Abstract
The introduction and establishment of fall armyworm (Spodoptera frugiperda) in Africa presents a major threat to agriculture in that continent and potentially to the entire Eastern Hemisphere. The species is subdivided into two subpopulations called the R-strain and C-strain that differ in their distribution on different plant hosts. This means that the scope of the economic risk posed by invasive fall armyworm is influenced by whether one or both strains are present. Multiple studies have found mitochondrial markers diagnostic of the two strains throughout Africa but there is substantial disagreement with a nuclear strain marker that makes conclusions about strain composition uncertain. In this study the issue of whether both strains are present in Africa was tested by an assay that can detect strain-biased mating behaviors. Western Hemisphere fall armyworm consistently showed evidence of strain-specific assortative mating in the field that was not found in surveys from multiple locations in Africa. The absence of strain mating biases and the disagreements between the strain diagnostic genetic markers indicates that the R-strain is rare (<1% of the population) or absent in Africa. Instead, it appears that the African fall armyworm populations are dominated by two groups, the C-strain and the descendants of interstrain hybrids. These results suggest that plant hosts associated with the R-strain may not be at high risk of fall armyworm infestation in Africa.
Introduction
Fall armyworm (Spodoptera frugiperda) is an important agricultural pest of the Western Hemisphere that has recently spread into Africa where it has the potential for significant economic impact [1]. The species has a broad host range having been found on greater than 80 plant species and cultivars [2]. However, infestations of agricultural importance are limited to a few crops in the Western Hemisphere, indicating that while capable of being broadly polyphagous, fall armyworm tends to be more specific in its host usage. The species can be subdivided into two subpopulations defined by their differential distribution in the field [3]. The C-strain (historically designated the corn-strain) is typically found on corn, sorghum, and cotton, while the R-strain (rice-strain) predominates on rice, alfalfa, pasture grasses, and millet, though subsequent observations indicate that the preference to rice may be variable [4–7]. There is genetic evidence that both strains are present in Africa suggesting that a variety of crop systems are at risk [8–11]. However, reports of fall armyworm damage to date have been primarily limited to C-strain preferred hosts, specifically corn and sorghum [12]. There are some anecdotal reports of infestations in R-strain host plants, but whether these occur consistently or incur significant economic damage has yet to be documented.
The fall armyworm strains were originally defined by the differential distribution of genetic markers among host plants in larval field collections [3, 13]. Most commonly used for population studies are mitochondrial haplotypes, with those defined by polymorphisms in the Cytochrome oxidase subunit I gene (COI) the best characterized [14–16]. Strain markers from nuclear DNA have been difficult to isolate and are currently limited to a small number of polymorphic loci located on the Z sex chromosome [17–19]. One such set of Z-linked markers are found in the Triosephosphate isomerase gene (Tpi), which is thought to have a general housekeeping function [20] and is highly conserved in noctuid moths [18]. The Tpi gene may be linked to loci important for speciation as polymorphisms can distinguish between the sibling species Helicoverpa armigera (Hübner) and H. zea (Boddie) and two "races" of Ostrinia nubialis (Hübner) [21, 22]. In fall armyworm the correspondence between the Tpi strain markers and host plants tends to be higher than that observed with mitochondrial markers, suggesting that Tpi is the more accurate indicator of strain identity [18, 23].
The preferential association of C-strain (COI-CS and TpiC) and R-strain (COI-RS and TpiR) markers to larvae found on different host plants has been demonstrated in both Americas, indicating a hemispheric distribution of the two strains [5, 6]. However, the correspondence between marker and plant host is not absolute. Inconsistencies include instances where specimens collected from a particular plant host do not have the expected strain marker [4, 18, 24, 25], as well as disagreements between the mitochondrial and nuclear markers [13, 18, 26]. Such inconsistencies could result from interstrain mating, where the resulting hybrids and their derivatives would have variable marker configurations and unknown plant host preferences.
Field studies of the two strains are difficult because of their morphological and behavioral similarities. Strain-specific differences have been reported for mating behavior [27–31], pesticide resistance [32–34], and pheromone composition [35–37]. However, fall armyworm can exhibit considerable phenotypic variation between regional populations and laboratory colonies that are independent of strain differences. For example, differential response to pheromone lures was found to vary by geography as much as by strain [38] and the observation of strain-specific mating behaviors can be colony dependent [39]. There are reports that South American fall armyworm display significant strain-differences in wing shape and size [40, 41], but it is not yet known whether this phenotypic distinction applies to populations from other locations. At this time the strains are considered to be morphologically indistinguishable with genetic markers remaining the most reliable means of strain identification.
Pheromone trap collections typically contain both host strains indicating a sympatric distribution. This would seem to require the existence of reproductive barriers between strains to maintain their continued divergence. Several mechanisms have been reported based primarily on laboratory studies. These include strain-specific assortative mating [28, 29] attributed to differences in female pheromone composition [36, 42] and the timing of mating behavior during scotophase [28, 30, 31]. Post-mating barriers have also been observed in the form of lower viability or fertility of interstrain hybrids [29, 39]. However, at this time there has been no conclusive demonstration that any of these mechanisms are actually restricting interstrain hybridization in the field.
This uncertainty is in large part due to the difficulty in identifying interstrain hybrids given the morphological equivalence of the two parental strains. For this reason, we recently developed a strategy for estimating the frequency of interstrain hybrid formation in field populations that used neutral single-base polymorphisms (SNPs) within the Tpi coding region that differ in their degree of strain-specificity [43]. In principle, the frequency of heterozygosity at the sites with high strain-specificity should be indicative of interstrain hybridization, while heterozygosity at sites with low specificity will reflect successful mating both within and between strains. Therefore, comparisons between the two should provide a direct measure of the frequency of interstrain relative to intrastrain hybridization. The results obtained from collections at multiple sites in the United States were consistent with at least a 4-fold reduction in hybridization frequency between strains versus within strains [43].
In this paper, the distribution of genetic markers in Africa was examined in more detail and compared with observations from the Western Hemisphere. Previously reported disagreements between the COI and Tpi markers in African populations [9, 10] were quantified and reinterpreted from the perspective of interstrain hybrids. The presence of the R-strain and the possible impact of hybrids on mating behavior were examined by the application of the Tpi heterozygosity methodology on the African fall armyworm populations. We first tested whether this methodology was sufficiently robust to detect strain-specific heterozygosity differences in larval collections where gender is not known, as well as with databases where multiple collections are pooled. The analysis was then applied to pheromone trap collections and larval samplings from Africa. The implications of the results to the strain composition of the African fall armyworm population are discussed.
Materials and methods
Fall armyworm were collected from pheromone traps (adult males) and larvae from corn or sorghum host plants (gender unknown) at various sites in the Western Hemisphere and Africa (Fig 1, Table 1). No endangered or protected species were involved in this study. We obtained permission from private farmers for access and data collection in their fields. The collections have been previously described, with their archived DNA preparations or additional specimens used for the new analysis in this study. All specimens were stored at -20°C until DNA preparation.
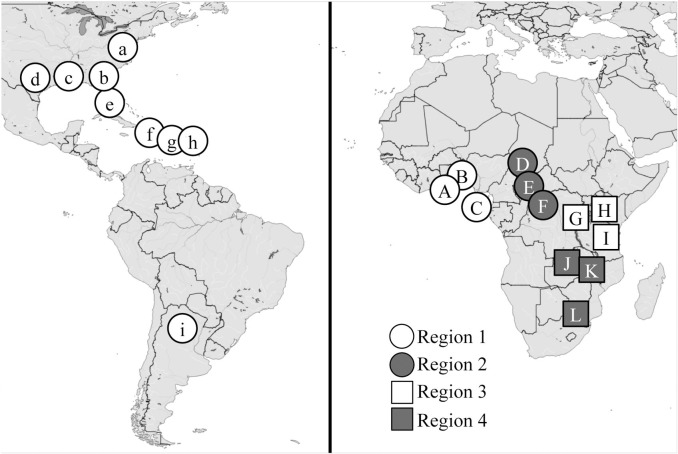
Fig 1. Map of the Western Hemisphere and Africa showing locations of fall armyworm collections.
Letters refer to Table 1 (parenthesis). Africa collections were grouped into regions designated by shapes and shadings. The map was generated using the free and open source software QGIS version 2.18 (http://www.qgis.org/en/site/about/index.html).
Table 1. Fall armyworm collections.
| Location (symbol in Fig 1) | Collection | Latitude | Longitude | Reference |
|---|---|---|---|---|
| i. Pooled larval collections | ||||
| Georgia (b) | USA-L | 33.09 | -84.39 | [44] |
| Mississippi (c) | USA-L | 33.25 | -90.91 | [45] |
| Puerto Rico (g) | Crb-L | 18.47 | -66.99 | [44] |
| St. Kitts (h) | Crb-L | 17.32 | -62.74 | [46] |
| Argentina (i) | Arg-L | -26.89 | -65.37 | [5] |
| Argentina (i) | Arg-L | -27.48 | -58.77 | [5] |
| Argentina (i) | Arg-L | -31.65 | -60.72 | [5] |
| Togo (B) | Region 1 | 6.17 | 1.30 | [10] |
| Ghana (A) | Region 1 | 7.34 | 0.41 | S1 Table |
| Ghana (A) | Region 1 | 6.37 | -1.36 | S1 Table |
| São Tomé and Príncipe (C) | Region 1 | 0.30 | 6.61 | [9] |
| Central African Republic (E) | Region 2 | 4.98 | 18.22 | S1 Table |
| Chad (D) | Region 2 | 8.68 | 16.23 | S1 Table |
| Chad (D) | Region 2 | 8.39 | 17.59 | S1 Table |
| Democratic Republic Congo (F) | Region 2 | 2.97 | 17.67 | [9] |
| Burundi (G) | Region 3 | -3.70 | 29.81 | [9] |
| Kenya (H) | Region 3 | 1.26 | 38.28 | [9] |
| Tanzania (I) | Region 3 | -7.30 | 37.50 | [9] |
| Democratic Republic Congo (J) | Region 4 | -11.93 | 28.29 | [9] |
| South Africa (L) | Region 4 | -23.18 | 30.51 | S1 Table |
| South Africa (L) | Region 4 | -26.63 | 26.12 | S1 Table |
| Zambia (K) | Region 4 | -13.23 | 30.23 | S1 Table |
| ii. Single pheromone trap collections | ||||
| Dominican Republic (f) | DR16T | 19.21 | -70.53 | [43] |
| Pennsylvania (a) | PA15T | 41.84 | -80.01 | [43] |
| Florida (e) | FL05T | 29.76 | -82.56 | [43] |
| Florida (e) | FL12T | 28.56 | -81.57 | [43] |
| Georgia (e) | GA07T | 30.79 | -83.95 | [43] |
| iii. Pooled pheromone trap collections | ||||
| Florida (e) | FL-T | 25.44 | -80.54 | [47, 48] |
| Pennsylvania (a) | PA-T | 40.82 | -77.72 | [49] |
| Texas (d) | TX-T | 26.10 | -98.09 | [47–49] |
| Puerto Rico (g) | PR-T | 18.47 | -66.99 | [44, 46] |
| Lomé, Togo (B) | TogTa-d | 6.17 | 1.30 | S1 Table |
DNA preparation and PCR amplification
Individual specimens were homogenized in 1.5 ml of phosphate buffered saline (PBS, 20 mM sodium phosphate, 150 mM NaCl, pH 8.0) using a tissue homogenizer (PRO Scientific Inc., Oxford, CT) or hand-held Dounce homogenizer and the homogenate transferred to a 2-ml microcentrifuge tube. Cells and tissue were pelleted by centrifugation at 6000g for 5 min. at room temperature. The pellet was resuspended in 800 μl Genomic Lysis buffer (Zymo Research, Orange, CA) by vortexing and incubated at 55°C for 5 min. Debris was removed by centrifugation at 10,000 rpm for 3 min. The supernatant was transferred to a Zymo-Spin III column (Zymo Research, Orange, CA) and processed according to manufacturer’s instructions. The DNA preparation was increased to a final volume of 100 μl with distilled water. Genomic DNA preparations of fall armyworm samples from previous studies were stored at -20°C.
Polymerase chain reaction (PCR) amplification for each gene segment was done separately, using a 30-μl reaction mix containing 3 μl 10X manufacturer’s reaction buffer, 1 μl 10mM dNTP, 0.5 μl 20-μM primer mix, 1 μl DNA template (between 0.05–0.5 μg), 0.5 units Taq DNA polymerase (New England Biolabs, Beverly, MA) and water. The thermocycling program was 94°C (1 min), followed by 33 cycles of 92°C (30 s), 56°C (45 s), 72°C (45 s), and a final segment of 72°C for 3 min. Typically 96 PCR amplifications were performed at the same time using either 0.2-ml tube strips or 96 well microtiter plates. Primers were synthesized by Integrated DNA Technologies (Coralville, IA). Amplification of CO1 used the primer pair CO1-891F (5’-TACACGAGCATATTTTACATC-3’) and CO1-1472R (5’-GCTGGTGGTAAATTTTGATATC-3’) to produce a 603-bp fragment. Amplification of the Tpi region was done with the primers Tpi412F (5’-CCGGACTGAAGGTTATCGCTTG -3’) and Tpi1140R (5’-GCGGAAGCATTCGCTGACAACC -3’) that spans a variable length intron to produce a fragment with a mean length of 500 bp.
For fragment isolations, 6 μl of 6X gel loading buffer was added to each amplification reaction and the entire sample run on a 1.8% agarose horizontal gel containing GelRed (Biotium, Hayward, CA) in 0.5X Tris-borate buffer (TBE, 45 mM Tris base, 45 mM boric acid, 1 mM EDTA pH 8.0). Fragments were visualized on a blue LED illuminator (Biotium, Hayward, CA) and cut out from the gel. Fragment isolation was performed using Zymo-Spin I columns (Zymo Research, Orange, CA) according to manufacturer’s instructions. DNA sequencing was performed directly from the gel purified PCR fragments by Sanger sequencing (Genewiz, South Plainfield, NJ).
Determination of strain-identity using COI
Preliminary identification of strain identity involved EcoRV restriction enzyme (New England Biolabs, Beverly, MA) of the PCR product produced by the CO1-891F/1472R primers. A strain-specific polymorphism at site 1182 produces in an EcoRV recognition site in the COI-RS haplotype but not with COI-CS [6, 14, 25]. Uncut fragments were further analyzed by DNA sequencing to confirm C-strain identity.
Analysis of Tpi polymorphisms
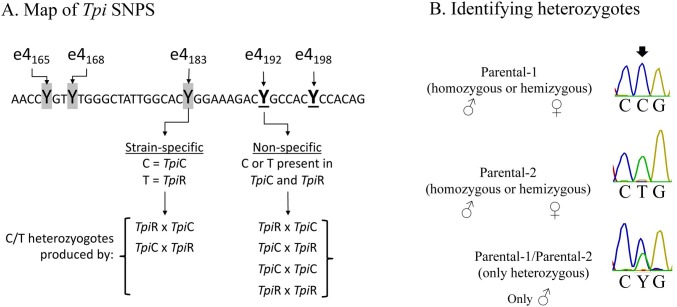
The single nucleotide polymorphisms (SNPs) used in this study are located in the fourth exon of the Tpi coding region. Three strain-specific SNPs are present, of which e4183 is representative and diagnostic in this study of the TpiC and TpiR haplotypes ([18], Fig 2A). Two other SNPs are found that also show high variability but display much less strain-specificity than e4183 [43]. For the purposes of this study they were considered non-specific, with site e4192 serving as the representative for both. The polymorphism associated with all five SNPs is a choice between C or T, neither of which alters the presumptive amino acid sequence.
Fig 2. Map of selected Tpi SNPs that differ in strain-specificity including a list of the crosses that will produce heterozygotes at these sites and a description of the method to identify heterozygotes.
(A) DNA sequence of the segment of the fourth exon of the coding region containing three strain-specific sites (shaded) and two non-specific sites (underlined and bold). All SNPs are associated with a C or T (designated Y by IUPAC convention). Below are the types of crosses that can produce heterozygotes at e4183 and e4192. (B) Example of DNA sequencing chromatographs diagnostic of the two parental alleles as present as a hemizygote or homozygote, and the chromatograph representing the heterozygous combination that shows an overlap of the parental curves.
The PCR amplified fragment produced by the primer pair Tpi412F/1140R contains both SNP sites and can be simultaneously read from a single sequencing run using Tpi412F as primer. The Tpi gene is Z-linked and so is hemizygous in females (ZW) and either homozygous or heterozygous in males (ZZ) with respect to the e4183 and e4192 SNPs. Sequencing of the PCR product amplified from genomic DNA can distinguish between homozygotes and heterozygotes, with the latter identified by overlapping chromatographs at the polymorphic site (Fig 2B). However, this method cannot distinguish hemizygotes from homozygotes, which is not relevant for trap collections where all specimens are males but is a consideration with larval collections where both sexes are present but not identified.
Detecting strain-specific assortative mating in field populations
The rationale for using the inbreeding coefficient F to assess strain mating behavior is based on the assumption that the frequency of heterozygosity of two SNPs that differ in strain distribution but are otherwise similar (i.e., are located in the same exon, have the same neutral polymorphic alternatives, and are detected by the same set of PCR amplification and DNA sequencing reactions) will depend on the frequency of mating between strains relative to that occurring within strains [43].
The frequencies of the C-allele and T-allele for each SNP were estimated using Hardy-Weinberg equilibrium analysis. The allele frequencies for C and T are given by p and q, respectively, such that p + q = 1. For each collection p was calculated by the equation p = freqCC + 0.5[freqY], where freqCC is the observed frequency of CC homozygotes and freqY the observed frequency of Y (CT) heterozygotes. The frequency of the T-allele (q) is then given by the equation q = 1—p. The local expected heterozygote frequency, He, is equal to the equation He = 2pq. The local observed heterozygote frequency, Ho, is given by the empirically determined freqY. Wright's local inbreeding coefficient, F = (He—Ho)/He, was calculated for SNPs e4183 and e4192.
The difference in strain-specificity between SNPs e4183 and e4192 means that heterozygosity at the two sites results from different sets of mating patterns. Heterozygotes at e4192 can be produced by mating both between (inter) and within (intra) strains while heterozygotes at the strain-specific e4183 site can only occur through interstrain hybridization (Fig 2A). Therefore, if reproduction barriers between strains exist such that intrastrain is more frequent than interstrain hybridization, then the frequency of observed heterozygosity at e4183 will be more severely affected than at e4192. This will result in F183 being greater than F192.
DNA sequence and statistical analysis
DNA sequence alignments and comparisons were performed using programs available on the Geneious 10.0.7 software (Biomatters, Auckland, New Zealand). Basic mathematical calculations and generation of graphs were done using Excel and PowerPoint (Microsoft, Redmond, WA). Other statistical analyses including ANOVA, t-tests, and chi-square were performed using GraphPad Prism version 7.00 for Mac (GraphPad Software, La Jolla California USA).
Results
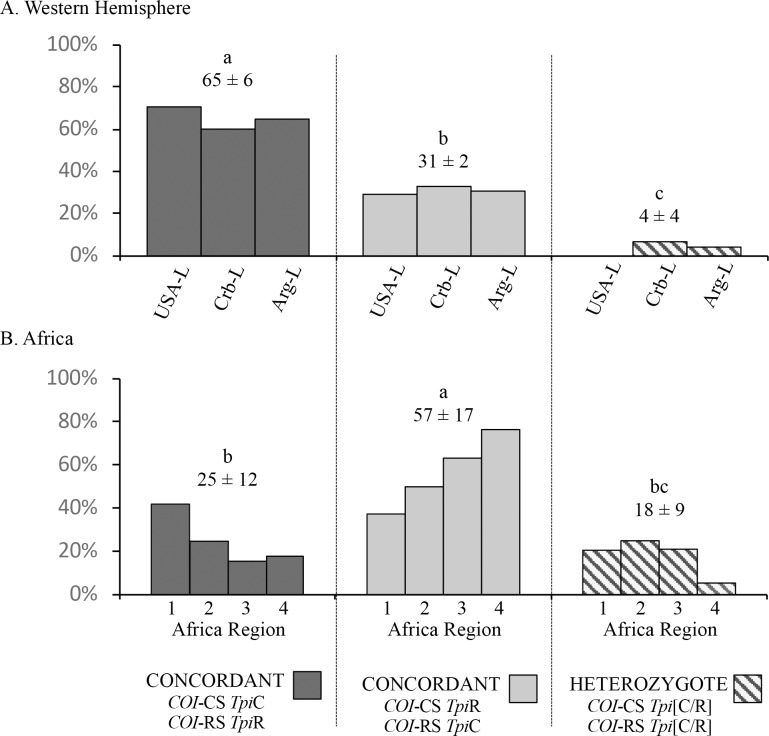
Earlier studies demonstrated significant inconsistencies in the distribution of the COI and Tpi genetic markers in the African fall armyworm populations [9]. A majority of the specimens (all collected from C-strain hosts) displayed the discordant combination of the R-strain COI-RS together with the C-strain TpiC marker. This high frequency of discordance between markers was compared to that observed from equivalent Western Hemisphere larval collections also from corn or sorghum hosts (Fig 3). Specimens from locations in Florida, the Caribbean, and Argentina, each gave similar results to each other, with a mean of 65% showing concordance (COI-CS TpiC or COI-RS TpiR) in their marker configuration that was significantly higher than the discordant frequency (Fig 3A). A mean of 4% of specimens was heterozygous for TpiR/TpiC, which is likely to be an underestimate because the larval collections were not identified by gender. Females (ZW) are hemizygous for Tpi and so added to the concordant and discordant categories but did not contribute to the number of Tpi heterozygotes (Fig 2B).
Fig 3. Comparisons of COI and Tpi marker configurations as categorized by their agreement in identifying strains (concordance), disagreement (discordance), and heterozygosity for the Tpi strain-specific alleles (TpiR/TpiC).
The mean frequency ± the standard deviation (SD) is listed for each category. Comparisons between the means were done using single-tailed ANOVA analysis (P < 0.0001, R2 = 0.8482, F = 16.76) followed by Tukey’s comparison testing with significant differences indicated by different lower-case letters. (A) Western Hemisphere (WH) fall armyworm populations. The categories are composed of the pooled larval collections (Table 1). (B) Africa fall armyworm populations categorized by regions (Fig 1).
A different pattern was observed with the African fall armyworm populations ([9, 10], Fig 3B). Only about 25% of the African specimens were concordant for COI and Tpi, compared to a mean of 57% for the discordant configuration, with both frequencies significantly different from that observed in the Western Hemisphere. The mean of 18% male heterozygotes was 4-fold higher than the mean of 4% observed in the Western Hemisphere. This difference was not statistically significant when analyzed by ANOVA for all three categories (Concordant, Discordant, and Heterozygote), but was significant by a direct pairwise comparison using a two-tailed t-test (P = 0.0483, t = 2.599, df = 5).
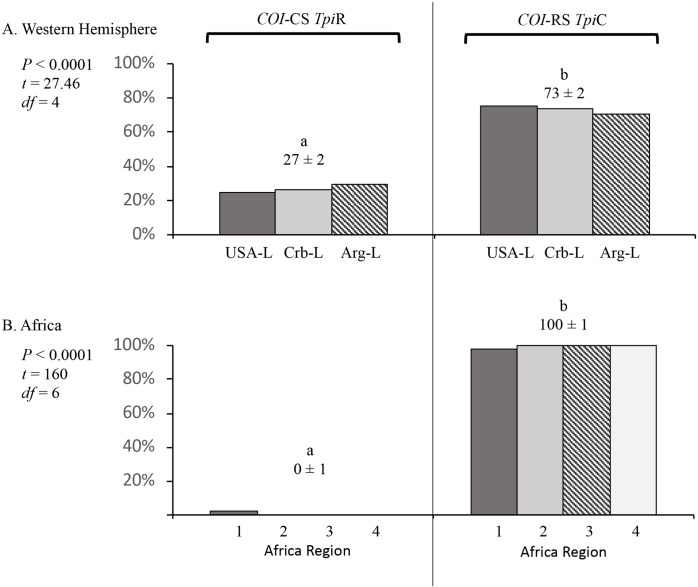
Predominance of the COI-RS TpiC discordants
A consistent observation from field studies in the Western Hemisphere is that the COI-RS TpiC configuration occurs more frequently than the reciprocal discordant genotype of COI-CS TpiR [23]. This is illustrated by the Western Hemisphere larval collections from C-strain plants where the proportion of the COI-CS TpiR configuration among discordants (27%) was significantly lower than the 73% mean frequency of COI-RS TpiC specimens (Fig 4A). A similar but more extreme trend was found in Africa, where in each of the four regions nearly all discordant specimens were COI-RS TpiC (Fig 4B).
Fig 4. Comparison of the COI-CS TpiR and COI-RS TpiC configuration frequencies that make up the discordant category described in Fig 3.
The mean frequency ± SD is listed for each category. Pair-wise comparisons between the means were calculated using single-tailed t-test with significant differences indicated by different lower-case letters. (A) Western Hemisphere collections. (B) Africa collections. The same collections were used as in Fig 3. All collections were from corn-strain preferred host plants.
Detecting strain-specific assortative mating in field populations
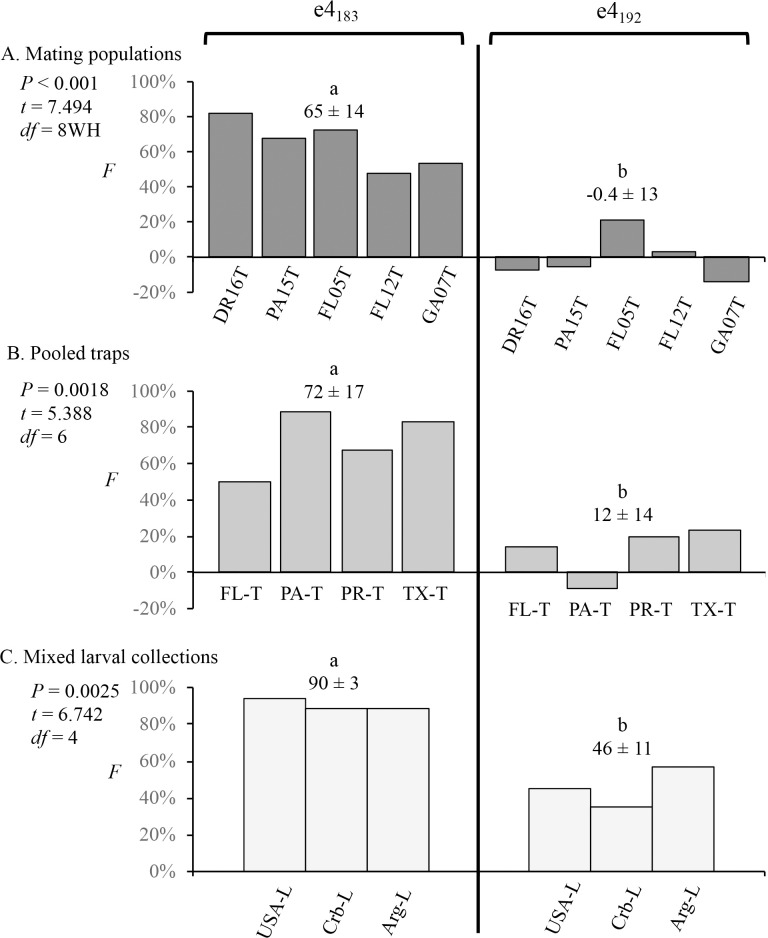
Earlier studies demonstrated that comparisons of heterozygosity frequencies of SNPs that differ in their degree of strain-specificity could provide evidence of reduced interstrain hybridization in field populations [43]. The initial studies were based on pheromone collections from the same location and time period, which were presumed to represent a single mating population with the allele frequencies observed used to calculate the inbreeding coefficient, F. Such collections consistently showed a higher F value for the strain-specific e4183 SNP (F183) compared to that of the non-specific e4192 SNP (F192) independent of location (Fig 5A).
Fig 5. Comparisons of inbreeding coefficients (F) for the e4183 and e4192 SNPs calculated for different mating populations and collection methods.
The mean F ± SD is listed for each category. Pairwise comparisons between the means were calculated using single-tailed t-test with significant differences indicated by different lower-case letters. (A) Inbreeding coefficients calculated for pheromone trap collections from a single time and location and therefore are representative of a single breeding population. (B) Inbreeding coefficients calculated for pooled pheromone trap collections from different times or locations within a state. (C) Inbreeding coefficients calculated for pooled larval collections from a given country or region.
A characteristic of this technique, at least in principle, is that external factors can be discounted as long as they equally affect both F values such that their relative difference is maintained. This was confirmed in two ways. In the first, pheromone trap collections from different times and locations were pooled resulting in allele frequencies derived from multiple mating populations (Fig 5B). In this case the calculated composite F coefficients were not representative of an actual field population but still consistently showed F183 greater than F192 at all locations. A second test pooled larval collections from different time periods. Because the gender of the larvae was unknown, an unspecified subset of these will be female, which have only one copy of the Tpi gene and are indistinguishable from males that are homozygous for a Tpi allele. Since Tpi heterozygotes are only possible in males, the inclusion of female larvae will result in an underestimate of heterozygote frequency and therefore an overestimate of F. The results of the larval collections were consistent with these expectations, with both F183 and F192 values generally higher than observed with the trap collections, but with F183 still significantly greater than F192 (Fig 5C).
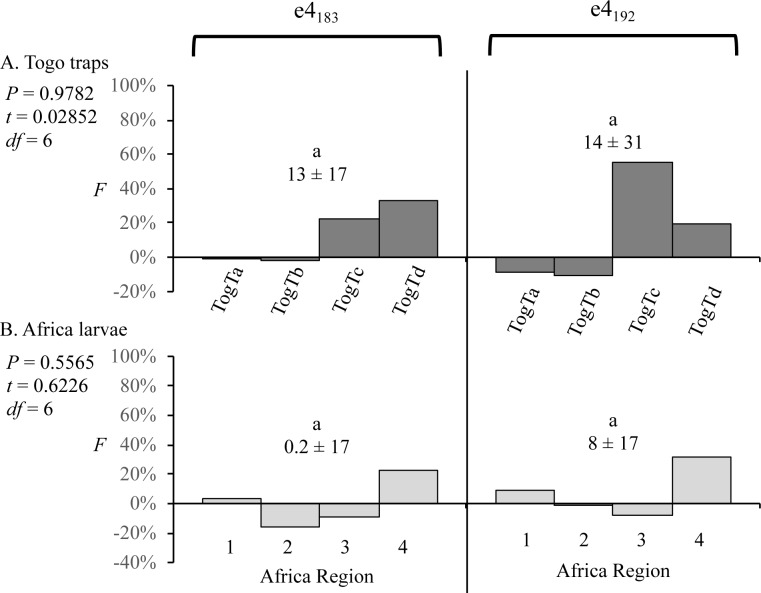
This methodology was applied to two different sets of African collections. The first were pheromone trap collections from four different time periods at a single location in Togo, with each time period collection assumed to be representative of the local mating population. There was substantial variation in F values between sites but no significant difference between mean F183 and F192 (Fig 6A). Similar results were obtained with the second collection set that was composed of pooled larval specimens from multiple locations within each of the four African regions (Fig 6B). Once again there was no statistically significant difference between F183 and F192 values. The African larval collections also differed from its Western Hemisphere counterpart in that the expected overestimate of F (in larval collections that presumably includes females) was not observed (compare Figs 5C and 6B). The reason for this difference is unknown.
Fig 6. Comparisons of inbreeding coefficients (F) for the e4183 and e4192 SNPs in African fall armyworm collections.
The mean F ± SD is listed for each category. Comparisons between the means were done using single-tailed t-test with significant differences indicated by different lower-case letters. Comparisons between the means were done using single-tailed t-test and no significant differences were found. (A) Inbreeding coefficients calculated for pheromone trap collections Togo. (B) Inbreeding coefficients calculated for pooled larval collections from four different regions in Africa (Fig 1).
Discussion
The inbreeding coefficient F describes the difference between the observed heterozygosity (Ho) of a particular allele and the heterozygosity expected (He) as calculated from the parental allele frequencies and based on assumptions of random mating and equal fitness of the resultant products. In the absence of reproductive barriers between parents the expected relationships will be Ho = He and F = 0, while the existence of reproductive barriers will result in Ho < He and F approaching +1. This methodology was used in a previous study to demonstrate the existence of reproductive barriers between fall armyworm host strains that could be detected in field populations. It showed that F for strain-specific e4183 SNP on average 4-fold larger than the F of the nearby e4192 SNP that is less strain-specific, consistent with a significantly reduced frequency of interstrain hybridization [43].
In this study the method was shown to be viable in composite collections from different time periods and locations, even when using larval specimens of undetermined gender. This allows for the pooling of collections with small sample sizes, making possible analysis of the larval African specimens grouped by geographical region. The one limitation are collections where only a single parental allele is present as heterozygotes in these cases are not possible.
Are both fall armyworm strains present in Africa?
The presence of the R-strain in Africa is based on mitochondrial markers primarily in the COI gene [8–11], but is made uncertain by three observations. First, there are widespread reports throughout the sub-Sahara region of fall armyworm infestation in corn and sorghum, two hosts preferred by the C-strain, but major infestations in host plants preferred by the R-strain have yet to be documented. Second, over 95% of the specimens tested from multiple locations in Africa expressed the C-strain diagnostic TpiC marker [9, 10], indicating that the R-strain population as defined by Tpi is rare. Third, and more problematic, is that the majority of the TpiC specimens express the R-strain COI-RS haplotype. These observations indicate that the COI strain markers are in substantial disagreement with both host plants and Tpi. Recently, two other mitochondrial genes (cyt b and COIII) were characterized in fall armyworm collected from Uganda and shown to exhibit strain-specific SNPs [11]. The strain identity defined by the cyt b and COIII markers were consistent with each other and with those found in COI. The equivalence of these mitochondrial markers with respect to identifying host strains indicates that the disagreement with Tpi goes beyond COI and involves multiple segments of the mitochondrial genome.
If the mitochondrial haplotypes are not reliable markers of strain identity in the African fall armyworm populations then there is little current evidence supporting the presence of an African R-strain. The absence of a significant R-strain population would be consistent with the comparative heterozygosity F study that found no evidence of strain-specific mating barriers in the Africa populations. In summary, at this point the best available evidence is that the R-strain is not present in significant numbers in Africa. The question then is what happened to the COI marker?
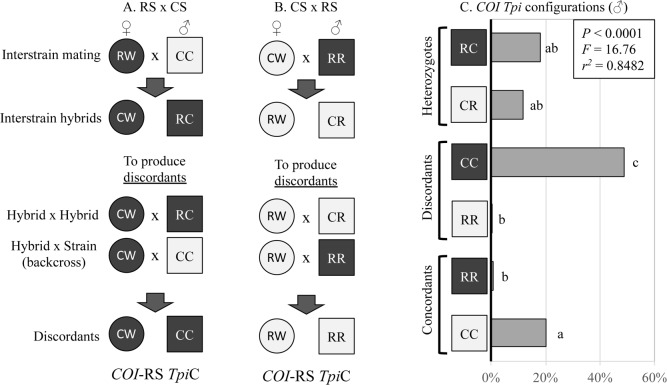
One possibility is that the African fall armyworm population is made up of the C-strain and interstrain hybrids, with the R-strain either absent or too low to be detected by the comparative heterozygosity studies. The rationale for this proposal is outlined in Fig 7. Discordance between COI and Tpi is presumed to have occurred due to interstrain crosses, which can occur in two directions with each producing the TpiR/TpiC heterozygote (Fig 7A and 7B). Subsequent mating of the hybrids to each other or a backcross to the parental male will generate the discordant configurations. Fig 7C shows the Africa haplotype data (from Fig 3) recalculated to estimate the proportions of only the male specimens, which should provide a more accurate assessment of heterozygote frequency. This was done by assuming a 1:1 sex ratio and reducing the concordant and discordant numbers by 50%. The results show that 28% of the African fall armyworm are interstrain heterozygotes, which is more than the proportion of COI-CS TpiC C-strain (20%). The predominant genotype is the COI-RS TpiC discordant that is present at 51%. Therefore, in this scenario the large majority (about 80%) of fall armyworm in Africa are derived from an interstrain hybridization event sometime in their past, with “true” C-strain (COI-CS TpiC) a minority and the “true” R-strain (COI-RS TpiR) mostly absent.
Fig 7. Mating scenarios to produce Tpi heterozygotes and COI Tpi discordant genotypes their relative frequencies in extrapolated male specimens from Africa.
The TpiR (R) and TpiC (C) alleles are defined by the e4183 polymorphism. Females are designated by a circle and are hemizygous for Tpi and so can only carry one Tpi allele, while males (square) and are either homozygous (RR, CC) or heterozygous (RC, CR) for TpiR and TpiC. Dark shading indicates a COI-RS mitochondrial haplotype while light shading identifies COI-CS. A) Female rice-strain (RS) mating male corn-strain (CS), while B) represents the reciprocal cross. C) Graph of the frequencies of each COI Tpi configuration within the male subset of the data described in Fig 3B (see text for explanation). Lower case letters indicate significant differences as determine by ANOVA and Tukey’s multiple comparison test.
Can e4183 heterozygotes be assumed to be interstrain hybrids?
A major assumption in this proposal is that heterozygosity at e4183 is an accurate marker for interstrain hybrids, which genetically is defined by heterozygosity at all the loci critical for driving strain divergence. There are two lines of evidence in support. The first and most compelling is that e4183 is an accurate marker of strain identity as indicated by surveys at multiple locations and times in the Western Hemisphere [18, 23, 44, 45, 50, 51]. This indicates a strong linkage of this SNP to the genes most critical for strain differentiation, directly implicating functions on the Z-chromosome located near Tpi by recombination distance. The second is the strain-specificity exhibited by mitochondrial markers that has been confirmed by multiple studies in the Western Hemisphere [5, 6, 19, 52]. Assuming the mitochondria are not involved in strain differentiation, this suggests a coincidental association that links the maternal inheritance pattern of mitochondria with the sex-specific inheritance of the Z and W chromosomes. In other words, the strain specificity of the COI and Tpi markers indicate that autosomal genes play at best a secondary role in strain differentiation. Since most sex-linked functions are located on the Z-chromosome rather than W, a Z-linked marker like e4183 should act as an accurate proxy for true interstrain hybrids.
Why do the African and Western Hemisphere populations differ?
Descriptions of how fall armyworm was introduced into Africa are completely speculative at this time but there are plausible scenarios for how an interstrain hybrid dominated population could have emerged. In the Western Hemisphere the distribution of the two strains is largely sympatric, particular in the habitats associated with C-strain preferred host plants [5, 6, 19, 52]. It is therefore likely that a contaminated shipment would carry both strains. Such a small invasive propagule would be subject to the variety of effects associated with population bottlenecks, most notably inbreeding depression due to recessive deleterious alleles. This will tend to reduce genetic variation and fitness within the normal breeding groups, which in this case are the two strains, thereby tending to increase the proportion of progeny produced by interstrain crosses. If this promotion of hybrid frequencies is greater than the reproductive barriers that limit productive mating between strains, it is plausible that a hybrid population could emerge and predominate.
The expected proportions of the two strains would depend on multiple factors that include the initial composition of the invasive propagule, the availability of host plants, and subsequent stochastic events. If the initial contaminated product was a C-strain host, a majority C-strain in the initial introductory population is likely, increasing the probability of its survival in the established population and conversely the marginalization and eventual loss of the R-strain.
What characteristics are expected in the hybrid and discordant genotypes?
The behavior of interstrain hybrids is unknown. The heterozygotes so far captured in Africa have all been from corn or sorghum indicating attraction to C-strain hosts, while the absence of reproductive barriers as assayed by the comparative heterozygosity analysis suggests an unrestricted capacity to mate with TpiR moths.
It is noteworthy that the COI-RS TpiC class is much greater than the reciprocal COI-CS TpiR discordants in Africa, just as it is in the Western Hemisphere. This indicates that the set of matings initiated by female R-strain crossed to male C-strain (Fig 7A) is much more frequent and/or productive than the reciprocal (Fig 7B). This observation does not appear to be consistent with other findings indicating that the female R-strain crossed to male C-strain mating (but not the reciprocal) produced daughters with significantly reduced fertility [39]. The reason for this apparent inconsistency is unknown but suggests the possibility of unknown complexity and variability in the nature of the reproductive barriers between strains.
In conclusion, consistent findings of discrepancies between the COI and Tpi strain markers and the absence of strain-specific mating barriers indicate that the R-strain subpopulation found in the Western Hemisphere is currently not present in significant numbers in Africa. This could have important consequences in assessing the threat of fall armyworm to different crop species. Evidence is presented that suggest the Africa fall armyworm populations is primarily composed of interstrain hybrids with a C-strain minority population.
Supporting information
(DOCX)
Acknowledgments
Thank you to Dr. Jean Thomas (USDA-ARS) and Dr. Robert Meagher (USDA-ARS) for advice on the manuscript. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
R.N.N. received funding from United States Department of Agriculture-Agricultural Research Service (6036-22000-030-00D and 6615-22000-016-00D) and the United States Agency for International Development (8044-22000-046-06R). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Day R, Abrahams P, Bateman M, Beale T, Clottey V, Cock M, et al. Fall Armyworm: Impacts and Implications for Africa. Outlooks on Pest Management. 2017;28(5):196–201. 10.1564/v28_oct_02. [DOI] [Google Scholar]
- 2.Luginbill P. The fall armyworm. US Dept Agric Tech Bull. 1928;34:1–91. [Google Scholar]
- 3.Pashley DP. Host-associated genetic differentiation in fall armyworm (Lepidoptera, Noctuidae)—a sibling species complex. Ann Entomol Soc Am. 1986;79(6):898–904. ISI:A1986E957200010. [Google Scholar]
- 4.Juárez ML, Murúa MG, García MG, Ontivero M, Vera MT, Vilardi JC, et al. Host association of Spodoptera frugiperda (Lepidoptera: Noctuidae) corn and rice strains in Argentina, Brazil, and Paraguay. J Econ Entomol. 2012;105(2):573–82. 10.1603/Ec11184 ISI:000302784300034. [DOI] [PubMed] [Google Scholar]
- 5.Murúa MG, Nagoshi RN, Dos Santos DA, Hay-Roe M, Meagher RL, Vilardi JC. Demonstration using field collections that Argentina fall armyworm populations exhibit strain-specific host plant preferences. J Econ Entomol. 2015;108(5):2305–15. WOS:000362828300018. 10.1093/jee/tov203 [DOI] [PubMed] [Google Scholar]
- 6.Nagoshi RN, Silvie P, Meagher RL, Lopez J, Machados V. Identification and comparison of fall armyworm (Lepidoptera: Noctuidae) host strains in Brazil, Texas, and Florida. Ann Entomol Soc Am. 2007;100(3):394–402. WOS:000246324900008. [Google Scholar]
- 7.Pashley DP. The current status of fall armyworm host strains. Fla Entomol. 1988;71:227–34. [Google Scholar]
- 8.Cock MJW, Beseh PK, Buddie AG, Cafa G, Crozier J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci Rep-Uk. 2017;7. WOS:000403874900086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagoshi RN, Goergen G, Tounou KA, Agboka K, Koffi D, Meagher RL. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci Rep-Uk. 2018;8 ARTN 3710 10.1038/s41598-018-21954-1 WOS:000426151800043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagoshi RN, Koffi D, Agboka K, Tounou KA, Banerjee R, Jurat-Fuentes JL, et al. Comparative molecular analyses of invasive fall armyworm in Togo reveal strong similarities to populations from the eastern United States and the Greater Antilles. Plos One. 2017;12(7). WOS:000406362700091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otim MH, Tay WT, Walsh TK, Kanyesigye D, Adumo S, Abongosi J, et al. Detection of sister-species in invasive populations of the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) from Uganda. Plos One. 2018;13(4). WOS:000429081600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stokstad E. FOOD SECURITY New crop pest takes Africa at lightning speed. Science. 2017;356(6337):473–4. WOS:000400545700013. 10.1126/science.356.6337.473 [DOI] [PubMed] [Google Scholar]
- 13.Prowell DP, McMichael M, Silvain JF. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Ann Entomol Soc Am. 2004;97(5):1034–44. WOS:000224104100023. [Google Scholar]
- 14.Nagoshi RN, Meagher RL, Adamczyk JJ, Braman SK, Brandenburg RL, Nuessly G. New restriction fragment length polymorphisms in the cytochrome oxidase I gene facilitate host strain identification of fall armyworm (Lepidoptera: Noctuidae) populations in the southeastern United States. J Econ Entomol. 2006;99(3):671–7. ISI:000238234500012. [DOI] [PubMed] [Google Scholar]
- 15.Pashley DP. Host-associated differentiation in armyworms (Lepidoptera: Noctuidae): an allozymic and mitochondrial DNA perspective In: Loxdale HD, der Hollander J, editors. Electrophoretic Studies on Agricultural Pests. Oxford: Oxford University Press; 1989. p. 103–14. [Google Scholar]
- 16.Lu YJ, Adang MJ. Distinguishing fall armyworm (Lepidoptera:Noctuidae) strains using a diagnostic mitochondrial DNA marker. Fla Entomol. 1996;79:48–55. [Google Scholar]
- 17.Lu YJ, Kochert GD, Isenhour DJ, Adang MJ. Molecular characterization of a strain-specific repeated DNA sequence in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Insect Mol Biol. 1994;3(2):123–30. [DOI] [PubMed] [Google Scholar]
- 18.Nagoshi RN. The fall armyworm triose phosphate isomerase (Tpi) gene as a marker of strain identity and interstrain mating. Ann Entomol Soc Am. 2010;103(2):283–92. 10.1603/An09046 ISI:000275386800017. [DOI] [Google Scholar]
- 19.Prowell DP, McMichael M, Silvain JF. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Ann Entomol Soc Am. 2004;97(5):1034–44. ISI:000224104100023. [Google Scholar]
- 20.Helfert S, Estevez AM, Bakker B, Michels P, Clayton C. Roles of triosephosphate isomerase and aerobic metabolism in Trypanosoma brucei. Biochem J. 2001;357:117–25. WOS:000169783500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dopman EB, Bogdanowicz SM, Harrison RG. Genetic mapping of sexual isolation between E and Z pheromone strains of the European corn borer (Oshinia nubilalis). Genetics. 2004;167(1):301–9. ISI:000221851100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagoshi RN, Gilligan TM, Brambila J. Combining Tpi and CO1 Genetic Markers to Discriminate Invasive Helicoverpa armigera From Local Helicoverpa zea (Lepidoptera: Noctuidae) Populations in the Southeastern United States. J Econ Entomol. 2016;109(5):2115–24. 10.1093/jee/tow177 WOS:000386136700020. [DOI] [PubMed] [Google Scholar]
- 23.Nagoshi RN. Improvements in the identification of strains facilitate population studies of fall armyworm subgroups. Ann Entomol Soc Am. 2012;105(2):351–8. ISI:000301448500023. [Google Scholar]
- 24.Murua MG, Nagoshi RN, Dos Santos DA, Hay-Roe MM, Meagher RL, Vilardi JC. Demonstration Using Field Collections that Argentina Fall Armyworm Populations Exhibit Strain-specific Host Plant Preferences. J Econ Entomol. 2015;108(5):2305–15. WOS:000362828300018. 10.1093/jee/tov203 [DOI] [PubMed] [Google Scholar]
- 25.Nagoshi RN, Meagher R. Fall armyworm FR sequences map to sex chromosomes and their distribution in the wild indicate limitations in interstrain mating. Insect Mol Biol. 2003;12(5):453–8. WOS:000185348300005. [DOI] [PubMed] [Google Scholar]
- 26.Nagoshi RN, Meagher RL. FR tandem-repeat sequence in fall army-worm (Lepidoptera: Noctuidae) host strains. Ann Entomol Soc Am. 2003;96(3):329–35. WOS:000183079900012. [Google Scholar]
- 27.Groot AT, Marr M, Heckel DG, Schofl G. The roles and interactions of reproductive isolation mechanisms in fall armyworm (Lepidoptera: Noctuidae) host strains. Ecol Entomol. 2010;35(s1):105–18. 10.1111/J.1365-2311.2009.01138.X ISI:000273301500011. [DOI] [Google Scholar]
- 28.Pashley DP, Hammond AM, Hardy TN. Reproductive isolating mechanisms in fall armyworm host strains (Lepidoptera, Noctuidae). Ann Entomol Soc Am. 1992;85(4):400–5. WOS:A1992JD98100004. [Google Scholar]
- 29.Pashley DP, Martin JA. Reproductive incompatibility between host strains of the fall armyworm (Lepidoptera: Noctuidae). Ann Entomol Soc Am. 1987;80:731–3. [Google Scholar]
- 30.Schofl G, Dill A, Heckel DG, Groot AT. Allochronic separation versus mate choice: Nonrandom patterns of mating between fall armyworm host strains. Am Nat. 2011;177(4):470–85. WOS:000289237700008. 10.1086/658904 [DOI] [PubMed] [Google Scholar]
- 31.Schofl G, Heckel DG, Groot AT. Time-shifted reproductive behaviours among fall armyworm (Noctuidae: Spodoptera frugiperda) host strains: evidence for differing modes of inheritance. J Evolution Biol. 2009;22(7):1447–59. WOS:000267129500009. [DOI] [PubMed] [Google Scholar]
- 32.Rios-Diez JD, Saldamando-Benjumea CI. Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) Strains From Central Colombia to Two Insecticides, Methomyl and Lambda-Cyhalothrin: A Study of the Genetic Basis of Resistance. J Econ Entomol. 2011;104(5):1698–705. 10.1603/Ec11079 ISI:000295923100031. [DOI] [PubMed] [Google Scholar]
- 33.Ingber DA, Mason CE, Flexner L. Cry1 Bt Susceptibilities of Fall Armyworm (Lepidoptera: Noctuidae) Host Strains. J Econ Entomol. 2018;111(1):361–8. WOS:000424973300046. 10.1093/jee/tox311 [DOI] [PubMed] [Google Scholar]
- 34.Adamczyk JJ Jr., Holloway JW, Leonard BR, Graves JB. Susceptibility of fall armyworm collected from different plant hosts to selected insecticides and transgenic Bt cotton. J Cotton Sci. 1997;1:21–8. [Google Scholar]
- 35.Groot AT, Marr M, Schofl G, Lorenz S, Svatos A, Heckel DG. Host strain specific sex pheromone variation in Spodoptera frugiperda. Front Zool. 2008;5. WOS:000263448100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lima ER, McNeil JN. Female sex pheromones in the host races and hybrids of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Chemoecology. 2009;19(1):29–36. WOS:000264260800005. [Google Scholar]
- 37.Unbehend M, Hanniger S, Meagher RL, Heckel DG, Groot AT. Pheromonal Divergence Between Two Strains of Spodoptera frugiperda. J Chem Ecol. 2013;39(3):364–76. WOS:000316754500004. 10.1007/s10886-013-0263-6 [DOI] [PubMed] [Google Scholar]
- 38.Unbehend M, Hanniger S, Vasquez GM, Juarez ML, Reisig D, McNeil JN, et al. Geographic Variation in Sexual Attraction of Spodoptera frugiperda Corn- and Rice-Strain Males to Pheromone Lures. Plos One. 2014;9(2). WOS:000331711900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kost S, Heckel DG, Yoshido A, Marec F, Groot AT. A Z-linked sterility locus causes sexual abstinence in hybrid females and facilitates speciation in Spodoptera frugiperda. Evolution. 2016;70(6):1418–27. 10.1111/evo.12940 WOS:000379268200022. [DOI] [PubMed] [Google Scholar]
- 40.Canas-Hoyos N, Marquez EJ, Saldamando-Benjumea CI. Differentiation of Spodoptera frugiperda (Lepidoptera: Noctuidae) Corn and Rice Strains From Central Colombia: A Wing Morphometric Approach. Ann Entomol Soc Am. 2014;107(3):575–81. WOS:000335662600004. [Google Scholar]
- 41.Canas-Hoyos N, Marquez EJ, Saldamando-Benjumea CI. Heritability of Wing Size and Shape of the Rice and Corn Strains of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Neotrop Entomol. 2016;45(4):411–9. WOS:000379710000011. 10.1007/s13744-016-0393-y [DOI] [PubMed] [Google Scholar]
- 42.Groot AT, Marr M, Schofl G, Lorenz S, Svatos A, Heckel DG. Host strain specific sex pheromone variation in Spodoptera frugiperda. Front Zool. 2008;5:20 ISI:000263448100001. 10.1186/1742-9994-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagoshi RN, Fleischer S, Meagher RL. Demonstration and quantification of restricted mating between fall armyworm host strains in field collections by SNP comparisons. J Econ Entomol. 2017;110(6):2568–75. WOS:000417754400040. 10.1093/jee/tox229 [DOI] [PubMed] [Google Scholar]
- 44.Nagoshi RN, Meagher RL, Jenkins DA. Puerto Rico fall armyworm has only limited interactions with those from Brazil or Texas but could have substantial exchanges with Florida populations. J Econ Entomol. 2010;103(2):360–7. ISI:000276169800020. [DOI] [PubMed] [Google Scholar]
- 45.Nagoshi RN, Meagher RL, Flanders K, Gore J, Jackson R, Lopez J, et al. Using haplotypes to monitor the migration of fall armyworm (Lepidoptera:Noctuidae) corn-strain populations from Texas and Florida. J Econ Entomol. 2008;101(3):742–9. ISI:000258337200014. [DOI] [PubMed] [Google Scholar]
- 46.Nagoshi RN, Fleischer S, Meagher RL, Hay-Roe M, Khan A, Murua MG, et al. Fall armyworm migration across the Lesser Antilles and the potential for genetic exchanges between North and South American populations. Plos One. 2017;12(2):e0171743 WOS:000393700100069. 10.1371/journal.pone.0171743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagoshi RN, Meagher RL, Hay-Roe M. Inferring the annual migration patterns of fall armyworm (Lepidoptera: Noctuidae) in the United States from mitochondrial haplotypes. Ecology and Evolution. 2012;2(7):1458–67. 10.1002/ece3.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagoshi RN, Rosas-Garcia NM, Meagher RL, Fleischer SJ, Westbrook JK, Sappington TW, et al. Haplotype profile comparisons between (Lepidoptera: Noctuidae) populations from Mexico with those from Puerto Rico, South America, and the United States and their implications to migratory behavior. J Econ Entomol. 2015;108(1):135–44. WOS:000350033700018. 10.1093/jee/tou044 [DOI] [PubMed] [Google Scholar]
- 49.Nagoshi RN, Fleischer SJ, Meagher RL. Texas is the overwintering source of fall armyworm in central Pennsylvania: Implications for migration into the northeastern United States. Environ Entomol. 2009;38(6):1546–54. ISI:000272581400004. [DOI] [PubMed] [Google Scholar]
- 50.Nagoshi RN, Meagher RL. Using intron sequence comparisons in the triose-phosphate isomerase gene to study the divergence of the fall armyworm host strains. Insect Mol Biol. 2016;25(3):324–37. 10.1111/imb.12223 WOS:000375669800012. [DOI] [PubMed] [Google Scholar]
- 51.Nagoshi RN, Murua MG, Hay-Roe M, Juarez ML, Willink E, Meagher RL. Genetic characterization of fall armyworm (Lepidoptera: Noctuidae) host strains in Argentina. J Econ Entomol. 2012;105(2):418–28. 10.1603/Ec11332 ISI:000302784300017. [DOI] [PubMed] [Google Scholar]
- 52.Nagoshi RN, Meagher RL. Seasonal distribution of fall armyworm (Lepidoptera: Noctuidae) host strains in agricultural and turf grass habitats. Environ Entomol. 2004;33(4):881–9. ISI:000223650800013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.