Abstract
The common human-biting tick, Ixodes pacificus, is the primary vector of the Lyme disease spirochete, Borrelia burgdorferi sensu stricto (ss) in western North America and has been found to harbor other closely-related spirochetes in the Borrelia burgdorferi sensu lato (sl) complex. Between 2008–2015, 11,066 adult and 3,815 nymphal I. pacificus and five adult and 144 nymphal Ixodes spinpalpis, a commonly collected wildlife tick, were collected from 42 California counties. Borrelia burgdorferi sl was detected in 1.2% and 3.8% I. pacificus adults and nymphs, respectively. Results from this study indicate genetic diversity and geographic structure of B. burgdorferi sl in California I. pacificus ticks, by sequence comparison of the16S rRNA gene, with B. burgdorferi ss, the agent of Lyme disease, found only in I. pacificus collected from the north and central coastal and Sierra Nevada foothill regions; B. burgdorferi ss was not detected in ticks tested from southern California. In contrast, Borrelia bissettiae, a member of the B. burgdorferi sl complex, was detected in both I. pacificus and I. spinipalpis, in the coastal region of both northern and southern California, but was absent from ticks in the Sierra Nevada foothills. In a similar pattern to B. bissettiae, Borrelia americana (a member of the B. burgdorferi sl complex) was detected in a single adult I. pacificus from the north coast and two I. spinipalpis nymphs from south-coastal California. This study highlights that the geographic area of Lyme disease acarological risk in California is the north-central and Sierra Nevada foothill regions of the state with little to no risk in the southern regions of the state.
Introduction
There is considerable diversity in the Borrelia burgdorferi sensu lato (sl) complex. Worldwide, members of the B. burgdorferi sl complex include at least 22 named genospecies worldwide, of which ten genospecies have been identified to date in North America: Borrelia burgdorferi sensu stricto (ss), B. americana, B. andersoni, B. bissettiae, B. californiensis, B. carolinensis, B. garinii, B. kurtenbachii, B. laneii, and B. mayonii [1–4]. In California, only B. burgdorferi ss, B. americana, B. bissettiae, B. californiensis, and B. lanei have been described in Ixodes pacificus ticks, along with currently-uncharacterized Borrelia species [2, 5, 6]. While B. burgdorferi ss is the primary etiologic agent of Lyme disease in North America, and B. mayonii causes Lyme disease in the upper Midwest, recent studies suggest that B. bissettiae, a known human pathogen in Europe [7–9], may also infect people in the southeastern United States [10] and California [11].
The recognition of variation within the B. burgdorferi sl complex has direct public health implications. By considering all B. burgdorferi sl positive ticks “positive,” the prevalence for infection, and thus the acarological index of Lyme disease risk, has been overestimated [12]. Furthermore, genospecies other than B. burgdorferi ss may cause human disease, which could manifest with potentially different etiologies.
In this study, molecular methods were used to characterize the Borrelia genospecies of B. burgdorferi sl-positive I. pacificus and I. spinipalpis to investigate large-scale spatial patterns of Borrelia genospecies present in California. Previous studies have identified B. burgdorferi sl and B. miyamotoi in California I. pacificus but did not further resolve B. burgdorferi to genospecies [12]. Ixodes pacificus is a common human-biting tick found along the Pacific Coast of the United States and is the primary vector of Lyme disease to people in this region (https://www.cdc.gov/ticks/geographic_distribution.html). These data enabled us to clarify the relative acarological risk of human exposure to pathogenic Borrelia in a heavily populated state in western North America.
Materials and methods
Tick collection
The California Department of Public Health (CDPH), Vector-Borne Disease Section performs routine surveillance and testing of sylvatic Ixodes ticks for Borrelia spp. spirochetes. This includes relapsing fever group Borrelia (e.g., B. miyamotoi) and members of the B. burgdoferi sl complex [12]. Ixodes pacificus adults and nymphs were collected throughout the state of California from 2008 to 2015. Ticks were collected from low vegetation, leaf litter, or other substrates (e.g., rocks or downed logs) by CDPH and county public health agencies using 1-m2 white double nap flannel “flag” attached to a 1.5-m wooden dowel. In most instances, ticks were collected from public lands such as regional or state parks. Ticks were collected all months of the year, with adult ticks most commonly collected in the winter months and the nymphs in the spring and summer months. Adult and nymphal I. spinipalpis ticks were collected opportunistically by flagging, during the same collection events; this tick species rarely attaches to people but parasitizes wildlife such as woodrats (Neotoma fuscipes and N. macrotis) and may be an important bridge vector for B. burgdorferi sl. Ticks were either maintained alive within 37-mL polystyrene containers (Fisher Scientific, USA) in sealed plastic bags with moistened paper toweling at 3°C or retained in 70% ethanol within 1.5-mL microcentrifuge snap-cap tubes.
Tick preparation
Ticks were tested individually by direct florescence antibody assay (DFA), using Borrelia generic fluorescent-labeled antibodies to detect Borrelia species spirochetes. Live ticks were dissected onto etched microscope slides and stained with FITC-labeled BacTrace Anti-Borrelia Species Antibody (KPA) [13, 14]. Half of each dissected tick was transferred to a 2-mL snap cap tube that contained 20ul sterile PBS and stored at -80°C for later use. At least 100 visual fields were examined at 400X magnification for the presence of Borrelia spirochetes.
Ticks that tested positive for Borrelia spirochetes by DFA were further analyzed to determine the Borrelia genospecies. DNA from frozen tick tissues was extracted using QIAGEN DNeasy Blood and Tissue Kit (Hercules, CA) according to manufacturer’s instructions.
Molecular analyses
DNA from DFA-positive ticks was screened for B. miyamotoi and B. burgdorferi sl using a TaqMan assay [15]. Forward and reverse primers were, respectively, 5’GCTGTAAACGATGCACACTTGGT3’ and 5′GGCGGCACACTTAACACGTTAG 3’ targeting a 1130bp 16S rRNA sequence as described [15]. The probes used were 6FAM-TTCGGTACTA ACTTTTAGTTA corresponding to B. burgdorferi sl and VIC-CGGTACTAACCTTTCGAT TA corresponding to B. miyamotoi with the 3’ ends modified with a minor groove binding protein. All reactions were performed in a final volume of 25 ul on a BioRad CFX96 Real-Time Detection System containing 2x SooFast Probes SuperMix (BioRad), primers (900 nM), and probes (200 nM) per reaction. Thermal cycling conditions were as follows: 95°C for 2 min, 45 cycles of 95°C for 5 sec, and 63°C for 30 sec.
A 1130bp section of the 16S rRNA gene was amplified from TaqMan positive B. burgdorferi sl ticks. Forward and reverse primers were, respectively 5’CTGGCAGTGCGTCTTAAGCA3’ [16] and 5’GACTTATCACCGGCAGTCTTA3’ [17]. PCRs were performed in 25ul volumes with final concentrations of 0.2uM for forward and reverse primers, 200uM dNTPs, and 0.625 units of Taq DNA polymerase per reaction. Thermal cycling conditions were: 94°C for 1 min, 45 cycles of 94°C for 1 min, 61.2°C for 30 sec, and 72°C for 90 sec, followed by final extension of 72°C for 7 min. The PCR products were visualized on a 2% Life Technologies E-gel stained with SYBRgreen (Carlsbad, CA).
PCR product was purified using either Affymatrix ExoSAP-IT (Santa Clara, CA) or QIAquick PCR Purification Kit, according to manufacturer’s instructions, respectively. Samples were sequenced by Quintara (http://www.quintarabio.com/). For each sample, forward and reverse sequences were obtained. The forward and reverse reads were aligned using ClustalOmega (http://www.ebi.ac.uk/Tools/msa/clustalo/) and edited manually. Electropherograms were examined for the presence of conflicting base calls using ApE (http://biologylabs.utah.edu/jorgensen/wayned/ape/) to address the possibility that a tick was co-infected with more than one genospecies of B. burgdorferi sl. In instances where a sample seemed to produce more than one PCR product, suggesting multiple B. burgdorferi sl infections, PCR products were cloned using a Qiagen PCR Cloning Kit. Inserts from 3–5 colonies were then Sanger sequenced as described above.
The acquired 16S rRNA sequences were aligned with 16S rRNA sequences from other Borrelia genospecies retrieved from GenBank. Sequences were aligned using ClustalOmega (http://www.ebi.ac.uk/Tools/msa/clustalo/). The 16S rRNA sequence from B. miyamotoi (Genbank accession number AB904793.1) served as the outgroup. After manual refinement, conserved regions were identified using the Gblocks feature of the Phylogeny.fr suite [18, 19]. The HKY+G nucleotide substitution model was selected using TOPALiv2’s model selection feature [20]. TOPALi was then used to launch MrBayes to construct a phylogenetic tree [21–23]. The tree was generated using two runs of 9,000,000 generations with 35% burn in and trees sampled every 1000 generations.
Results
In total, 11,066 I. pacificus adults, collected from 2008 to 2015, were screened for Borrelia spp. by DFA. Of these, 228 adults (2.1%) were DFA positive for Borrelia spirochetes and, of these, 128 (1.2%) were B. burgdorferi sl positive and 96 (0.9%) were B. miyamotoi positive when tested by TaqMan assay; four positive ticks were not able to amplify. A subset of 27 of the B. burgdorferi sl-positive adult ticks were characterized to genospecies by sequence comparison. This subset of positive ticks was selected to optimize the number of ticks tested from different regions of the state. Borrelia burgdorferi ss was detected in 11 counties, B. bissettiae was detected in two counties, and B. americana was detected from one county (Table 1).
Table 1. Adult and nymphal Ixodes pacificus and Ixodes spinipalpis collected in California and tested for Borrelia spp., 2008–2015.
| Region | County | Tick species | Life stage | # tested | # samples positive for B. burgdorferi s.l. | % prevalence of B. burgdorferi s.l. | # in subset with B. burgdorferi s.l. genomospecies determined a | B. americana | B. bissettiae | B. burgdorferi ss |
|---|---|---|---|---|---|---|---|---|---|---|
| Central Coast | ||||||||||
| Monterey | Ixodes pacificus | Adult | 140 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 35 | 0 | 0.0 | ||||||
| San Benito | Ixodes pacificus | Adult | 47 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 4 | 0 | 0.0 | ||||||
| San Luis Obispo | Ixodes pacificus | Adult | 298 | 1 | 0.3 | 1 | 1 | |||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Central Valley | ||||||||||
| Colusa | Ixodes pacificus | Adult | 13 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Glenn | Ixodes pacificus | Adult | 171 | 2 | 1.2 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Kern | Ixodes pacificus | Adult | 57 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Sacramento | Ixodes pacificus | Adult | 88 | 3 | 3.4 | |||||
| Ixodes pacificus | Nymphs | 190 | 18 | 9.5 | 1 | 1 | ||||
| Ixodes spinipalpis | Nymphs | 11 | 1 | 9.1 | ||||||
| San Joaquin | Ixodes pacificus | Adult | 13 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 3 | 0 | 0.0 | ||||||
| Stanislaus | Ixodes pacificus | Adult | 211 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Yuba | Ixodes pacificus | Adult | 424 | 7 | 1.7 | |||||
| Ixodes pacificus | Nymphs | 30 | 0 | 0.0 | ||||||
| North Coastal | ||||||||||
| Alameda | Ixodes pacificus | Adult | 466 | 5 | 1.1 | |||||
| Ixodes pacificus | Nymphs | 29 | 0 | 0.0 | ||||||
| Contra Costa | Ixodes pacificus | Adult | 285 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 264 | 4 | 1.5 | 1 | 1 | ||||
| Ixodes spinipalpis | Nymphs | 1 | 0 | 0.0 | ||||||
| Humboldt | Ixodes pacificus | Adult | 30 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 2 | 0 | 0.0 | ||||||
| Lake | Ixodes pacificus | Adult | 253 | 2 | 0.8 | 2 | 2 | |||
| Ixodes pacificus | Nymphs | 492 | 14 | 2.8 | 7 | 7 | ||||
| Marin | Ixodes pacificus | Adult | 682 | 14 | 2.1 | 4 | 1 | 3 | ||
| Ixodes pacificus | Nymphs | 331 | 24 | 7.3 | 10 | 10 | ||||
| Mendocino | Ixodes pacificus | Adult | 61 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 19 | 0 | 0.0 | ||||||
| Napa | Ixodes pacificus | Adult | 385 | 3 | 0.8 | |||||
| Ixodes pacificus | Nymphs | 342 | 3 | 0.9 | ||||||
| San Mateo | Ixodes pacificus | Adult | 620 | 15 | 2.4 | 6 | 1 | 2 | 3 | |
| Ixodes spinipalpis | Adult | 1 | 0 | 0.0 | ||||||
| Ixodes pacificus | Nymphs | 96 | 4 | 4.2 | ||||||
| Ixodes spinipalpis | Nymphs | 5 | 1 | 20.0 | ||||||
| Santa Clara | Ixodes pacificus | Adult | 182 | 3 | 1.6 | 3 | 3 | |||
| Ixodes pacificus | Nymphs | 134 | 9 | 6.7 | 6 | 6 | ||||
| Santa Cruz | Ixodes pacificus | Adult | 893 | 3 | 0.3 | 1 | 1 | |||
| Ixodes pacificus | Nymphs | 476 | 16 | 3.4 | 6 | 1 | 5 | |||
| Ixodes spinipalpis | Nymphs | 4 | 1 | 25.0 | ||||||
| Solano | Ixodes pacificus | Adult | 121 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Sonoma | Ixodes pacificus | Adult | 216 | 1 | 0.5 | |||||
| Ixodes pacificus | Nymphs | 337 | 3 | 0.9 | 1 | 1 | ||||
| Trinity | Ixodes pacificus | Adult | 56 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Sierra-Nevada Foothills | ||||||||||
| Amador | Ixodes pacificus | Adult | 256 | 3 | 1.2 | |||||
| Ixodes pacificus | Nymphs | 116 | 7 | 6.0 | ||||||
| Butte | Ixodes pacificus | Adult | 441 | 8 | 1.8 | 1 | 1 | |||
| Ixodes pacificus | Nymphs | 309 | 17 | 5.5 | 3 | 3 | ||||
| Calaveras | Ixodes pacificus | Adult | 537 | 7 | 1.3 | 1 | 1 | |||
| Ixodes pacificus | Nymphs | 30 | 0 | 0.0 | ||||||
| El Dorado | Ixodes pacificus | Adult | 312 | 10 | 3.2 | 5 | 5 | |||
| Ixodes pacificus | Nymphs | 233 | 21 | 9.0 | 2 | 2 | ||||
| Ixodes spinipalpis | Nymphs | 12 | 3 | 25.0 | ||||||
| Inyo | Ixodes pacificus | Adult | 2 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Mariposa | Ixodes pacificus | Adult | 181 | 1 | 0.6 | 1 | 1 | |||
| Ixodes pacificus | Nymphs | 15 | 0 | 0.0 | ||||||
| Nevada | Ixodes pacificus | Adult | 551 | 7 | 1.3 | 1 | 1 | |||
| Ixodes pacificus | Nymphs | 173 | 6 | 3.5 | ||||||
| Placer | Ixodes pacificus | Adult | 10 | 1 | 10.0 | |||||
| Ixodes pacificus | Nymphs | 40 | 0 | 0.0 | ||||||
| Shasta | Ixodes pacificus | Adult | 460 | 26 | 5.7 | |||||
| Ixodes pacificus | Nymphs | 90 | 0 | 0.0 | ||||||
| Sierra | Ixodes pacificus | Adult | 28 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Siskiyou | Ixodes pacificus | Adult | 13 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Tuolumne | Ixodes pacificus | Adult | 64 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 3 | 0 | 0.0 | ||||||
| Southern Region | ||||||||||
| Los Angeles | Ixodes pacificus | Adult | 360 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 11 | 0 | 0.0 | ||||||
| Orange | Ixodes pacificus | Adult | 659 | 2 | 0.3 | |||||
| Ixodes spinipalpis | Adult | 4 | 1 | 25.0 | 1 | 1 | ||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Ixodes spinipalpis | Nymphs | 111 | 20 | 18.0 | 8 | 2 | 6 | |||
| Riverside | Ixodes pacificus | Adult | 180 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 1 | 0 | 0.0 | ||||||
| San Bernardino | Ixodes pacificus | Adult | 678 | 3 | 0.4 | |||||
| Ixodes pacificus | Nymphs | 1 | 0 | 0.0 | ||||||
| San Diego | Ixodes pacificus | Adult | 58 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
| Santa Barbara | Ixodes pacificus | Adult | 496 | 1 | 0.2 | |||||
| Ixodes pacificus | Nymphs | 9 | 0 | 0.0 | ||||||
| Ventura | Ixodes pacificus | Adult | 68 | 0 | 0.0 | |||||
| Ixodes pacificus | Nymphs | 0 | 0 | |||||||
a Due to screening a subset of samples positive for B. burgdorferi s.l., a true prevalence of genospecies by county could not be determined.
Similarly, 204 of the 3,815 nymphal I. pacificus were positive for Borrelia spp. by DFA. Of these, 146 (3.8%) of DFA-positive nymphs tested positive for B. burgdorferi sl and 52 (1.4%) tested positive for B. miyamotoi by TaqMan assay; six positive ticks were not able to amplify. Of the 37 Borrelia-positive I. pacificus nymphs that were genotyped, 36 were positive for B. burgdorferi ss from nine counties, and one was positive for B. bissettiae (Table 1).
Five I. spinipalpis adult ticks were collected from two counties; a single female from Orange County was positive for B. bissettiae (Table 1). In addition, 144 I. spinipalpis nymphs were tested from six counties. Of these 26 (18.1%) I. spinipalpis nymphs that were B. burgdorferi sl positive, two (1.4%) were B. americana positive, and six (4.2%) were B. bissettiae positive (Table 1). None of the I. spinipalpis adults or nymphs tested positive for either B. burgdorferi ss or B. miyamotoi.
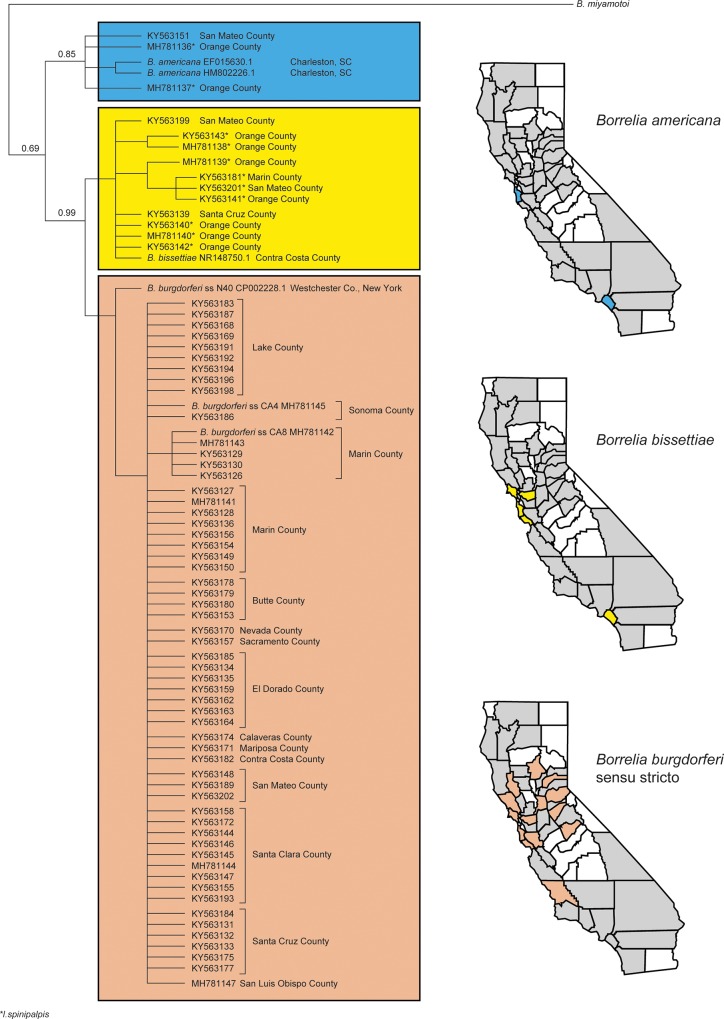
The 16S rRNA sequence fragments obtained from sequencing a subset of positive amplicons (27 from adult ticks, 45 from nymphal ticks) were used to construct a phylogenetic tree (Table 2). Borrelia spp. from Ixodes ticks clustered into three clades, each containing a sequence from a GenBank-obtained Borrelia genospecies (Fig 1). The clade that contained B. burgdorferi ss was the largest with 58 samples and two controls (CA4 and CA8). The clade that included a B. bissettiae control (CA389) also included 11 tick-derived samples from along the northern and southern coastal regions of the state. In northern California, B. bissettiae was detected in I. pacificus whereas in southern California, B. bissettiae was detected in I.spinipalpis only. The B. americana clade included two positive I. spinipalpis nymphs from Orange County in southern California, one positive I. pacificus adult from the north-coastal county of San Mateo, and GenBank-derived sequence controls from Charleston County South Carolina (accession numbers HM802226, EU081286) (Table 2). Branch lengths are non-informative.
Table 2. Summary of California Borrelia positive ticks.
| Borrelia spp. | County | Tick species | Life stage/sex | Tick no. | GenBank Accession No. | Latitude, Longitude |
|---|---|---|---|---|---|---|
| Borrelia americana | Orange | I. spinipalpis | Nymph | 12–0751 | MH781136 | 33.573899, -117.839928 |
| Borrelia americana | Orange | I. spinipalpis | Nymph | 12–0785 | MH781137 | 33.573899, -117.839928 |
| Borrelia americana | San Mateo | I. pacificus | Female | 12–0040 | KY563151 | 37.363597, -122.246426 |
| Borrelia americana | Charleston, South Carolina | I. minor | Control | HM802226.1 | ||
| Borrelia americana | Charleston, South Carolina | I. minor | Control | EF015630.1 | ||
| Borrelia bissettiae | Marin | I. pacificus | Female | 08–0745 | KY563181 | 37.835500, -122.478300 |
| Borrelia bissettiae | Orange | I. spinipalpis | Nymph | 12–0780 | KY563143 | 33.573899, -117.839928 |
| Borrelia bissettiae | Orange | I. spinipalpis | Female | 12–0438 | MH781138 | 33.573899, -117.839928 |
| Borrelia bissettiae | Orange | I. spinipalpis | Nymph | 12–0776 | MH781139 | 33.573899, -117.839928 |
| Borrelia bissettiae | Orange | I. spinipalpis | Nymph | 12–0743 | KY563141 | 33.573899, -117.839928 |
| Borrelia bissettiae | Orange | I. spinipalpis | Nymph | 12–0454 | KY563140 | 33.573899, -117.839928 |
| Borrelia bissettiae | Orange | I. spinipalpis | Nymph | 12–0451 | MH781140 | 33.573899, -117.839928 |
| Borrelia bissettiae | Orange | I. spinipalpis | Nymph | 12–0755 | KY563142 | 33.573899, -117.839928 |
| Borrelia bissettiae | San Mateo | I. pacificus | Female | 13–1009 | KY563199 | 37.390048, -122.257426 |
| Borrelia bissettiae | San Mateo | I. pacificus | Female | 13–1040 | KY563201 | 37.390048, -122.257426 |
| Borrelia bissettiae | Santa Cruz | I. pacificus | Nymph | 11–1420 | KY563139 | 37.014408, -122.084290 |
| Borrelia bissettiae | Contra Costa | I. pacificus | CA389—Control | NR148750.1 | ||
| Borrelia burgdorferi ss | Butte | I. pacificus | Nymph | 11–1646 | KY563178 | 39.52121, -121.44802 |
| Borrelia burgdorferi ss | Butte | I. pacificus | Nymph | 11–1702 | KY563179 | 39.52121, -121.44802 |
| Borrelia burgdorferi ss | Butte | I. pacificus | Nymph | 11–1735 | KY563180 | 39.52121, -121.44802 |
| Borrelia burgdorferi ss | Butte | I. pacificus | Female | 12–1336 | KY563153 | 39.52121, -121.44802 |
| Borrelia burgdorferi ss | Calaveras | I. pacificus | Male | 11–1827 | KY563174 | 38.022044, -120.549008 |
| Borrelia burgdorferi ss | Contra Costa | I. pacificus | Nymph | 08–1104 | KY563182 | 37.900529, -122.256376 |
| Borrelia burgdorferi ss | El Dorado | I. pacificus | Nymph | 09–0752 | KY563185 | 38.801096, -120.893394 |
| Borrelia burgdorferi ss | El Dorado | I. pacificus | Nymph | 10–0518 | KY563134 | 38.770251, -121.040021 |
| Borrelia burgdorferi ss | El Dorado | I. pacificus | Female | 10–0556 | KY563135 | 38.770251, -121.040021 |
| Borrelia burgdorferi ss | El Dorado | I. pacificus | Male | 11–0460 | KY563159 | 38.770251, -121.040021 |
| Borrelia burgdorferi ss | El Dorado | I. pacificus | Female | 11–0480 | KY563162 | 38.770251, -121.040021 |
| Borrelia burgdorferi ss | El Dorado | I. pacificus | Female | 11–0489 | KY563163 | 38.770251, -121.040021 |
| Borrelia burgdorferi ss | El Dorado | I. pacificus | Male | 11–0570 | KY563164 | 38.801096, -120.893394 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Female | 09–0426 | KY563183 | 38.910458, -122.592294 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Male | 09–0671 | KY563187 | 38.961918, -122.741246 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Nymph | 11–1019 | KY563168 | 39.017000, -122.813000 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Nymph | 11–1073 | KY563169 | 39.017000, -122.813000 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Nymph | 13–0797 | KY563191 | 39.262280, -122.950110 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Nymph | 13–0800 | KY563192 | 39.262280, -122.950110 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Nymph | 13–0916 | KY563194 | 39.262280, -122.950110 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Nymph | 13–0937 | KY563196 | 39.017000, -122.813000 |
| Borrelia burgdorferi ss | Lake | I. pacificus | Nymph | 13–0963 | KY563198 | 39.017000, -122.813000 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 10–0106 | KY563126 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 10–0112 | KY563127 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 10–0113 | MH781141 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 10–0120 | KY563128 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 10–0682 | KY563129 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 10–0853 | KY563136 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 10–1031 | MH781142 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 10–1045 | KY563130 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 11–2405 | KY563156 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Nymph | 11–2530 | MH781143 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Male | 11–2554 | KY563154 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Female | 12–0166 | KY563149 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Marin | I. pacificus | Male | 12–1134 | KY563150 | 38.006443, -122.494629 |
| Borrelia burgdorferi ss | Mariposa | I. pacificus | Male | 10–1001 | KY563171 | 37.292633, -120.147953 |
| Borrelia burgdorferi ss | Nevada | I. pacificus | Female | 10–0719 | KY563170 | 39.330100, -120.986900 |
| Borrelia burgdorferi ss | Sacramento | I. pacificus | Nymph | 11–2083 | KY563157 | 38.653200, -121.210900 |
| Borrelia burgdorferi ss | San Luis Obispo | I. pacificus | Female | 15–0797 | MH781147 | 35.422511, -120.739472 |
| Borrelia burgdorferi ss | San Mateo | I. pacificus | Female | A13-0477 | KY563202 | 37.277536, -122.223845 |
| Borrelia burgdorferi ss | San Mateo | I. pacificus | Male | 12–0051 | KY563148 | 37.363597, -122.246426 |
| Borrelia burgdorferi ss | San Mateo | I. pacificus | Male | 13–0194 | KY563189 | 37.472000, -122.280000 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Female | 11–0326 | KY563158 | 37.325300, -122.178900 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Nymph | 11–1358 | KY563172 | 37.324000, -122.176000 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Nymph | 11–1673 | KY563144 | 37.186126, -121.537900 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Nymph | 11–1685 | KY563145 | 37.186126, -121.537900 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Nymph | 11–1686 | MH781144 | 37.186126, -121.537900 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Nymph | 11–1688 | KY563146 | 37.186126, -121.537900 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Nymph | 11–2111 | KY563147 | 37.186126, -121.537900 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Female | 11–2322 | KY563155 | 37.405632, -122.305901 |
| Borrelia burgdorferi ss | Santa Clara | I. pacificus | Female | 13–0855 | KY563193 | 37.277758, -122.151275 |
| Borrelia burgdorferi ss | Santa Cruz | I. pacificus | Female | 09–0532 | KY563184 | 37.014408, -122.084290 |
| Borrelia burgdorferi ss | Santa Cruz | I. pacificus | Nymph | 10–0146 | KY563131 | 37.014408, -122.084290 |
| Borrelia burgdorferi ss | Santa Cruz | I. pacificus | Nymph | 10–0172 | KY563132 | 37.014408, -122.084290 |
| Borrelia burgdorferi ss | Santa Cruz | I. pacificus | Nymph | 10–0201 | KY563133 | 37.014408, -122.084290 |
| Borrelia burgdorferi ss | Santa Cruz | I. pacificus | Nymph | 11–1429 | KY563175 | 37.014408, -122.084290 |
| Borrelia burgdorferi ss | Santa Cruz | I. pacificus | Nymph | 11–1514 | KY563177 | 37.014408, -122.084290 |
| Borrelia burgdorferi ss | Sonoma | I. pacificus | Nymph | 09–0824 | KY563186 | 38.343912, -122.547333 |
| Borrelia burgdorferi ss | Sonoma | I. pacificus | CA4—Control | MH781145 | ||
| Borrelia burgdorferi ss | Sonoma | I. pacificus | CA8—Control | MH781146 | ||
| Borrelia burgdorferi ss | Westchester, New York | I. scapularis | N40—Control | CP002228.1 |
Fig 1. Borrelia genospecies detected in Ixodes pacificus and Ixodes spinipalpis ticks in California counties, 2008–2015.
Discussion
This is the first study that characterizes the genetic diversity and large-scale geographic sub-structuring of B. burgdorferi sl over a large region of western North America. Borrelia burgdorferi sl includes B. burgdorferi ss, the agent of Lyme disease in North America, as well as other closely related spirochetes that have not yet been implicated as human pathogens, such as B. bissettiae and B. americana.
Borrelia burgdorferi sensu stricto
Previous studies in western North America have highlighted northwestern California and the western slopes of the northern Sierra Nevada foothills as regions with moderate to high risk of exposure to the Lyme disease bacteria, B. burgdorferi ss. In northern California, I. pacificus nymphal tick infection prevalence average is 5% [12], but can be as high as 20 to 40% in some localities [24–26]. This prevalence is similar to many regions highly endemic for Lyme disease in the eastern and mid-western United States [27, 28]. Nevertheless, while I. pacificus ticks are found in many areas of western North America and present a risk of transmitting Lyme disease to people, this risk is not uniform throughout the region. For example, despite thousands of ticks tested to date, the only ticks found positive for B. burgdorferi ss from southern California are one adult I. pacificus and two Dermacentor occidentalis from Los Angeles County [29], and a single Ixodes peromysci nymph from Santa Barbara County [30]. Although D. occidentalis attaches to humans, it is not a competent vector of B. burgdorferi ss [31]. Ixodes peromysci is an uncommon tick that feeds predominately on Peromyscus spp. mice, and previously has been considered to be endemic only to the Channel Islands, off the coast of southern California [32]. To date, only a single I. pacificus has tested positive for B. burgdorferi ss from southern California. Our current findings further indicate that the acarological risk of acquiring Lyme disease in southern California is exceedingly low [29].
Borrelia bissettiae
Borrelia bissettiae (formerly B. bissettii) [6, 33] is a potential human pathogen in the United States and in Europe. In the Czech Republic, B. bissettiae was detected by PCR from sera of seven patients suspected to have Lyme borreliosis [8]. This spirochete was detected also by PCR from cardiac-valve tissue from a patient with endocarditis and aortic valve stenosis [7] and from a lymphocytomic breast tissue lesion from a Slovenian patient [9]. In the United States, B. bissettiae was detected by PCR from plasma cultured from a resident of southeastern North America [10]. In northwestern California, serum specimens from three residents of a rural community at high risk of tick-exposure and who were PCR positive for B. burgdorferi sl, were found to have been infected with B. bissettiae by sequence analysis [11]. However, none of those individuals had a clinical history compatible with Lyme disease [11, 34].
In this present study, B. bissettiae was detected in I. pacificus and I. spinipalpis adults and nymphs in coastal regions of both northern and southern California. This spirochete was first isolated from an adult I. pacificus from Del Norte County in the far north-coastal quadrant of California [33, 35]. Subsequently, it was detected in I. pacificus and I. spinipalpis in a few other regions of western North America [6, 36]. Recent studies have detected B. bissettiae in wild rodents and Ixodes spp. ticks in the midwestern and southeastern United States, Europe, and recently in South America [37–40]. Interestingly, B. bissettiae is recorded rarely from the northeastern United States, a region that harbors a remarkably high tick-infection prevalence with B. burgdorferi ss.
In California, B. bissettiae is found commonly in association with dusky-footed woodrats (Neotoma fuscipes), big-eared woodrats (Neotoma macrotis), Allen’s chipmunks (Neotamias senex), and I. spinipalpis [5, 36, 41, 42]. In addition, B. bissettiae has been detected in the bird tick Ixodes auritulus [14] and in sylvatic bird blood samples [43]. Genetic sub-structuring of Californian B. burgdorferi sl has been reported on a finer-scale within a single California county: B. burgdorferi ss was found in ticks from inland areas with higher than average temperatures whereas B. bissettiae was found in ticks from coastal areas with cooler temperatures [5]. These local regional differences in tick diversity may align with habitat types (e.g., chaparral, riparian, oak-woodland), which in turn can support different host species and potential reservoirs for different Borrelia genospecies [5].
Borrelia americana
Borrelia americana was first isolated from Ixodes minor nymphs and birds in South Carolina as well as from I. pacificus from California [44]. Since then, it has been detected from ticks outside the United States, with recent detections in Ixodes persulcatus in China [45]. The pathogenic status of this spirochete is unclear but B. americana-like DNA reportedly has been amplified from patients with Lyme disease–like symptoms from the southern United States [46]. The first detection of B. burgdorferi sl in southern California was an isolate from an I. pacificus tick collected in Orange County [47], later named CA-29-91 [48], and ultimately renamed B. americana [44]. More recently, B. americana was detected in I. pacificus from Los Angeles and Alameda counties [5, 29]. In the current study, it is notable that B. americana was detected in both the north-coastal (San Mateo County) and south-coastal (Orange County) regions of the state, as well as in two tick species, e.g., an I. pacificus adult from San Mateo County and two I. spinipalpis nymphs from Orange County (Table 1).
Borrelia miyamotoi
Other Borrelia spp. that cause human disease in North America include relapsing fever Borrelia that are molecularly and clinically distinct from B. burgdorferi sl infections. While most relapsing fever Borrelia, such as B. hermsii, are typically associated with argasid (soft) ticks in the genus Ornithodoros, B. miyamotoi is vectored by Ixodes species ticks in Europe, North America, and Asia. This spirochete recently was identified as an emerging pathogen in Russia, the Netherlands, Japan, and northeastern United States, and is associated with an acute febrile illness and subsequent relapsing fevers if left untreated [49]. Although no human cases of B. miyamotoi infection have been confirmed in California, serological, ecological, and epidemiological data offer presumptive evidence that B. miyamotoi occasionally infects people in northwestern California [50]. Molecular strain differences among B. miyamotoi appears to align with its associated tick species, with little geographic substructuring [51]. Similar to a 1% infection prevalence in other Ixodes ticks (both adults and nymphs) in surveillance conducted in the United States, Canada,and in Europe [15, 52, 53], B. miyamotoi is found in approximately 1% of I. pacificus nymphs and adults in California [12, 54]. While there is evidence of B. miyamotoi in rodents [15, 55], this similarity of infection prevalence among geographic regions, with diverse vectors and potential reservoir hosts, suggests a strong reliance on transovarial transmission to maintain infection in an area. Unlike B. burgdorferi sl, B. miyamotoi can be maintained transovarially and can be found in larval I. pacificus [12, 56]. In California, B. miyamotoi was detected primarily in I. pacificus from the northern region of the state, and was rarely detected in southern Californian I. pacificus [12]. To date, B. miyamotoi has not been found in I. spinipalpis nor any other wildlife tick in western North America.
Conclusion
Our findings demonstrate large-scale geographic structuring of the B. burgdorferi sl complex in western North America with concomitant differential acarological risk of exposure to Lyme borreliosis spirochetes. In southern California, people are at an exceeding low acarological risk of exposure to B. burgdorferi ss, the agent of Lyme disease in North America. In this study, ticks infected with B. burgdorferi ss were found in the Sierra Nevada foothills, north coastal, and central coastal regions of California, as far south as San Luis Obispo County. The geographic distribution of B. burgdorferi ss in California coincides with epidemiological findings, with the highest incidence of Lyme disease reported in northern California [57]. Notably, only a single I. pacificus has tested positive for B. burgdorferi ss from southern California, despite decades of testing and thousands of ticks tested [CDPH, unpublished data; 21]. While the risk of acquiring Lyme disease may be low in southern California, the risk of exposure to other tick-borne pathogens, such as spotted-fever group rickettsia may be higher in this region of the state [58]. Public health education messages should highlight this differential risk of tick-borne diseases to health care providers and the public.
While people are not at acarological risk of exposure to B. burgdorferi ss in southern California, this study did find three other Borrelia in ticks from this region: B. bissettiae, B. americana, and B. miyamotoi. Likewise, the acarological risk of exposure to B. bissettiae is variable among California regions, with an evident association with coastal regions of the state, including coastal areas of southern California. Of note, no I. pacificus from the Sierra Nevada foothills were positive for B. bissettiae, while B. burgdorferi ss is found commonly in I. pacificus from that region.
This study provides an assessment of acarological risk for known human tick-borne disease pathogens as well as potentially novel human pathogenic Borrelia species over a broad geographic area. Prior understanding of regional risk of known and potential tick-borne disease agents can assist with advancing diagnostics and epidemiologic investigations of human tick-borne disease cases. Results from this study indicate that additional research is warranted to evaluate fine scale landscape or reservoir host distribution range and tick-borne disease prevalence in California.
Acknowledgments
We gratefully acknowledge the excellent laboratory assistance of Mary Joyce Pakingan, Marina De Leon, Gordon Lau, and Robert Payne. Thanks to Ervic Aquino for assisting with graphics. We also thank VBDS Public Health Biologists and staff from many of California’s local mosquito and vector control agencies for their assistance with tick collections. Lastly, we would like to acknowledge Vicki Kramer, Chief of the Vector-Borne Disease Section for her ongoing support of enhanced surveillance of tick-borne diseases in California.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Margos G, Vollmer SA, Ogden NH, Fish D. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect Genet Evol. 2011; 11:1545–63. 10.1016/j.meegid.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margos G, Federova N, Kleinjan JE, Harberger C, Schwan TG, Sing A et al. Borrelia lanei sp. nov. extends the diversity of Borrelia species in California. Int J Syst Evol Microbiol. 2017; 67:3872–3876. 10.1099/ijsem.0.002214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudenko N, Golovchenko M, Mokracek A, Piskunova N, Ruzek D, Mallatova N, et al. Detection of Borrelia bissettii in cardiac value tissue of a patient with endocarditis and aortic value stenosis in the Czech Republic. J Clin Microbiol.2008; 46:3540–3543. 10.1128/JCM.01032-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016; 16:556–564. 10.1016/S1473-3099(15)00464-8 Epub 2016 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedorova N, Kleinjan JE, James D, Hui LT, Peeters H, Lane RS. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick-borne Dis. 2014; 5:951–61. 10.1016/j.ttbdis.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 6.Margos G, Lane RS, Federova N, Koloczek J, Piesman J, Hojgaard A, et al. Borrelia bissettiae sp. Nov. and Borrelia californiensis sp. nov. prevail in diverse enzootic transmission cycles. Int J Syst Evol Microbiol. 2016; 66:1447–1452. 10.1099/ijsem.0.000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011; 2:123–8. 10.1016/j.ttbdis.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudenko N, Golovchenko M, Ruzek D, Piskunova N, Mallatova N, Grubhoffer L. Molecular detection of Borrelia bissettii DNA in serum samples from patients in the Czech Republic with suspected borreliosis. FEMS Microbiol Lett. 2009; 292:274–281. 10.1111/j.1574-6968.2009.01498.x [DOI] [PubMed] [Google Scholar]
- 9.Maraspin V, Klevisar N, Sabjiic R, Lusa L, Strle F. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016; 63:914–921. 10.1093/cid/ciw417 [DOI] [PubMed] [Google Scholar]
- 10.Golovchenko M, Vancová M, Clark K, Oliver JH Jr, Grubhoffer L, Rudenko N. A divergent spirochete strain isolated from a resident of the southeastern United States was identified by multilocus sequence typing as Borrelia bissettii. Parasit Vectors. 2016; 9:68 10.1186/s13071-016-1353-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard YA, Fedorova N, Lane RS. Genetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north-coastal California residents. J Clin Microbiol. 2011;49:945–54. 10.1128/JCM.01689-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padgett KA, Bonilla DL, Kjemtrup A, Vilcins IM, Yoshimizu MH, Hui L, et al. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus 2014; PLoS One. 2014; 9(10):e110853 10.1371/journal.pone.0110853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persing DH, Telford SR, Spielman A, Barthold SW. Detection of Borrelia burgdorferi infection in Ixodes dammini ticks with the polymerase chain reaction J Clin Microbiol. 1990; 28:566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padgett KA, Bonilla DL. Novel exposure sites for nymphal Ixodes pacificus within picnic areas. Ticks Tick-borne Dis. 2011; 2: 191–195. 10.1016/j.ttbdis.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 15.Barbour AG, Bunkis J, Travinsky B, Gatewood Hoen A, Diuk-Wasser MA, Fish D, et al. Niche partitioning of Borrelia burdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009; 81:1120–1131. 10.4269/ajtmh.2009.09-0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbour AG, Carter CJ, Bundoc V, Hinnebusch J. The nucleotide sequence of a linear plasmid of Borrelia burgdorferi reveals similarities to those of circular plasmids of other prokaryotes. J Bacteriol. 19; 178:6635–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marconi RT, Garon CF. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992; 30:2830–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008; July 1;36(Web Server issue):W465–9. 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000; 17:540–542. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 20.Milne I, Lindner D, Bayer M, Husmeier D, McGuire G, Marshall DF, et al. TOPALi v2: a rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics. 2009; 25:126–127. 10.1093/bioinformatics/btn575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001; 17:754–755. [DOI] [PubMed] [Google Scholar]
- 22.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003; 19:1572–1574. [DOI] [PubMed] [Google Scholar]
- 23.Altekar G, Dwarkadas S, Huelsenbeck JP, Ronquist F. Parallel Metropolis coupled Markov chair Monte Carlo for Bayesian phylogenetic inference. Bioinformatics. 2004; 20:407–415. 10.1093/bioinformatics/btg427 [DOI] [PubMed] [Google Scholar]
- 24.Tälleklint-Eisen L, Lane RS. 1999. Variation in the density of questing Ixodes pacificus (Acari:Ixodidae) nymphs infected with Borrelia burgdorferi at different spatial scales in California. J Parasitol. 1999; 85:824–831. [PubMed] [Google Scholar]
- 25.Lane RS, Mun J, Peribanez MA, Stubbs HA. Host-seeking behavior of Ixodes pacificus (Acari: Ixodidae) nymphs in relation to environmental parameters in dense-woodland and woodland-grass habitats. J Vec Biol. 2007; 32:342–357. [DOI] [PubMed] [Google Scholar]
- 26.Swei A, Meentemeyer R, Briggs CJ. Influence of abiotic and environmental factors on the density and infection prevalence of Ixodes pacificus (Acari: Ixodidae) with Borrelia burgdorferi. J Med Entomol. 2014; 48:20–28. [DOI] [PubMed] [Google Scholar]
- 27.Stafford KC, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998; 36:1240–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee X, Coyle DR, Johnson DK, Murphy MW, McGeehin MA, Murphy RJ, et al. , Prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum in Ixodes scapularis (Acrari: Ixodidae) nymphs collected in managed red pine forests in Wisconsin. J Med Entomol. 51:694–701. [DOI] [PubMed] [Google Scholar]
- 29.Lane RS, Fedorova N, Kleinjan JE, Maxwell M. Eco-epidemiological factors contributing to the low risk of human exposure to ixodid tick-borne borreliae in southern California, USA. Ticks Tick Borne Dis. 2013; 4:377–385. 10.1016/j.ttbdis.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 30.MacDonald AJ, Hyon DW, Brewington JB, O’Connor KE, Swei A, and Briggs CJ. Lyme disease risk in southern California: abiotic and environmental drivers of Ixodes pacificus (Acari: Ixodidae) density and infection prevalence with Borrelia burgdorferi. Parasit Vectors 2017; 10:7 10.1186/s13071-016-1938-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane RS, Brown RN, Piesman J, Peavey CA. Vector competence of Ixodes pacificus and Dermacentor occidentalis (Acari: Ixodidae) for various isolates of Lyme disease spirochetes.J Med Entomol. 1994; 31: 417–424. [DOI] [PubMed] [Google Scholar]
- 32.Furman DP, Loomis EC. The ticks of California (Acari: Ixodida). University of California Press; Berkeley: 1984. [Google Scholar]
- 33.Postic D, Ras NM, Lane RS, Hendson M, Baranton G. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127). J Clin Microbiol. 1998; 36:3497–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane RS, Manweiler SA, Stubbs HA, Lennette ET, Madigan JE, Lavoie PE. Risk factors for Lyme disease in a small rural community in northern California. Am J Epidemiol. 1992; 13611:1358–1368. [DOI] [PubMed] [Google Scholar]
- 35.Bissett ML, Hill W. Chracterization of Borrelia burgdorferi strains isolated from Ixodes pacificus ticks in California. J. Clin Microbiol. 1987; 25:2296–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown RN, Peot MA, Lane RS. Sylvatic maintenance of Borrelia burgdorferi (Spirochetales) in northern California; untangling the web of transmission. J Med Entomol. 2006; 43:743–751. [DOI] [PubMed] [Google Scholar]
- 37.Schneider BS, Zeidner NS, Burkot TR, Maupin GO, Piesman J. Borrelia isolates in Northern Colorado identified as Borrelia bissettii.J. Clin Microbiol. 2000; 38:3103–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliver JH Jr, Lin T, Gao L, Clark KL, Banks CW, Durden LA, et al. An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc Natl Acad Sci U S A. 2003; 100:11642–5. 10.1073/pnas.1434553100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbieri AM, Venzal JM, Marcili A, Almeida AP, González EM, Labruna MB. Borrelia burgdorferi sensu lato infecting ticks of the Ixodes ricinus complex in Uruguay: first report for the Southern Hemisphere. Vector Borne Zoonotic Dis. 2013; 13:147–53. 10.1089/vbz.2012.1102 [DOI] [PubMed] [Google Scholar]
- 40.Raileanu C, Moutailler S, Pavel I, Porea D, Mihalca AD, Savuta G. Borrelia diversity and co-infection with other tick borne pathogens in ticks. Front Cell Infect Microbiol. 2017; 7: 36 10.3389/fcimb.2017.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vredevoe LK, Stevens JR, and Schneider BS. Detection and characterization of Borrelia bissettii in rodents from the central California coast. J. Med. Entomol. 2004;41: 736–745. [DOI] [PubMed] [Google Scholar]
- 42.Hacker GM, Brown RN, Fedorova N, Girard YA, Higley M, Clueit B, et al. Spatial clustering of Borrelia burgdorferi sensu lato within populations of Allen’s chipmunks and dusky-footed woodrats in northwestern California. PLoS One, 2018; 10.1371/journal.pone.0195586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman EA, Eisen L, Eisen RJ, Fedorova N, Hasty JM, Vaughn C, et al. Borrelia burgdorferi sensu lato spirochetes in wild birds in northwestern California: associations with ecological factors, bird behavior and tick infestation. PLoS One. 2015; 10(2):e0118146 10.1371/journal.pone.0118146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudenko N, Golovchenko M, Lin T, Gao L, Grubhoffer L, Oliver JH Jr. Delineation of a new species of the Borrelia burgdorferi Sensu Lato Complex, Borrelia americana sp. nov. J Clin Microbiol. 2009; 47:3875–80. 10.1128/JCM.01050-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu PF, Niu QL, Liu ZJ, Yang JF, Chen Z, Guan GQ,et al. Molecular epidemiological surveillance to assess emergence and re-emergence of tick-borne infections in tick samples from China evaluated by nested PCRs. Acta Trop. 2016; 158:181–8. 10.1016/j.actatropica.2016.02.027 [DOI] [PubMed] [Google Scholar]
- 46.Clark KL, Leydet BF, Threlkeld C. Geographical and genospecies distribution of Borrelia burgdorferi sensu lato DNA detected in humans in the USA. J Med Microbiol. 2014; 63:674–84. 10.1099/jmm.0.073122-0 [DOI] [PubMed] [Google Scholar]
- 47.Meyers MH, Moore DR, Gellert G, Euler GL, Prendergast TH, Badri M, et al. Isolation of Borrelia burgdorferi from ticks in southern California. Western J Med 1992;157:455–456. [PMC free article] [PubMed] [Google Scholar]
- 48.Schwan TG, Schrumpf ME, Karstens RH, Clover JR, Wong J, Daugherty M. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J Clin Microbiol. 1993;31:3096–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Inf. 2015; 21:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krause PJ, Carroll M, Fedorova N, Brancato J, Dumouchel C, Akosa F, et al. Human Borrelia miyamotoi infection in California: serodiagnosis is complicated by multiple endemic Borrelia species. PLoS One 2018; 13(2):e0191725 10.1371/journal.pone.0191725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook VJ, Fedorova N, Macdonald WP, Lane RS, Barbour AG. Unique strain of Borrelia miyamotoi in Ixodes pacificus ticks, California, USA. Emerg Infect Dis. 2016; 22:2205–2207. 10.3201/eid2212.152046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geller J, Nazarova L, Katargina O, Golovljova I. Borrelia burgdorferi sensu lato prevalence in tick populations in Estonia. Parasit Vectors. 2013; 9;6:202 10.1186/1756-3305-6-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dibernardo A, Cote T, Ogden NH, Lindsay LR. The prevalence of Borrelia miyamotoi infection, and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasit Vectors. 2014; 15:183 10.1186/1756-3305-7-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynn GE, Graham CB, Horiuchi K, Eisen L, Johnson TL, Lane RS, et al. Prevalence and Geographic Distribution of Borrelia miyamotoi in host-seeking Ixodes pacificus (Acari: Ixodidae) nymphs in Mendocino County, California. J. Med Entomol. 2018; 553:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salkeld D, Nieto NC, Bonilla DL, Yoshimizu MH, Padgett KA. Borrelia miyamotoi infections in small mammals, California, USA. EID. 2018; 24(12): 2356–2359. 10.3201/eid2412.171632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoon Dis. 2001;1:21–34. [DOI] [PubMed] [Google Scholar]
- 57.California Department of Public Health. Vector-Borne Disease Section Annual Report, 2017. Kjemtrup, A. and Kramer, V. editors. Sacramento, California, 2018. pp 1–32. https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/VBDSAnnualReports.aspx
- 58.Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, Porse CC, et al. The eco-epidemiology of Pacific Coast Tick Fever in California. PLoS Neg Trop Dis. 2016; 10.1371/journal.pntd.0005020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.