; 61: pii e02244–16. 10.1128/AAC.02244-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maluta RP, Logue CM, Casas MRT, Meng T, Guastalli EAL, Rojas TCG, et al. Overlapped sequence types (STs) and serogroups of avian pathogenic (APEC) and human extra-intestinal pathogenic (ExPEC) Escherichia coli isolated in Brazil. 2017; 61 pii: e02244–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fratamico PM, Bagi LK, Bush EJ, Solow BT. Prevalence and characterization of shiga toxin-producing Escherichia coli in swine feces recovered in the National Animal Health Monitoring System’s Swine 2000 study. Appl Environ Microbiol. 2004; 70:7173–8. 10.1128/AEM.70.12.7173-7178.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Q-W, Xu X-H, Lan F-J, Zhao Z-C, Wu Z-Y, Cao Y-P, et al. Molecular characteristic of mcr-1 producing Escherichia coli in a Chinese university hospital. Ann Clin Microbiol Antimicrob. 2017;16:32 10.1186/s12941-017-0207-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Mu X, Zhang P, Zhao D, Ji J, Quan J, et al. Detection and characterization of a clinical Escherichia coli ST3204 strain coproducing NDM-16 and MCR-1. Infect Drug Resist. 2018;11:1189–95. 10.2147/IDR.S175041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheikh J, Dudley EG, Sui B, Tamboura B, Suleman A, Nataro JP. EilA, a HilA-like regulator in enteroaggregative Escherichia coli. Mol Microbiol. 2006; 61:338–50. 10.1111/j.1365-2958.2006.05234.x [DOI] [PubMed] [Google Scholar]
- 51.Nüesch-Inderbinen MT, Hofer E, Hächler H, Beutin L, Stephan R. Characteristics of enteroaggregative Escherichia coli isolated from healthy carriers and from patients with diarrhoea. J Med Microbiol. 2013; 62:1828–34. 10.1099/jmm.0.065177-0 [DOI] [PubMed] [Google Scholar]
- 52.Nolan LK, Horne SM, Giddings CW, Foley SL, Johnson TJ, Lynne AM, Skyberg J. Resistance to serum complement, iss, and virulence of avian Escherichia coli.Vet Res Commun. 2003;27:101–10. [DOI] [PubMed] [Google Scholar]
- 53.Bozcal E, Eldem V, Aydemir S, Skurnik M. The relationship between phylogenetic classification, virulence and antibiotic resistance of extraintestinal pathogenic Escherichia coli in İzmir province, Turkey. PeerJ. 2018; 6:e5470 10.7717/peerj.5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derakhshandeh A, Firouzi R, Motamedifar M, Motamedi Boroojeni A, Bahadori M, Arabshahi S, et al. Distribution of virulence genes and multiple drug-resistant patterns amongst different phylogenetic groups of uropathogenic Escherichia coli isolated from patients with urinary tract infection. Lett Appl Microbiol. 2015;60:148–54. 10.1111/lam.12349 [DOI] [PubMed] [Google Scholar]
- 55.Matussek A, Jernberg C, Einemo I-M, Monecke S, Ehricht R, Engelmann I, et al. Genetic makeup of Shiga toxin-producing Escherichia coli in relation to clinical symptoms and duration of shedding: a microarray analysis of isolates from Swedish children. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2017; 36:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrington SM, Sheikh J, Henderson IR, Ruiz-Perez F, Cohen PS, Nataro JP. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect Immun. 2009; 77:2465–73. 10.1128/IAI.01494-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barreteau H, El Ghachi M, Barnéoud-Arnoulet A, Sacco E, Touzé T, Duché D, et al. Characterization of colicin M and its orthologs targeting bacterial cell wall peptidoglycan biosynthesis. Microb Drug Resist Larchmt N. 2012; 18:222–9. [DOI] [PubMed] [Google Scholar]
- 58.Ross BN, Rojas-Lopez M, Cieza RJ, McWilliams BD, Torres AG.The role of long polar fimbriae in Escherichia coli O104:H4 adhesion and colonization.PLoS One. 2015;10:e0141845 10.1371/journal.pone.0141845 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RAR)
(FAS)
(TXT)
(RAR)
Data Availability Statement
All relevant data are within the manuscript; The Whole Genome Shotgun project of EC-PAG-733 was deposited at DDBJ/ENA/GenBank under the accession QKXE00000000.