Abstract
Connectivity features based on resting-state (RS) functional magnetic resonance imaging (fMRI) demonstrate great promise as biomarkers to guide diagnosis and treatment in major depressive disorder (MDD). However, there is a pressing need for valid, reliable biomarkers closer to the bedside for clinical research and practice. This study directly compared RS-fMRI connectivity features with transcranial magnetic stimulation (TMS) neurophysiological measures, long interval cortical inhibition (LICI) and cortical silent period (CSP), in female adolescents with MDD. LICI-200 showed the most significant associations with RS functional connectivity features, demonstrating its potential to evaluate the neurochemical underpinnings of network features in MDD.
Keywords: TMS, fMRI, connectivity, resting state, depression
1. Introduction
Major depressive disorder (MDD) is diagnosed clinically using functional and mood- related impairments that are suggestive of but not specific to underlying neuropathology. There is significant variation in the etiology of MDD and in the response to treatment between patients (Kupfer et al., 2012). Rapidly implementable biomarkers are needed to characterize patient phenotypes and inform treatment plans. This is especially true for adolescents, in whom disease processes and neuromodulatory treatments interact with the developing brain. Patients often undergo multiple trials of antidepressants and psychotherapy. Recurrence rates of depression in adolescents may be as high as 70% with standard treatments. At least 30% of adolescents with depression attempt suicide during the course of their illness (Cousins and Goodyer, 2015). Currently, investigational neurostimulatory approaches are only considered for adolescents after medical management has failed (Lee et al., 2017; McIntyre et al., 2014).
Distinct biotypes of MDD have been identified in adults using connectivity features based on resting-state (RS) functional magnetic resonance imaging (fMRI) (Drysdale et al., 2017), with clusters of functional connectivity features correlating with different clinical phenotypes. RS- fMRI connectivity studies have also elucidated mechanisms involved in neuromodulatory interventions for MDD, such as repetitive transcranial magnetic stimulation (rTMS). Intrinsic network connectivity is altered by rTMS (Liston et al., 2014), which may be related to its antidepressant effect (Fox et al., 2012). RS-fMRI connectivity markers also predict responsiveness to rTMS treatment (Avissar et al., 2017; Drysdale et al., 2017). Effective neurostimulatory treatment requires targeting brain regions or networks, which may be specific to a patient’s biotype, necessitating the use of biomarkers to guide treatment. Presently, the expense and data processing requirements of fMRI studies create practical barriers for the clinical implementation of fMRI-based biomarkers.
In addition to its therapeutic applications, TMS is an inexpensive, noninvasive means of assessing cortical physiology that has been used to explore the neurochemical underpinnings of MDD. TMS indirectly measures GABAB receptor tone via stimulation paradigms such as long interval cortical inhibition (LICI) and cortical silent period (CSP), which elicit distinct motor responses quantified with electromyography (EMG) (Florian et al., 2008). GABAB deficits in MDD (Bajbouj et al., 2006; Levinson et al., 2010) may explain the efficacy of serotonergic antidepressants such as fluoxetine, which may potentiate GABAergic neurotransmission. In adolescents with MDD, pre-treatment LICI deficits may be associated with treatment resistance and suicidal behaviors (Croarkin et al., 2014; Lewis et al., 2018), warranting further evaluation of LICI as a biomarker in MDD.
This study examined the feasibility of a multi-modal approach to biomarkers in adolescent MDD by examining associations between TMS-based measurements of cortical inhibition and RS-fMRI connectivity features in the same participants. This approach may highlight common neurophysiological substrates between TMS and RS-fMRI and contribute to the development of robust biomarkers in psychiatric diseases.
2. Methods
2.1. Participants
Five individuals (mean age 15.5 ± 2.1 years, 5 females) were enrolled in this pilot study. All participants were recruited from an ongoing study of adolescent depression with TMS biomarkers. All participants were diagnosed with MDD based on a structured diagnostic interview, the Schedule for Affective Disorders and Schizophrenia for School Aged Children (Kaufman et al., 1997), with a board certified child and adolescent psychiatrist (PEC). Three participants were taking fluoxetine and one participant was taking escitalopram at the time of the study. All study procedures were approved by the Mayo Clinic Institutional Review Board. Participants underwent TMS testing (LICI and CSP) and then an MRI session on the same day. Participation was limited to participants judged able to provide informed assent who had parents with the capacity to provide informed consent. Co-Occurring eating disorders, bipolar disorders, eating disorders and obsessive compulsive disorder were exclusionary. Participants were screened for safety to undergo both TMS and MRI. A personal or family history of seizures was exclusionary. All participants and parents provided written informed assent and consent prior to participation in the original and this pilot study.
2.2. TMS
Two Magstim 200 stimulators connected by a BiStim module (Magstim Co. Ltd., Whitland, Wales, UK) were used to generate magnetic pulses, delivered by a figure-of-eight electromagnetic coil (70 mm diameter, each loop). Participants sat comfortably during the sessions and wore earplugs. Participants were instructed to maintain muscle relaxation, which was monitored with audio feedback. Motor evoked potential (MEP) data were collected with surface EMG electrodes at the contralateral (right) abductor pollicis brevis (APB) muscle. The TMS coil was placed tangentially to each participant’s head at a 45 degree angle over the left primary motor cortex, “motor hotspot.” Procedures to determine coil position and motor thresholds were followed as previously published (Daskalakis et al., 2002). Cortical inhibition was assessed using CSP and LICI paradigms.
2.2.1. Cortical Silent Period
To measure the CSP, a single TMS pulse was delivered to the left primary motor cortex at 140% motor threshold while the participant actively contracted the right APB at 20% of maximum force (measured by dynamometer). The pulse induced a temporary interruption of motor activity on EMG, the duration of which was measured as the time from the start of the pulse to the spontaneous resumption of activity. Ten CSP trials were performed, and CSP durations were averaged for each participant.
2.2.2. Long Interval Cortical Inhibition
To measure LICI, a suprathreshold conditioning stimulus (calibrated to produce a 1-mV peak-to-peak MEP amplitude) was delivered to the left primary motor cortex before an identical suprathreshold test stimulus was delivered, with interstimulus intervals (ISIs) of 100 ms, 150 ms, and 200 ms. Paired-pulse trials were repeated ten times for each ISI and mean MEP amplitudes for the conditioned and test stimuli were calculated. LICI values were calculated as the ratio of the mean conditioned MEP amplitude (resulting from the test stimulus) to the mean unconditioned MEP amplitude.
2.3. MRI
T1 anatomic (MPRAGE, TR/TE=7.37/3.04ms, inversion time=900ms, flip angle=8°, voxel size=1×l×1.2mm3, axial direction) and RS-fMRI (GE EPI, 3.3mm isocubic voxel size, 48 slices, TR/TE=2900/30ms, flip angle=90°, axial directions, total acquisition time=449.5 s, total number of time points=155TRs) data were collected using a GE 3 Tesla scanner equipped with 8-channel head-coil array. During scanning, participants were instructed to keep focusing on a gray cross in the center of black screen (Biswal et al., 1995), and respirations and cardiac pulses were simultaneously recorded. All imaging data were aligned with the N27 brain template, and RS-fMRI data were preprocessed and denoised with anatomy-based image correction (ANATICOR) and physiologic estimation by temporal independent component analysis (PESTICA) (Beall, 2010; Beall and Lowe, 2007; Jo et al., 2013; Jo et al., 2010). At the motion correction stage, we performed motion censoring for head motion artifacts using estimated translational and rotational displacement with respect to the x, y, and z axes. The threshold set for motion censoring was an estimated displacement of less than 0.3 mm for the Euclidean L2 norm of motion displacement between time series volumes and less than 0.5 mm translations in any of the three directions or less than 0.58° of maximum rotations around any of the axes. One participant had 6% of time points censored by these criteria, and the others did not have time-point loss in RS-fMRI data (Cha et al., 2017; Jo et al., 2013). For each participant, a connectivity matrix was obtained from full Pearson correlation coefficients between time series separately averaged within FreeSurfer’s regions of interest (ROIs; 167 regions) (Destrieux et al., 2010).
2.4. Group Statistics
We calculated Pearson correlation coefficients matrices between RS-fMRI connectivity matrices and TMS-related measures (LICIs and CSP values) for the entire participant group. We applied Bonferroni-type multiple comparison correction to compensate for 4 observations (CSP, LICI 100, 150, and 200) by two different thresholds; (i) uncorrected p0.001 / 4 = 0.00025 (Fig 1-A), and (ii) FDR q<0.05 / 4 = 0.0125 (Fig 1-B). In addition, we also tested the significance of the results by the network-based statistics with a threshold level of family-wise-error-corrected p<0.01 (Zalesky et al., 2010).
Figure 1: Significant correlations and anti-correlations between functional connectivity features and measures of cortical inhibition (LICIs and CSP).
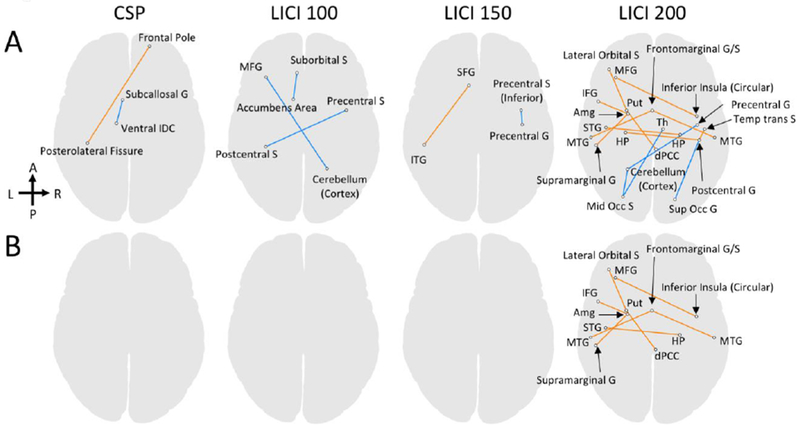
Panel A shows the regions-of-interest (ROI) pairs for which the resting-state (RS) functional connectivity significantly correlated with CSP, LICI-100, LICI-150, and LICI-200 scores at a thresholded level uncorrected p<0.001/4=0.00025 (FDR q<0.015, |t|>20.8). Significant correlations and anti-correlations are colored in orange and in blue, respectively. Panel B displays the ROI pairs for which the RS functional connectivity significantly correlated with CSP, LICI-100, LICI-150, and LICI-200 scores at a thresholded level FDR q<0.05/4=0.0125 (uncorrected p<0.00008, |t|>23.9). G and S in the ROI labels denote gyrus and sulcus, respectively. Abbreviations: Amg=Amygdala, Put=Putamen, HP=hippocampus, Th=thalamus, IDC=inferior dicencephalon, dPCC=dorsal posterior cingulate cortex, SFG=superior frontal gyrus, MFG=middle frontal gyrus, IFG=inferior frontal gyrus, STG=superior temporal gyrus, MTG=middle temporal gyrus, ITG=inferior temporal gyrus, Temp tans S=temporal transverse sulcus, Mid Occ S=middle occipital sulcus, Sup Occ G=superior occipital gyrus. A complete list of the ROIs used in the connectivity analysis is available in the supplementary materials.
3. Results
Means (standard deviations) of cortical inhibition measures in this sample were consistent with prior reports of cortical inhibition in adolescents with MDD (Croarkin et al., 2014): LICI-100 = 0.142 (0.086), LICI-150 = 0.238 (0.141), LICI-200 = 0.406 (0.42); CSP = 0.158 (0.022). Significant correlations and anti-correlations between connectivity features and LICI-100, LICI-150, LICI-200, and CSP are shown in figure 1. CSP demonstrated the fewest and LICI-200 demonstrated the most correlations with connectivity features. In the network-based statistics, we did not find any significant correlation between RS functional connectivity and TMS-related measures, likely due to the small sample size.
4. Discussion
To our knowledge this is the first attempt to examine concurrent RS-fMRI and TMS measures in adolescents with MDD. Our preliminary data confirm the feasibility of an approach to biomarkers in adolescent MDD that directly compares finite measurements of cortical inhibition with connectivity features in the same participants. TMS-based measurements of cortical inhibition, such as LICI, may contribute to connectivity-guided targeting strategies for rTMS treatment of MDD (Fox et al., 2012), as well as similar targeting approaches using task-based fMRI (Luber et al., 2017). In our study, LICI-200 showed the most correlations with functional connectivity features and consequently the greatest potential as a cross-modality biomarker of cortical inhibitory network dysfunction in MDD. Prior work suggests that impaired LICI measures may be associated with treatment resistance and suicidal behaviors in adolescents with depression (Croarkin et al., 2014; Lewis et al., 2018). Drawing definitive conclusions regarding connectivity patterns in this small sample is problematic. Larger, hypothesis-driven work could provide insights into the neural circuitry of treatment resistance in adolescent depression, inform the understanding of the neurochemical basis of functional connectivity measures, and hone bedside measures of cortical inhibition for clinical practice.
Limitations of this study include the small sample size, the lack of a control group, participants’ medication use, and use of motor cortical excitability measures. These results are preliminary and the small sample size leaves many unanswered questions regarding the reliability and validity of the TMS and rsfMRI measurements described in this report. Further experiments in larger samples with healthy individuals will be essential to develop and validate this approach. Further work could determine if LICI-200 generally has more associated connectivity features, or if the association between LICI-200 and connectivity features is specific to MDD in adolescents. Prior work has demonstrated that antidepressant medications can increase cortical inhibition (Manganotti et al., 2001; Minelli et al., 2010; Robol et al., 2004), and thus it is possible that antidepressant use contributed to the levels of cortical inhibition measured here. Arguably, findings with motor cortical measures such as CSP and LICI may not directly generalize to the pathophysiology of MDD. However, similar measures such as the motor threshold are routinely used to dose clinical rTMS (McClintock et al, 2018). Hence, a greater neurobiological understanding of motor cortical measures is important as such measures (CSP and LICI) could be more rapidly integrated into clinical practice as compared to other modalities.
It remains unclear whether LICI has any direct association with specific connectivity features, networks, or with network components that are local to the stimulation site or to relevant motor circuits. Other TMS paradigms have been used to query functional connectivity, often in comparison with network changes identified by fMRI (Fox et al., 2012). Such techniques may further determine how LICI is associated with connectivity changes in MDD. TMS-based measurements of GABAergic neurotransmission may correlate with discrete RS functional connectivity features in adolescents with MDD and provide an efficient and accessible proxy for RS studies, with utility in future clinical research and practice. With enhanced understanding and validation, motor cortical measures such as LICI-200 may one day assist with treatment selection and monitoring.
Supplementary Material
Highlight:
Resting state connectivity features demonstrate promise as biomarkers in psychiatry
TMS neurophysiology measures are inexpensive biomarkers of cortical physiology
Few studies have examined correlates of fMRI and TMS measures in adolescents
LICI-200 showed the most associations with RS functional connectivity features
Acknowledgements:
This research was supported by grants from the Brain and Behavior Research Foundation (Young Investigator Award 20883), the Mayo Clinic Foundation (Departmental Small Grant Award), and the National Institute of Mental Health (K23 MH100266 and R01MH113700). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
GAW has received research support from Medtronic, Inc., Neuralynx, Inc., and Neuropace, Inc. PEC has received research grant support from Pfizer, Inc., has received equipment support from Neuronetics, Inc.; and receives supplies and genotyping services from Assurex Health, Inc. for an investigator-initiated study. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. and a site primary investigator for a study funded by NeoSync, Inc. CPL is a site investigator on multicenter studies funded by Neuronetics, Inc. and NeoSync, Inc. IB, JS, JDP, and HJJ have no financial disclosures.
Presented at the 2017 Annual Meeting of the American College of Neuropsychopharmacology, Palm Springs, California, December 4, 2017.
References
- Avissar M, Powell F, Ilieva I, Respino M, Gunning FM, Liston C, et al. , 2017. Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimul. 10, 919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P, 2006. Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol. Psychiatry 59, 395–400. [DOI] [PubMed] [Google Scholar]
- Beall EB, Lowe MJ, 2007. Isolating physiologic noise sources with independently determined spatial measures. Neuroimage 37, 1286–1300. [DOI] [PubMed] [Google Scholar]
- Beall EB, 2010. Adaptive cyclic physiologic noise modeling and correction in functional MRI. J. Neurosci. Methods 187, 216–228. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Cha J, Jo HJ, Gibson WS, Lee JM, 2017. Functional organization of the human posterior cingulate cortex, revealed by multiple connectivity-based parcellation methods. Hum. Brain. Mapp 38, 2808–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins L, Goodyer IM, 2015. Antidepressants and the adolescent brain. J Psychopharmacol. 29, 545–555. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Nakonezny PA, Husain MM, Port JD, Melton T, Kennard BD, et al. , 2014. Evidence for pretreatment LICI deficits among depressed children and adolescents with nonresponse to fluoxetine. Brain Stimul. 7, 243–251. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R, 2002. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 543, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E, 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. , 2017. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med 23, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian J, Müller-Dahlhaus M, Liu Y, Ziemann U Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. 2008. J. Physiol 586, 495–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, Pascual-Leone A, 2012. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, et al. , 2013. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. J. Appl. Math. 2013 https://doi:10.1155/2013/935154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW, 2010. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 52, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. ,1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child. Adolesc. Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML, 2012. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet 379, 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Lewis CP, Daskalakis ZJ, Croarkin PE, 2017. Transcranial direct current stimulation: Considerations for research in adolescent depression. Front.Psychiatry 8 https//doi:10.3389/fpsyt.2017.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ, 2010. Evidence of cortical inhibitory deficits in major depressive disorder. Biol. Psychiatry 67, 458–464. [DOI] [PubMed] [Google Scholar]
- Lewis CP, Nakonezny PA, Blacker CJ, Vande Voort JL, Port JD, Worrell GA, et al. Cortical inhibitory markers of lifetime suicidal behavior in depressed adolescents. 2018. Neuropsychopharmacology. https//doi:10.1038/s41386-018-0040-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. , 2014. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry 76, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber BM, Davis S, Bernhardt E, Neacsiu A, Kwapil L, Lisanby SH, et al. , 2017. Using neuroimaging to individualize TMS treatment for depression: Toward a new paradigm for imaging-guided intervention. Neuroimage 148, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Bortolomasi M, Zanette G, Pawelzik T, Giacopuzzi M, Fiaschi A, 2001. Intravenous clomipramine decreases excitability of human motor cortex. A study with paired magnetic stimulation. J. Neurol. Sci. 184, 27–32. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, 2018. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J. Clin. Psychiatry, 79. doi: 10.4088/JCP.16cs10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, et al. , 2014. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 156, 1–7. [DOI] [PubMed] [Google Scholar]
- Minelli A, Bortolomasi M, Scassellati C, Salvoro B, Avesani M, Manganotti P, 2010. Effects of intravenous antidepressant drugs on the excitability of human motor cortex: a study with paired magnetic stimulation on depressed patients. Brain Stimul. 3, 15–21. [DOI] [PubMed] [Google Scholar]
- Florian J, Müller-Dahlhaus M, Liu Y, Ziemann U„ 2008. Inhibitory circuits and the nature of their interactions in the human motor cortex - a pharmacological TMS study. J.Physiol. 586, 495–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robol E, Fiaschi A, Manganotti P, 2004. Effects of citalopram on the excitability of the human motor cortex: a paired magnetic stimulation study. J. Neurol. Sci 221, 41–46. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET, 2010. Network-based statistic: identifying differences in brain networks. Neuroimage 53, 1197–1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


