Abstract
Aims/hypothesis
Our objectives were to explore whether the phenomenon of HbA1c ‘tracking’ occurs in individuals with type 1 diabetes, how long after diagnosis does tracking take to stabilise, and whether there is an effect of sex and age at diagnosis on tracking.
Methods
A total of 4525 individuals diagnosed with type 1 diabetes between 1 January 1995 and 1 May 2015 were identified from The Health Improvement Network (THIN) database. Mixed models were applied to assess the variability of HbA1c levels over time with random effects on general practices (primary care units) and individuals within practices.
Results
4525 individuals diagnosed with type 1 diabetes were identified in THIN over the study period. The greatest difference in mean HbA1c measurement (−7.0 [95% CI −8.0, −6.1] mmol/mol [0.6%]) was seen when comparing measurements made immediately after diagnosis (0–1 year since diagnosis) with those at 10 or more years (the reference category). The mean difference in HbA1c for the successive periods compared with 10 or more years after diagnosis declined and was no longer statistically significant after 5 years. In the stratified analysis using sex and age group there was considerable heterogeneity with adult onset type 1 diabetes appearing to track earlier and at a lower mean HbA1c.
Conclusions/interpretation
In individuals with type 1 diabetes, glycaemic control measured by HbA1c settles onto a long-term ‘track’ and this occurs on average by 5 years following diagnosis. Age at diagnosis modifies both the rate at which individuals settle into their track and the absolute HbA1c tracking level for the next 10 years.
Electronic supplementary material
The online version of this article (10.1007/s00125-018-4574-6) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Glycated haemoglobin, HbA1c, Tracking, Type 1 diabetes mellitus

Introduction
Clinical practice suggests that HbA1c remains remarkably stable in individuals with type 1 diabetes. Some individuals are consistently able to achieve good glycaemic control at repeated clinical follow-up, while others struggle to do so for any meaningful period of time. In these latter individuals, life events (such as pregnancy) or planned interventions (including structured education or continuous subcutaneous insulin infusion therapy) associate with improvements in HbA1c. However, this improved HbA1c is often not maintained beyond a few years [1, 2]. This stability of HbA1c that can manifest over decades, or even a lifetime, has been referred to as glycaemic ‘tracking’ [3].
Glycaemic tracking is distinct from HbA1c variability. Year to year variability in HbA1c is a characteristic described in individuals with pre-existing diabetes and associates with both micro- and macrovascular disease [4, 5]. Glycaemic tracking is also not simply the inverse of glucose variability, which is the daily change in blood glucose that includes symptomatic hypo- and hyperglycaemia.
It is vitally important to explore the phenomenon of glycaemic tracking. If tracking is an inherent part of the natural history of type 1 diabetes, this would emphasise the importance of early metabolic control after diagnosis. There may therefore be a window of opportunity where focused interventions set the scene for long-term glycaemic control and facilitate cost effective allocation of time and resources. Exploring the phenomenon of glycaemic tracking may also provide insights into underlying mechanisms, and from there, testable approaches to influencing the final level of HbA1c tracking.
Glycaemic tracking remains to be well characterised. Crucially, previous reports [3–18] have largely examined individuals with pre-existing type 1 diabetes (i.e. not from the time of diagnosis). Those few studies that have followed individuals from the time of diagnosis have been of limited sample size and short duration, have examined individuals across a narrow age group (primarily paediatric) and have not adjusted for important confounders (e.g. socioeconomic status). These studies are summarised in the electronic supplementary material (ESM) Table 1 and illustrate the need to study the phenomenon of glycaemic tracking in a more robust manner.
To explore the concept of glycaemic tracking formally, we performed a large, UK population-based cohort study involving over 4000 individuals with newly diagnosed type 1 diabetes and over 50,000 longitudinally collected HbA1c measurements. We wished to establish: (1) whether the phenomenon of HbA1c tracking occurs in individuals with type 1 diabetes; (2) how long after diagnosis does tracking take to stabilise; and (3) the effect of sex and age of diagnosis at tracking.
Methods
The Health Improvement Network (THIN) database is a large primary care dataset derived from general practices (primary care units) across the UK [19]. More than 675 practices contribute to the dataset resulting in over 14 million patient records of which around 4 million are active participants. The database consists of individuals’ demographic details, diagnosis, prescriptions and laboratory results. The database is generalisable to the UK population, and has been utilised for numerous epidemiological studies, including type 1 diabetes [20].
The use of the THIN data for research was approved by the South-East Multicenter Research Ethics Committee in 2003, without the need for informed consent. As per the requirements for ethical approval, further registration and authorisation for this project were obtained from the Scientific Review Committee of the data provider (IQVIA: 17THIN015).
Population
Individuals diagnosed with type 1 diabetes between 1 January 1990 and 1 May 2015 were identified using appropriate Read codes (https://digital.nhs.uk/article/1104/Read-Codes) and based on an algorithm recently published by Sharma et al 2016 [21]. To be classified as having type 1 diabetes, participants need to have a type 1 diabetes specific Read code, be aged less than 40 years at diagnosis and have been prescribed insulin but not oral hypoglycaemic medications. Individuals were eligible to be included in the cohort if they were diagnosed at least 1 year after registration with the practice or a year after the practice was eligible to take part, whichever was the latest. The date of diagnosis used was the one recorded in the database. Participating general practices were eligible to take part following: (1) introduction of the computerised system; (2) the date practices were deemed as having acceptable mortality rates. This was important to ensure that practices were recording important information and comorbidities accurately.
Measurement of HbA1c
All HbA1c values recorded in the database were extracted for the cohort of individuals with type 1 diabetes. Where the unit of measurement was percentage, values were converted to mmol/mol for analysis. Duplicates and implausible measurements (<20 mmol/mol [<4%] and >195 mmol/mol [>20%]) were removed before the analysis. Initial HbA1c at the time of diagnosis is often measured in a secondary care setting and these data were therefore not available for inclusion.
Statistical analysis
The analysis aimed to answer the three aforementioned questions: does HbA1c tracking occur among individuals with type 1 diabetes; if tracking occurs, how soon after diagnosis does this manifest; if tracking occurs, what is the impact of sex and age at diagnosis on the natural history of tracking?
Sociodemographic characteristics, HbA1c levels and time since diagnosis were summarised using descriptive statistics. Exploratory plots were produced for the mean levels of HbA1c vs time since diagnosis of type 1 diabetes. Similar plots were also produced stratifying by 10 year age bands and sex. The time since diagnosis with type 1 diabetes represents the duration between the date when a particular HbA1c measurement was taken and the initial date of diagnosis. This was divided into 11 categories: 0–1 years, 1–2 years, 2–3 years,…, 9–10 years and ≥10 years to facilitate modelling because our exploratory analyses indicated that the association between HbA1c level and time was likely to be non-linear.
Glycaemic tracking was defined as a period in which there was no statistically significant (p < 0.05) difference in HbA1c across adjacent years in comparison with the HbA1c measurement at ≥10 years from diagnosis.
We used linear mixed effects models to assess the variability of HbA1c levels over time with random effects on practices and individuals within practices. These models, also known as multilevel or hierarchical linear models, constitute both fixed effects and random effects. The fixed part is similar to standard linear regression but the addition of random effects allows the model to account for the potential effect of variability at different grouping or clustering levels. In other words, the random effects are variance components associated with each level. In our study, there are three variance components, namely: (1) within individual variability (the residual error associated with repeated measurements from an individual), (2) between individuals within practice variation (as several individuals share the same practice), and (3) between practice variation. Individuals within the same practice may be correlated because of a shared random intercept, through a shared random slope on a covariate or both. Thus, both adjusted and unadjusted two-level random intercepts and slopes (for individuals within practices) models were fitted using the ≥10 years duration group as reference. The adjusted model included age at diagnosis (10 year bands), sex and the Townsend index.
The Townsend index, ranging from 1 to 5, is a measure of material deprivation, calculated using social indices such as income, education and employment specific to each participant’s neighbourhood [22, 23]. Socioeconomic status has been convincingly related to glycaemic control [24] and the Townsend index has been used extensively as a covariate in diabetes studies using THIN database [25–27]. In the adjusted model, the group with the longest duration of type 1 diabetes (≥10 years) was preferred as the reference category; this is because if individuals were to track, i.e., if the proposed hypothesis was true, then we would expect to see no significant difference between this group and groups with a lower duration of type 1 diabetes. The duration after which there is no significant difference in the final HbA1c will be the time point at which tracking manifests. In addition to this, a stratified analysis will be conducted using sex and age group as stratification factors.
In a sensitivity analysis, the random intercepts and slopes model were fitted including data only from individuals with 10 or more complete years of follow-up. All analyses were performed using Stata 14 (StataCorp, College Station, TX, USA).
Results
There were 4525 individuals diagnosed with type 1 diabetes in the THIN database over the study period 1995–2015 from a total of 617 practices. The majority were male (60.6%) and the highest proportion (38.4%) were diagnosed between the ages of 10 and 20 years. The mean ± SD HbA1c level was 72.6 ± 20.6 mmol/mol (8.8 ± 4%) and the median follow-up after diagnosis was 5 years (Table 1).
Table 1.
Baseline characteristics of participants and HbA1c summary statistics
| Characteristics | All participants N = 4525 |
Participants contributing for 10 years and above n = 938 |
|---|---|---|
| Age group (years) | ||
| 0–10 | 1294 ± 28.6 | 293 ± 31.3 |
| 10–20 | 1737 ± 38.4 | 324 ± 34.5 |
| 20–30 | 798 ± 17.6 | 138 ± 14.7 |
| 30–40 | 696 ± 15.4 | 183 ± 19.5 |
| Sex | ||
| Male | 2743 ± 60.6 | 574 ± 61.2 |
| Female | 1782 ± 39.4 | 364 ± 38.8 |
| Townsend index | ||
| 1 | 1020 ± 22.5 | 229 ± 24.4 |
| 2 | 869 ± 19.2 | 178 ± 19.0 |
| 3 | 923 ± 20.4 | 190 ± 20.3 |
| 4 | 828 ± 18.3 | 175 ± 18.7 |
| 5 | 650 ± 14.4 | 137 ± 14.6 |
| Missing | 235 ± 5.2 | 29 ± 3.0 |
| Follow-up period (years) | ||
| Mean (SD) | 6.0 ± 4.6 | 13.2 ± 2.4 |
| Median (25th–75th percentile) | 5.0 (2.1–9.2) | 13.2 (11.2–14.8) |
| HbA1c (mmol/mol)a | ||
| Mean (SD) | 72.6 ± 20.6 | 73.7 ± 19.5 |
| Median (25th–75th percentile) | 69.4 (58.5–82.5) | 70.5 (60.7–83.6) |
| HbA1c (%)a | ||
| Mean (SD) | 8.8 ± 4.0 | 8.9 ± 3.9 |
| Median (25th–75th percentile) | 8.5 (7.5–9.7) | 8.6 (7.7–9.8) |
Data presented as mean ± SD or median (25th–75th percentile)
aNumber of measurements in study period: 41,583 for all participants group, 16,989 for participants contributing for 10 years and above
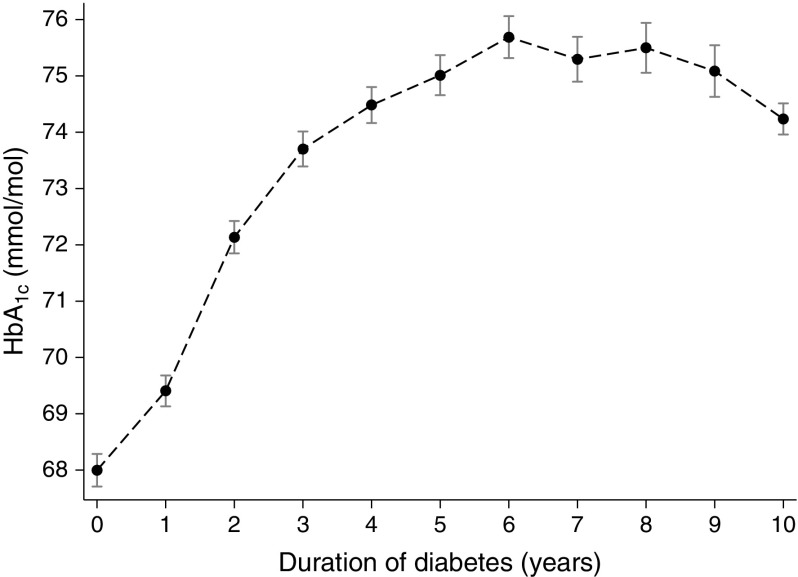
HbA1c increased with time from diagnosis in individuals with type 1 diabetes and stabilised by 5 years to an average of 75.0 mmol/mol (9.0%) following this period (Fig. 1). However, the timing of stabilisation was dependent on age at diagnosis and sex (ESM Fig. 1).
Fig. 1.
Mean HbA1c by year from diagnosis of type 1 diabetes in 4525 patients with newly diagnosed diabetes. For example, duration time 0 represents the HbA1c values captured from date of diagnosis to year 1; time 1 represents the HbA1c measurements from year 1 to 2, and time 9 represents HbA1c measurements from year 9 to 10
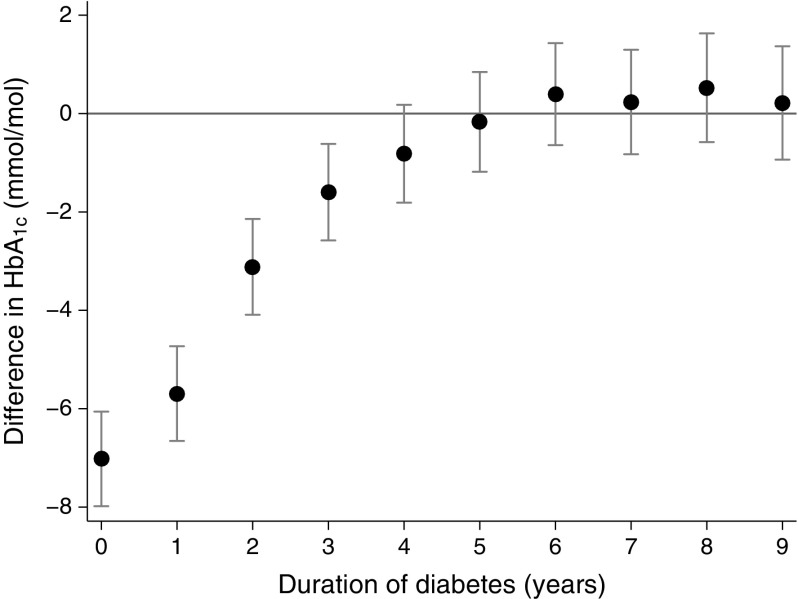
The largest difference between mean HbA1c measurements was between those taken in the first year following diagnosis (time: 0 years in Fig. 2) and those taken after 10 or more years (the reference category) (−7.0 [95% CI −8.0, −6.1] mmol/mol [0.6%]; Fig. 2). The mean HbA1c difference for the successive periods after diagnosis (1–2 years, 2–3 years, etc.) compared with after 10 or more years declined considerably and was no longer statistically significant 5 years following diagnosis (a duration time of 4 years in figures illustrates HbA1cs measured between 4 and 5 years after diagnosis); the mean HbA1c difference for this duration after diagnosis was −0.8 (95% CI −1.8, 0.2) mmol/mol (0.7%). The findings remained similar when analysis was restricted only to participants contributing HbA1c measurements for 10 years and above (ESM Fig. 2).
Fig. 2.
Mean (95% CI) difference in HbA1c between the mean for the reference group (≥10 years post diagnosis) and the mean for each year after diagnosis. Duration time 0 represents the HbA1c values captured from date of diagnosis to year 1; time 1 represents the HbA1c measurements from year 1 to 2, etc. Models were constructed using a random intercept and slopes model adjusting for age, sex and Townsend index. The time point at which the 95% CI for the yearly difference crosses the null value (zero) is considered the starting point of tracking
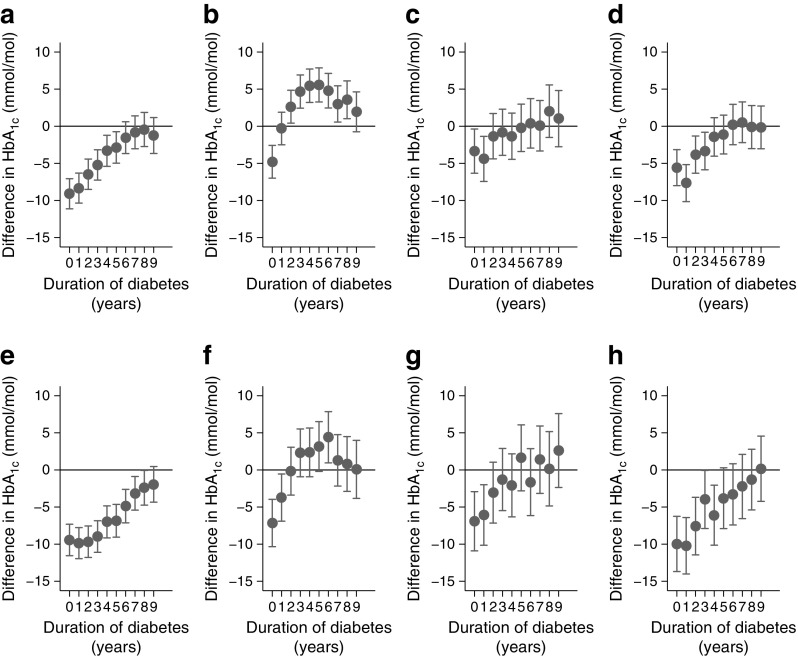
There was considerable heterogeneity in the timing of tracking in the stratified analysis using sex and age group. Adult onset type 1 diabetes seem to track earlier (Fig. 3) and at a lower mean HbA1c (ESM Fig. 1). Children diagnosed between 0 and 10 years tracked from 6–7 years and 8–9 years after diagnosis for boys and girls, respectively (Fig. 3a, e). For the 10–20 years diagnosis age group, tracking occurred at 9–10 years in males and 7–8 years in females (Fig. 3b, f). For the 20–30 years group, tracking occurred at 2–3 years for both sexes (Fig. 3c, g). Among the 30–40 years age group, tracking was at 4–5 years in men (Fig. 3d) and 5–6 years in women (Fig. 3h).
Fig. 3.
Difference in HbA1c between the reference group (≥10 years) and each year after the time of diagnosis of type 1 diabetes stratified by age at diagnosis and sex. Duration time 0 represents the HbA1c values captured from date of diagnosis to year 1; time 1 represents the HbA1c measurements from year 1 to 2, etc. (a, e) Diagnosis between ages 0 and 10 years; (b, f) between ages 10 and 20 years; (c, g) between ages 20 and 30 years; (d, h) between ages 30 and 40 years; male group (a–d) and female group (e–h). Models were constructed using a random intercept and slopes model adjusting for age, sex and Townsend index. The difference is given for each year with a point estimate and its 95% CI. The time point where the above difference crosses the null value (zero) is considered the starting point of tracking
Discussion
We show that in individuals with type 1 diabetes, glycaemic control measured by HbA1c settles onto a long-term ‘track’ and that this occurs on average by 5 years following diagnosis. We also show that the age at diagnosis modifies both the rate at which individuals settle into their track and the absolute HbA1c tracking level for the next 10 years. Our analytical approach utilising a random intercept and slope model ensures that our overall results are not confounded by trajectories at an individual level.
This is the first study to demonstrate the phenomenon of glycaemic tracking on a large number of unselected, newly diagnosed individuals with type 1 diabetes across a broad age group and with a long period of follow-up. Crucially, this study is unique in that the association has been adjusted for important potential confounders such as local clinical practice and Townsend score. The study is weakened by the fact that adequate follow-up data are not available for greater than 10 years and that the diagnosis of type 1 diabetes was taken prima facie from the national THIN general practice database. For this reason, we limited the analysis to those diagnosed with type 1 diabetes under the age of 40 years, on insulin alone and with no history of oral hypoglycaemic prescriptions. Finally, the HbA1c assay has evolved over the course of the time span during which this study extends. Although appropriate conversions were meticulously undertaken, inherent differences in methodologies should be taken into account when interpreting differences in HbA1c before and after 2009 in the UK. Similarly, several important changes in diabetes management have been introduced over the study period such as the introduction of novel insulin formulations, widespread adoption of intensive insulin treatment schemes and revision of education systems. These may have contributed towards an improvement of glycaemic control at a national level and although data is missing, they should be considered in the context of this study.
It is notable that the overall HbA1c levels in the UK are suboptimal. This has been seen before with regard to other countries [28]. Furthermore there is a discrepancy between the sexes and this may relate to the higher insulin resistance [29] and behavioural issues [30] that may be more prevalent in younger women and girls with type 1 diabetes.
Several hypotheses, focusing on endogenous and/or exogenous factors, have been suggested to underlie the phenomenon of glycaemic tracking. Residual beta cell function, measured by stimulated C-peptide, decays with time following type 1 diabetes diagnosis [31] and low C-peptide is associated with higher HbA1c [32, 33]. Therefore, the loss of C-peptide following diagnosis may explain the rising HbA1c in the initial 5 years. Alternatively, or in addition, individual patient-related factors may contribute. Habituation of the day to day approach to managing chronic disease can make any long-lasting change difficult [34]. Studies involving paediatric and adolescent individuals with type 1 diabetes indicate that age, sex, body mass index, socioeconomic factors, physical activity levels, frequency of glucose monitoring and personality traits can also influence temporal HbA1c trends [16, 35–37]. It may be that a combination of these endogenous and exogenous factors contributes to the phenomenon of tracking and that both need to be addressed for effective long-term glycaemic control.
There are two clear implications of our results. First, there is a 5 year window during which longer-term HbA1c and therefore risk of diabetes complications is determined. Thus, urgent and appropriate targeting of therapies to this period of type 1 diabetes natural history should be considered. This may include, for example, the development of a newly diagnosed type 1 diabetes pathway with structured education and aggressive glucose control. Second, the benefits of addressing HbA1c after the first 5 years should be explored. It has been suggested that efforts would be better directed at risk factors other than HbA1c in individuals with established type 1 diabetes [13]. However, such an approach may result in loss of the tracking phenomenon and deterioration in HbA1c so this needs to be carefully explored. Unfortunately, data are missing with regards to both effectiveness and timing of a focused clinical intervention targeted at changing the track and this clinical need becomes even more evident in light of the study findings. Furthermore, it is worth noting that HbA1c provides only one indication of glycaemic control. Day to day glucose variability is of greater concern to individuals and is poorly reflected in the HbA1c measure. Indeed, ‘real world’ experience of new interventions such as structured education and insulin pump therapy suggest they have had a greater benefit on glucose variability than meaningful long-term changes to HbA1c [38, 39].
This is the first comprehensive study of the phenomenon of HbA1c tracking and supports the need to optimise glycaemic control early in the natural history of type 1 diabetes. Studies are now needed to explore the mechanisms underlying this phenomenon and how best to optimise tracking in newly diagnosed individuals.
Electronic supplementary material
(PDF 243 kb)
Acknowledgements
We are grateful to S. Heller, University of Sheffield, Sheffield, UK for critical reading and comments on this manuscript.
Contribution statement
All authors made a substantial contribution to the design of the study, acquisition of data, or analysis and interpretation of data, and gave final approval of the version to be published. KN, KT and NT contributed to study design, statistical analysis and writing the manuscript. NM contributed to the statistical analysis, writing the manuscript and preparing the figures. PN contributed to study conception, design, analysis and writing the manuscript. KN is the guarantor of this work.
Abbreviation
- THIN
The Health Improvement Network
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability
THIN data governance does not allow us to share individual patient data and therefore where possible metadata is presented. Researchers may apply for individual patient data access at https://www.iqvia.com/contact.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Footnotes
Krishnarajah Nirantharakumar and Nuredin Mohammed are joint first authors.
Change history
4/25/2018
Abstract
In Table 1 the data for age group, sex and Townsend index were incorrectly identified as mean ± SD instead of n (%). The table is corrected here.
Contributor Information
G. Neil Thomas, Email: g.n.thomas@bham.ac.uk.
Parth Narendran, Email: p.narendran@bham.ac.uk.
References
- 1.Brorsson AL, Viklund G, Ortqvist E, Lindholm Olinder A. Does treatment with an insulin pump improve glycaemic control in children and adolescents with type 1 diabetes? A retrospective case-control study. Pediatr Diabetes. 2015;16:546–553. doi: 10.1111/pedi.12209. [DOI] [PubMed] [Google Scholar]
- 2.Speight J, Amiel SA, Bradley C, et al. Long-term biomedical and psychosocial outcomes following DAFNE (Dose Adjustment For Normal Eating) structured education to promote intensive insulin therapy in adults with sub-optimally controlled type 1 diabetes. Diabetes Res Clin Pract. 2010;89:22–29. doi: 10.1016/j.diabres.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Hofer SE, Raile K, Frohlich-Reiterer E, et al. Tracking of metabolic control from childhood to young adulthood in type 1 diabetes. J Pediatr. 2014;165:956–961. doi: 10.1016/j.jpeds.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Forsander G, Persson B, Sundelin J, Berglund E, Snellman K, Hellstrom R. Metabolic control in children with insulin-dependent diabetes mellitus 5 y after diagnosis. Early detection of patients at risk for poor metabolic control. Acta Paediatr. 1998;87:857–864. doi: 10.1111/j.1651-2227.1998.tb01551.x. [DOI] [PubMed] [Google Scholar]
- 5.Jorde R, Sundsfjord J. Intra-individual variability and longitudinal changes in glycaemic control in patients with type 1 diabetes mellitus. Diabet Med. 2000;17:451–456. doi: 10.1046/j.1464-5491.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- 6.Luyckx K, Seiffge-Krenke I. Continuity and change in glycemic control trajectories from adolescence to emerging adulthood: relationships with family climate and self-concept in type 1 diabetes. Diabetes Care. 2009;32:797–801. doi: 10.2337/dc08-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge JA, James T, Shine B. Persistent individual tracking within overall improvement in HbA1c in a UK paediatric diabetes clinic over 15 years. Diabet Med. 2010;27:1284–1288. doi: 10.1111/j.1464-5491.2010.03057.x. [DOI] [PubMed] [Google Scholar]
- 8.Helgeson VS, Snyder PR, Seltman H, Escobar O, Becker D, Siminerio L. Brief report: trajectories of glycemic control over early to middle adolescence. J Pediatr Psychol. 2010;35:1161–1167. doi: 10.1093/jpepsy/jsq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanathan V, Sneeringer MR, Miller A, Eugster EA, DiMeglio LA. The utility of hemoglobin A1c at diagnosis for prediction of future glycemic control in children with type 1 diabetes. Diabetes Res Clin Pract. 2011;92:65–68. doi: 10.1016/j.diabres.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson C, Wernham EM, Elder CJ, Wright NP. Early glycaemic control is predictive of long-term control: a retrospective observational study. Pract Diabetes. 2013;30:16–18. doi: 10.1002/pdi.1734. [DOI] [Google Scholar]
- 11.Shalitin S, Phillip M. Which factors predict glycemic control in children diagnosed with type 1 diabetes before 6.5 years of age? Acta Diabetol. 2012;49:355–362. doi: 10.1007/s00592-011-0321-x. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera SM, Srivastava NT, Behzadi JM, Pottorff TM, Dimeglio LA, Walvoord EC. Long-term glycemic control as a result of initial education for children with new onset type 1 diabetes: does the setting matter? Diabetes Educ. 2013;39:187–194. doi: 10.1177/0145721713475845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill GV, Wallymahmed ME, Wallymahmed A, MacFarlane IA, Woodward A. Glycaemic streaming in type 1 diabetes: implications for intervention? Pract Diabetes. 2013;30:229–232. doi: 10.1002/pdi.1781. [DOI] [Google Scholar]
- 14.Samuelsson U, Steineck I, Gubbjornsdottir S. A high mean-HbA1c value 3-15 months after diagnosis of type 1 diabetes in childhood is related to metabolic control, macroalbuminuria, and retinopathy in early adulthood—a pilot study using two nation-wide population based quality registries. Pediatr Diabetes. 2014;15:229–235. doi: 10.1111/pedi.12085. [DOI] [PubMed] [Google Scholar]
- 15.Clements MA, Lind M, Raman S, et al. Age at diagnosis predicts deterioration in glycaemic control among children and adolescents with type 1 diabetes. BMJ Open Diabetes Res Care. 2014;2:e000039. doi: 10.1136/bmjdrc-2014-000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawes T, Franklin V, Farmer G. HbA1c tracking and bio-psychosocial determinants of glycaemic control in children and adolescents with type 1 diabetes: retrospective cohort study and multilevel analysis. Pediatr Diabetes. 2014;15:372–383. doi: 10.1111/pedi.12100. [DOI] [PubMed] [Google Scholar]
- 17.Schwandt A, Hermann JM, Rosenbauer J, et al. Longitudinal trajectories of metabolic control from childhood to young adulthood in type 1 diabetes from a large German/Austrian registry: a group-based modeling approach. Diabetes Care. 2017;40:309–316. doi: 10.2337/dc16-1625. [DOI] [PubMed] [Google Scholar]
- 18.Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes. 2016;17:327–336. doi: 10.1111/pedi.12295. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 20.Dafoulas GE, Toulis KA, McCorry D, et al. Type 1 diabetes mellitus and risk of incident epilepsy: a population-based, open-cohort study. Diabetologia. 2017;60:258–261. doi: 10.1007/s00125-016-4142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma M, Petersen I, Nazareth I, Coton SJ. An algorithm for identification and classification of individuals with type 1 and type 2 diabetes mellitus in a large primary care database. Clin Epidemiol. 2016;8:373–380. doi: 10.2147/CLEP.S113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarman B, Townsend P, Carstairs V. Deprivation indices. BMJ. 1991;303:523. doi: 10.1136/bmj.303.6801.523-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris R, Carstairs V. Which deprivation? A comparison of selected deprivation indexes. J Public Health Med. 1991;13:318–326. [PubMed] [Google Scholar]
- 24.Espelt A, Borrell C, Palencia L, et al. Socioeconomic inequalities in the incidence and prevalence of type 2 diabetes mellitus in Europe. Gac Sanit. 2013;27:494–501. doi: 10.1016/j.gaceta.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Toulis KA, Hanif W, Saravanan P, et al. All-cause mortality in patients with diabetes under glucagon-like peptide-1 agonists: a population-based, open cohort study. Diabetes Metab. 2017;43:211–216. doi: 10.1016/j.diabet.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Toulis KA, Nirantharakumar K, Ryan R, Marshall T, Hemming K. Bisphosphonates and glucose homeostasis: a population-based, retrospective cohort study. J Clin Endocrinol Metab. 2015;100:1933–1940. doi: 10.1210/jc.2014-3481. [DOI] [PubMed] [Google Scholar]
- 27.Toulis KA, Willis BH, Marshall T, et al. All-cause mortality in patients with diabetes under treatment with dapagliflozin: a population-based, open-cohort study in the Health Improvement Network Database. J Clin Endocrinol Metab. 2017;102:1719–1725. doi: 10.1210/jc.2016-3446. [DOI] [PubMed] [Google Scholar]
- 28.McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036–1050. doi: 10.1111/dme.12676. [DOI] [PubMed] [Google Scholar]
- 29.Greenfield JR, Samaras K, Chisholm DJ. Insulin resistance, intra-abdominal fat, cardiovascular risk factors, and androgens in healthy young women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2002;87:1036–1040. doi: 10.1210/jcem.87.3.8324. [DOI] [PubMed] [Google Scholar]
- 30.d’Emden H, Holden L, McDermott B, et al. Disturbed eating behaviours and thoughts in Australian adolescents with type 1 diabetes. J Paediatr Child Health. 2013;49:E317–E323. doi: 10.1111/jpc.12014. [DOI] [PubMed] [Google Scholar]
- 31.Hao W, Gitelman S, DiMeglio LA, Boulware D, Greenbaum CJ, Type 1 Diabetes TrialNet Study G Fall in C-peptide during first 4 years from diagnosis of type 1 diabetes: variable relation to age, HbA1c, and insulin dose. Diabetes Care. 2016;39:1664–1670. doi: 10.2337/dc16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckingham B, Cheng P, Beck RW, et al. CGM-measured glucose values have a strong correlation with C-peptide, HbA1c and IDAAC, but do poorly in predicting C-peptide levels in the two years following onset of diabetes. Diabetologia. 2015;58:1167–1174. doi: 10.1007/s00125-015-3559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks AM, Oram R, Home P, Steen N, Shaw JA. Demonstration of an intrinsic relationship between endogenous C-peptide concentration and determinants of glycemic control in type 1 diabetes following islet transplantation. Diabetes Care. 2015;38:105–112. doi: 10.2337/dc14-1656. [DOI] [PubMed] [Google Scholar]
- 34.Turner J, Kelly B. Emotional dimensions of chronic disease. West J Med. 2000;172:124–128. doi: 10.1136/ewjm.172.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frey MA, Templin T, Ellis D, Gutai J, Podolski CL. Predicting metabolic control in the first 5 yr after diagnosis for youths with type 1 diabetes: the role of ethnicity and family structure. Pediatr Diabetes. 2007;8:220–227. doi: 10.1111/j.1399-5448.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 36.Dovc K, Telic SS, Lusa L, et al. Improved metabolic control in pediatric patients with type 1 diabetes: a nationwide prospective 12-year time trends analysis. Diabetes Technol Ther. 2014;16:33–40. doi: 10.1089/dia.2013.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilliard ME, Wu YP, Rausch J, Dolan LM, Hood KK. Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. J Adolesc Health. 2013;52:28–34. doi: 10.1016/j.jadohealth.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2014;2:133–140. doi: 10.1016/S2213-8587(13)70144-X. [DOI] [PubMed] [Google Scholar]
- 39.Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J (2010) Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev: CD005103. 10.1002/14651858.CD005103.pub2 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 243 kb)
Data Availability Statement
THIN data governance does not allow us to share individual patient data and therefore where possible metadata is presented. Researchers may apply for individual patient data access at https://www.iqvia.com/contact.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.