Abstract
An important question remains as to how the brain differentially processes first (pricking) pain mediated by Aδ-nociceptors versus second (burning) pain mediated by C-nociceptors. In the present cross-over randomized, within-subjects controlled study, brain activity patterns were examined with event-related fMRI while pricking and burning pain were selectively evoked using a diode laser. Stimuli evoking equivalent pain intensities were delivered to the dorsum of the left foot. Different laser parameters were used to elicit pricking (60 ms pulse duration) and burning (2.0 s pulse duration) pain. Whole brain group analysis showed that several brain areas were commonly activated by pricking and burning pain including bilateral thalamus, bilateral anterior insula, bilateral posterior parietal lobule, contralateral dorsolateral prefrontal cortex, ipsilateral cerebellum, and mid anterior cingulate cortex. These findings show that pricking and burning pain were associated with activity in many of the same nociceptive processing brain regions. This may be expected given that Aδ and C nociceptive signals converge to a great extent at the level of the dorsal horn. Other brain regions showed differential processing. Stronger activation in the pricking pain condition was found in the ipsilateral hippocampus, bilateral parahippocampal gyrus, bilateral fusiform gyrus, contralateral cerebellum and contralateral cuneus/parieto-occipital sulcus. Stronger activation in the burning pain condition was found in the ipsilateral dorsolateral prefrontal cortex. These differential activation patterns suggest preferential importance of Aδ versus C-fiber signals for these specific brain regions.
Keywords: burning pain, event-related fMRI, diode laser, pricking pain
Introduction
Different types of nociceptive afferents evoke qualitatively different sensations. First pain is described as ‘sharp’ or ‘pricking’ and is mediated by myelinated Aδ-fibers. Second pain is described as ‘dull’ or ‘burning’ and is mediated by the slower unmyelinated C-fibers. It remains largely unclear how the signals conveyed by these fibers are differentially processed in the human brain. Knowledge about the cortical representation of signals originating from C- and Aδ-fiber nociceptors may provide new insights into the biological significance of these distinct pain pathways. Recently, reports emerged on differential involvement of nociceptive fibers in pain syndromes [27,44], suggesting that insights on differential nociceptor processing may be helpful in establishing better diagnoses. The ability to reliably differentiate between pain-mediating fibers may also provide unique utility in predicting the efficacy of treatments.
Few studies modeled brain responses associated with first and second pain. In a laser evoked potential (LEP) study, brain activation in response to second pain was found bilaterally in the secondary somatosensory cortex (SII) and the anterior cingulate cortex (ACC) [43], while a magnetoencephalography (MEG) study found second pain-related activation in the contralateral primary somatosensory cortex (SI) and bilateral SII [33]. However, both studies did not compare findings to the cortical processing of first pain. Studies directly comparing brain activation patterns of first and second pain consistently found largely overlapping activation patterns for both fibers, but differential processing was also found. In a MEG study, common activation was found in SII, while differential activation for first pain was found in SI and for second pain in ACC [51]. In another MEG study, common activation was found in bilateral SII, contralateral posterior parietal cortex, and in a few subjects in cingulate, insula, prefrontal, and inferior temporoparietal regions, but no differential activation was found [24]. In a recent functional magnetic resonance imaging (fMRI) study, common activation was found in bilateral thalamus, SII, posterior ACC, and ipsilateral middle insula, while significantly stronger activation for second pain was found in ACC, pre-SMA, and bilateral anterior insula [54]. Although these studies yielded valuable information, some issues arise. C-fiber stimulation can also reflect innocuous warmth processing, and pain intensity scores were frequently not reported. Further, studies did not equalize perceived pain intensity between first and second pain, which could confound perceptual quality and intensity of pain.
In the present study, event-related fMRI was used to selectively evaluate brain activity associated with pricking and burning pain induced by a diode laser. Perceived pain intensity was equalized between first and second pain. We hypothesized that the spatial representation of nociceptive signals conducted by the two fiber types would be similar to a large extent. However, differences in spatial processing were also expected, because of distinct qualitative differences in pain sensations. In addition we explored the possibility that input from the fibers could be differentially processed in brain regions not necessarily related to the perception of pain.
Materials and Methods
Subjects
Ten healthy volunteers (5 females and 5 males; 1 left-handed) completed this study. Of these ten participants, one subject reported a significantly higher pain intensity rating (VAS rating of 76 on a 0–100 scale) during the burning pain condition in the fMRI session than the other subjects. Statistical analysis identified the pain rating of this subject as a significant outlier; therefore this subject was excluded from further analysis. Four additional subjects were screened for this study but were excluded from full participation based on the following criteria: dual sensation of pricking and burning pain upon stimulation (n=1), inconsistent pain ratings (n=1), or insensitivity to laser stimuli within the safety margins (n=2). Mean age of the remaining nine subjects who completed the study was 29.7 ± 8.7 SD (standard deviation), ranging from 22 to 49 years. Participants who completed the study had at least 4 years of college education, none of them suffered from a serious medical of psychiatric illness, they were good sleepers (mean 7.1 ± 1.1 SD hours a night), all except one subject didn’t smoke, they used small amounts of caffeinated drinks (mean 1.3 ± 0.8 SD per day), used alcohol moderately (mean 2.1 ± 2.3 SD glasses a week), and exercised light to moderately. One female was in her early follicular phase of her menstrual cycle, two females were in their mid-luteal phases, and two women were in their premenstrual phases when tested. The University of Maryland Institutional Review Board for the Protection of Human Subjects approved the procedures and protocol for this study and the participants’ informed consent was obtained in accordance with the Declaration of Helsinki.
Stimulation paradigms
A diode laser (LASS-10M, Lasmed LLC, CA, USA; patented in 2004) with power of 20 W, wavelength of 980 nm, and an adjustable spot diameter between 1 and 5 mm diameters was used to apply the painful stimuli to the skin of the dorsum of the left foot. Selective activation of Aδ-fibers was accomplished by using a diode laser with high rate heating, high energy, brief pulses, and small spot sizes. Selective activation of C-fibers was accomplished by low rate heating with long pulses, low energy and larger spot sizes [62,64,66,68]. The diode laser has been previously used to selectively activate transient receptor potential vanilloid (TRPV1 and TRPV2) positive cells [32], to selectively activate C- and A-δ fibers in rats [62], to elicit a heat-activated inward current Iheat in dorsal root ganglion neurons of rats in vitro, and to elicit laser-evoked potentials (LEPs) associated with activation of Aδ- and C-nociceptors in humans [28,67]. The diode laser distributes energy principally at the level of cutaneous nociceptive endings (20–570 μm) [59], due to less absorption (~0.5 cm’1 for 970 nm) thereby avoiding superficial skin lesions [36,45]. Accordingly, it allows for simultaneous and homogeneous heating at skin depth 20–570 μm [40,67]. Other commonly used lasers such as the CO2 or Thulium-YAG laser show higher absorption (−63 cm’1 for 2010 nm for Thulium and −300 cm’1 for 10.2 μm for CO2 lasers) and more superficial distribution of energy [57]. As they require conduction through tissue to reach nociceptive endings, risk of burns is higher than with the diode laser. Further, the delay time between superficial skin heating and heating at 450 μm skin depth could be up to several seconds [60]. Different parameters of laser stimulation were used to elicit pricking and burning pain sensations. For pricking pain, short pulses of 60 ms with a spot size of 1 mm in diameter were applied and the laser beam was positioned 5 cm from the skin surface. The mean intensity used to evoke pricking pain was 715 mJ/mm2, ranging from 390 to 868 mJ/mm2. For burning pain, long pulses of 2 seconds duration with a spot size of 5 mm in diameter were applied with the laser beam positioned 15 cm from the skin surface. The mean intensity used to evoke burning pain was 468 mJ/mm2, ranging from 428 to 499 mJ/mm2. An aiming beam of 670 nm wavelength was used to identify the stimulated area. The differential activation of the burning and pricking pain was confirmed by the subjects’ verbal reports. For this purpose, subjects were asked to consider several mechanical- and temperature-related descriptors, partly adapted from the McGill pain questionnaire, and to identify which descriptors applied to their sensation. Reports of “pricking” and “sharp” were interpreted as associated with Aδ-fiber stimulation while reports of “warm/hot” and “burning” were considered to be associated with C-fiber stimulation. Intensities for pricking and burning pain were chosen based on subjects’ perceived pain threshold and pain perception. Before MRI scanning, in a separate screening session, sequences of laser stimuli was given which started with low, often undetectable levels, and intensities were increased stepwise until pain of around 20 on a visual analogue scale (VAS) ranging between 0 (no pain) and 100 (maximum tolerable pain) was reported. This relatively low pain intensity was chosen to avoid burns that could potentially occur with more intense stimulation, particularly for less pain sensitive individuals. Then, this stimulus level was repeated and adjusted if necessary until a response near 20 was given reliably for 10 consecutive stimuli of similar intensity. Mean pain intensity ratings for the pricking pain stimuli was 22.4 (±SD=6.7) and mean pain intensity for the burning pain stimuli was 25.4 (±SD=6.6) in the screening session. To avoid sensitization or fatigue of the nociceptors, the stimulation site on the dorsum of the foot was slightly different for the pricking and burning pain stimulation. Also, the temperature of the skin surface can affect the sensitivity to the laser pulse; with lower skin temperatures, higher energy levels need to be used to reach the same level of pain perception. Therefore the temperature of the foot surface was monitored carefully at the start and end of each functional run. The foot was kept warm by means of a reheatable bean sock between sessions.
Experimental design
Subjects participated in two sessions; a screening session and a MRI scanning session one or two days later. In the screening session, subjects were tested for their sensitivity to the experimental procedure. During the MRI scans, laser stimuli were presented in separate blocks of burning or pricking pain stimuli, with 30 stimuli in each block. The order of presentation of pricking and burning pain stimuli in this MRI session was counter-balanced across subjects. An event-related design was used and the inter-stimulus-interval varied between 14 and 18 seconds in a random sequence trial by trail to reduce anticipatory effects and artifacts occurring at fixed frequencies. A stimulus-free baseline period of 30 seconds was given before and after the presentation of each block of stimuli. After this last baseline period, subjects were asked to rate the average pain intensity across all stimuli in the block on a computerized VAS (DAPSYS; Brian Turnquist, Johns Hopkins University, http://www.dapsys.net) using an MR-compatible trackball (Fellowes, www.fellowes.com), which controls a cursor moving along a vertical VAS. The extremes of the VAS were labeled “no sensation” and “most intense pain imaginable,” with a marker for “just painful” located at the lower quarter of the scale. Subjects were instructed to use the range from “no sensation” to “just painful” to rate non-painful sensations and to use the range from “just painful” to “most intense pain imaginable” to rate painful sensations. Ratings were stored as numbers from 0 to 100 with 25 corresponding to “just painful.” This scale was chosen to give the subject the opportunity to rate intensities below pain thresholds in case the average stimulus intensity was not painful. Subjects practiced with this computerized VAS scale on the screening session. Scores on this 25–100 pain intensity scale were converted to a 0–100 pain intensity scale to allow comparison with rating data collected during the screening session. Subjects were also asked to rate average pain unpleasantness using the same trackball on a 0 (not unpleasant at all) to 100 (most unpleasant imaginable) scale. Lastly, they were asked to choose descriptors representing their laser-evoked sensations from a list, partly adapted from the McGill pain questionnaire.
For stimulus presentation, the laser beam was mounted in a MRI compatible micromanipulator stand which could be adjusted in vertical and horizontal directions. The stand was fixed to a movable table to allow easy repositioning after application of each stimulus. One investigator stayed in the scanner room during the MRI sessions to change the position of the laser beam after each stimulus. Before each stimulus was applied, the distance of the laser collimator to the skin was confirmed by measurements with a ruler without touching the skin.
Image acquisition
Functional MRI scans were acquired using echo planar imaging (EPI) on a 3.0 T Tim Trio scanner (Siemens Medical Solutions, Malvern, PA) with an 8-channel head coil with parallel imaging capability. A gradient echo single shot EPI sequence was used to provide 3.594 × 3.594-mm acquired resolution over a 23-cm field of view (FOV). T2* weighting from this sequence was accomplished with a gradient echo time (TE) of 30 ms and flip angle 90°. The repetition time (TR) was 2,000 ms, which allowed the whole brain to be covered using 24 slices, with a slice thickness of 6 mm and no gaps between slices. An interleaved slice sequence was used. A 3D T1 MP-RAGE volumetric scan was acquired for anatomical reference of the functional scans with parameters: 3.44 ms TE, 2250 ms TR, 900 ms TI, flip angle 9°, 96 slices, slice thickness of 1.5 mm and a 0.9 × 0.9 mm in-plane resolution over a 23 cm FOV.
Image processing and analysis
Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov) was used for image processing and analysis. The first four volumes were removed from the functional scan series to allow for signal equilibration. Remaining volumes were corrected for slice timing differences, and spatially aligned to the first volume for motion correction. Spiking artifacts were reduced (Despike routine in AFNI) and the time series were temporally smoothed using a moving three-point weighted average. After co-registration to the T1-weigthed structural volume, the functional volumes were normalized in stereotactic space (Talairach template) and spatially smoothed using a 5-mm full-width, half-maximum Gaussian blur. Linear, second-order, and third-order trends within the time series were removed, and voxelwise normalization was achieved by dividing the signal intensity at each time point by the voxels mean intensity.
A general linear model (GLM) was used to model temporally discrete responses to pricking or burning pain stimuli. Responses were modeled with a voxelwise regression of fMRI signal time courses assuming a standard gamma-response function. Based on the timing of the stimulus delivery it was predicted that the peak of burning pain condition would be delayed compared to the pricking pain condition. This was indeed confirmed when GLM regressions were performed at multiple lag times of event-related average waveforms from significant clusters. The peak amplitude for the burning pain condition occurred approximately 2 seconds later than the peak for the pricking pain condition. Further, the waveform for the pricking pain condition was best matched with a 2 second delay on the gamma function. Based on this observation, a lag of 2 seconds was used for data derived from the pricking pain condition and a lag of 4 seconds was used for data derived from the burning pain condition.
Individual activation maps were created to determine individual differences in activation, based on a region of interest (ROI) approach. ROIs included the thalamus, the foot representation of the SI, SII, the posterior insula, the anterior insula, rostral ACC, mid-ACC, the supplementary motor area (SMA), and the dorsolateral prefrontal cortex (DLPFC). Anatomic criteria for these a priori ROIs have been given elsewhere [38]. Significant voxel clusters were identified on group activation maps for pricking and burning pain separately.
Additionally, group analyses were performed on whole brain activation. A group overlap map was created of voxel clusters that were commonly activated in both conditions. Further, group contrast maps were calculated of activation that differed significantly between the pricking and burning pain conditions. Functionally activated regions were identified based on anatomical locations with the underlying structural images. The data were corrected for multiple comparisons by using minimum cluster size thresholds as determined by the AFNI routine AlphaSim. The minimum cluster size threshold for the ROI activation analyses was 4 voxels in original space which corresponds to ~310 mm3 with p< 0.01. The minimum cluster size threshold in the group overlap analysis was 4 voxels in original space which corresponds to ~310 mm3 at an overall p< 0.01, with a minimum individual voxel threshold of p< 0.01 in each region. In the group contrast analysis, the minimum cluster size was 6 voxels in original space which corresponds to ~465 mm3 at an overall p< 0.05, with a minimum individual voxel threshold of p< 0.05 in each region.
Results
Behavioral data
Stimulus intensities for the pricking and burning pain conditions were determined on an individual basis in a screening session preceding the MRI session. Subjects were also asked to rate their overall pain intensity and pain unpleasantness after each functional scan in the MRI session. No significant differences in pain intensity or unpleasantness ratings were found between the screening and the MRI sessions, or between the burning and pricking pain conditions within each session (Figure 1). Therefore, the stimuli felt equally painful in both sessions, and, importantly, pain intensities were perceptually equalized among subjects for the different pain conditions, despite some variations in foot skin temperatures. The foot dorsum was found to be significantly cooler in the pricking than the burning pain condition (mean=31.3°C, ±SD=0.90 for pricking pain and mean=32.3°C, ±SD=0.52 for burning pain; p<0.036), even though these conditions were presented in a counterbalanced order across subjects. No significant effect of order of stimulation was found, i.e., the foot was not warmer when the pricking pain condition followed the burning pain condition. Further, as can be expected, the temperature of the foot cooled down significantly over the time course of each functional scan (p<0.045), but no significant interaction was found for temperature change between the start and end of each functional run and pain conditions.
Figure 1.

Pain intensity and pain unpleasantness ratings.
The boxplots presented on the left side of the figure represent pain intensity ratings for each session and pain stimulation separately. Additionally, individual scores for each subject are presented as open dots in the box plots. A, mean pain intensity rating over 10 trials as determined in the screening session for the burning and pricking conditions separately. B, average pain intensity rating over a block of 30 trials in the MRI session for the burning and pricking conditions separately. No significant differences in pain intensity ratings were found between conditions of burning and pricking pain in the screening session or the MRI session. The boxplots presented on the right side of the figure represent pain unpleasantness ratings for each session and pain stimulation separately. A, average pain unpleasantness rating over 10 trials in the screening session. B, average unpleasantness rating over a block of 30 trials in the MRI session. Also, no significant differences in pain unpleasantness ratings were found between conditions of burning and pricking pain in the screening session or the MRI session.
Subjects further choose among a set of descriptors to characterize the sensory qualities they felt after each functional run in the MRI session. In general, subjects used the same descriptors in the fMRI session as the training session. For the pricking pain condition all subjects used the descriptors ‘sharp’ and ‘stinging’, while for the burning pain condition all subjects used the descriptors ‘burning’ and/or ‘hot’. However, three subjects also used the descriptors ‘burning’ and/or ‘hot’ in the pricking pain conditions while three (partly different) subjects also used ‘stinging’ and ‘sharp’ in the burning pain condition. Upon query, these sensations were described as distinctly different from those evoked by the other stimulus condition. The ‘burning’ and/or ‘hot’ sensation in the pricking pain condition was described as a ‘sharp hot prick’, not associated with a dull burning pain as described in the burning pain condition. The ‘stinging’ or ‘sharp’ sensation in the burning pain condition was described as a sensation which was clearly associated with the concomitant burning sensation, and different from the pinprick sensation evoked in the other stimulus condition. In the training session it was also verified that subjects did not experience “double sensations” with these stimuli, indicating that perceptions were not based on volleys of A- and C-fiber inputs driven together with the same stimulus.
Imaging results
Single subject analysis
Pricking and burning painful stimuli commonly activated brain regions frequently identified in pain neuroimaging studies, including the thalamus, SI, SII, anterior and posterior insula, DLPFC, cerebellum, rostral and mid ACC, and SMA. The time course of the BOLD signal change in selected classical pain ROIs associated with the presentation of pricking and burning painful stimuli is presented in Figure 2. As evident in this figure, the activation associated with the burning pain stimuli peaked about 2 seconds later than the activation associated with the pricking pain stimuli. This pattern was consistent for all the brain regions responding to both types of stimuli. Several mechanisms could account for this finding. First, this temporal difference could be explained by the fact that the stimulus duration for the burning pain condition was nearly 2 seconds longer than the pricking pain condition, and that afferent activation in the burning pain condition would be maximal near the end of that stimulation period. Activation time of the nociceptors was different due to the stimulation parameters necessary to selectively activate the fibers. Second, the difference in conduction velocities between Aδ- and C-fibers are also likely to contribute to the temporal difference between pricking pain and burning pain related activation. Third, differences in central processing of information conducted by the afferents, such as spinothalamic versus a more propriospinal pathway could potentially result in differences in arrival time at the cortical level. Different lags for the pricking and burning pain conditions were used for further analysis, such that an additional 2 second delay was used for the burning pain compared to the pricking pain condition.
Figure 2.

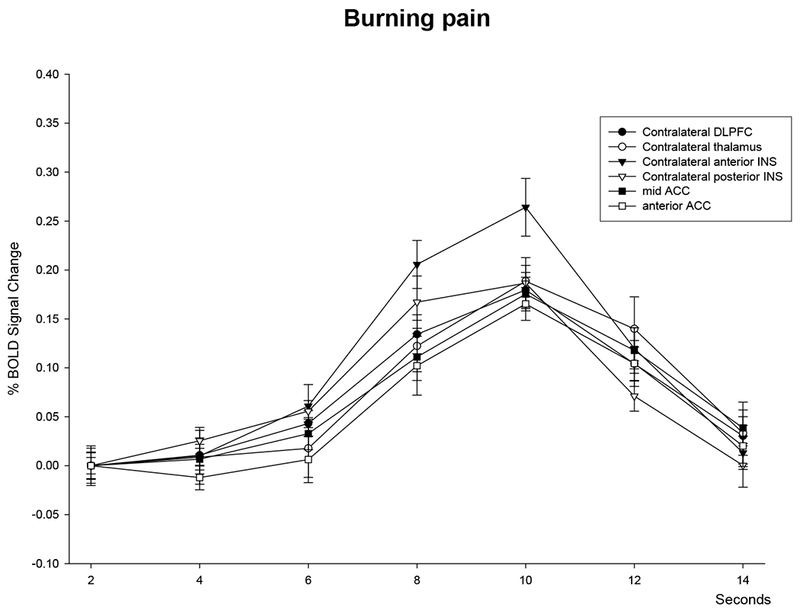
Time course of brain activation following pricking and burning pain.
Time course of BOLD signal change in selected ROIs following laser stimulation in the (A) pricking pain and (B) burning pain conditions. Stimulation onset was at 0 seconds. The right side of the brain is contralateral to stimulation. Error bars represent standard errors.
Table 1 represents the individual variation in activation of several regions previously associated with pain processing and selected for the ROI analysis. Many areas show consistent activation within and between subjects, however some areas, such as SI and the thalamus in the burning pain condition, only show activation in a limited number of subjects. The thalamus only showed activation in about half of the subjects for the burning pain condition, yet in almost all subjects for the pricking pain condition.
Table 1:
Individual subject activation in pain-related regions for each condition.
| Region | Side | Pricking pain (n = 9) | Burning pain (n = 9) |
|---|---|---|---|
| Thalamus | Ipsilateral | 7 | 5 |
| Contralateral | 9 | 4 | |
| SI | Ipsilateral | 3 | 3 |
| Contralateral | 7 | 3 | |
| SII | Ipsilateral | 9 | 8 |
| Contralateral | 8 | 6 | |
| Anterior-INS | Ipsilateral | 8 | 7 |
| Contralateral | 8 | 7 | |
| Posterior-INS | Ipsilateral | 7 | 6 |
| Contralateral | 8 | 6 | |
| DLPFC | Ipsilateral | 7 | 7 |
| Contralateral | 8 | 8 | |
| Rostral-ACC | Midline | 7 | 6 |
| Mid-ACC | Midline | 7 | 6 |
| SMA | Midline | 9 | 8 |
Abbreviations: ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; INS, insula; SMA, supplementary motor cortex
Group analysis
Following individual subject analysis, the activation common to the burning and pricking pain conditions was identified by a group overlap map (Figure 3; overall p<0.01, corrected for cluster size). Significant overlap in activation was found for pricking and burning pain in several brain areas, including the bilateral thalamus, bilateral anterior insula, bilateral posterior parietal lobule, mid-ACC, contralateral dorsolateral prefrontal cortex, and ipsilateral cerebellum (Table 2 and Figure 3).
Figure 3.

Activation and overlap maps.
Activation maps for the pricking (column 1) and burning (column 2) pain conditions separately, and the overlap map of activation between these conditions (column 3; superimposition of the two activation maps). The left side of each image corresponds to the right side of the brain, which is contralateral to stimulation. Functional activation maps are overlaid on a normalized anatomical MRI in Talairach space. The coordinates of slice cuts through the axial and sagittal planes are presented in the images. The color bar represents the extent of activation in z-scores of the pricking and burning pain activation maps. The minimum cluster size threshold in the group overlap analysis was 4 voxels in original space at an overall p< 0.01.
Table 2:
Brain regions that show overlap in activation in pricking and burning pain conditions.
| Pricking pain |
Burning pain |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Side | TAL coordinates | Z-score | TAL coordinates | Z-score | ||||
| x | y | z | x | y | z | ||||
| Thalamus | Ipsilateral | −8 | −1 | 9 | 4.05 | −5 | −17 | 10 | 3.95 |
| Contralateral | 6 | −2 | 7 | 3.62 | 11 | −3 | 13 | 3.80 | |
| Anterior Insula | Ipsilateral | −37 | 14 | 8 | 4.35 | −39 | 11 | 3 | 3.43 |
| Ipsilateral | −37 | −8 | 16 | 3.34 | −30 | −5 | 19 | 3.15 | |
| Contralateral | 39 | 5 | 19 | 4.15 | 43 | 17 | 20 | 3.88 | |
| Anterior Cingulate Cortex | Midline | −2 | 17 | 30 | 3.35 | −1 | 19 | 37 | 3.38 |
| Dorsolateral Prefrontal Cortex | Contralateral | 36 | 3 | 24 | 4.47 | 42 | 12 | 31 | 5.11 |
| Posterior Parietal Lobe | Ipsilateral | −57 | −33 | 25 | 4.22 | −56 | −39 | 26 | 4.07 |
| Contralateral | 50 | −30 | 15 | 3.51 | 47 | −37 | 20 | 3.14 | |
| Cerebellum | Ipsilateral | −36 | −50 | −29 | 3.43 | −38 | −53 | −37 | 3.46 |
Abbreviations: TAL, Talairach coordinates (x,y,z). Note that activation maps for each condition separately show activation in more regions than shown here. Data presented in this table represents overlap in activation between conditions.
Pricking and burning pain also evoked differential responses in selective brain regions. Group contrast maps were used to identify differential activation between pricking and burning pain conditions. Several areas showed stronger activation following pricking pain compared to burning pain (overall p<0.05, corrected for cluster size), including the ipsilateral hippocampus, bilateral parahippocampal gyrus, bilateral fusiform gyrus, contralateral cerebellum, and contralateral cuneus/parieto-occipital sulcus. Only one area showed stronger activation with burning compared to pricking pain: a region in the ipsilateral middle frontal gyrus, comprising part of the DLPFC (Table 3, Figure 4).
Table 3:
Brain regions showing a significant contrast for pricking versus burning pain.
| Region | Side | TAL coordinates | Volume | Z-score | ||
|---|---|---|---|---|---|---|
| x | y | z | (mm3) | |||
| Pricking > burning | ||||||
| Hippocampus | Ipsilateral | −29 | −16 | −10 | 805 | 3.43 |
| Parahippocampal gyrus | Ipsilateral | −11 | −34 | −4 | 465 | 2.52 |
| Contralateral | 20 | −34 | −1 | 476 | 3.14 | |
| Fusiform gyrus | Ipsilateral | −36 | −43 | −9 | 901 | 3.62 |
| Contralateral | 30 | −40 | −12 | 585 | 3.56 | |
| Cerebellum | Contralateral | 8 | −66 | −25 | 1133 | 3.23 |
| Cuneus/parieto-occipital sulcus | Contralateral | 7 | −80 | 17 | 1047 | 2.72 |
| Burning > pricking | ||||||
| Dorsolateral Prefrontal Cortex | Ipsilateral | −34 | 27 | 34 | 511 | −2.61 |
Figure 4.

Contrast maps.
Brain regions differentially activated by the burning and pricking pain stimulation. The left side of each image corresponds to the right side of the brain, which is contralateral to stimulation. Functional run activation is overlaid on a normalized anatomical MRI in Talairach space. The coordinates of slice cuts through the axial and coronal planes are presented in the images. Orange regions represent stronger activation in the pricking versus burning condition, while blue areas represent stronger activation in the burning versus pricking condition. In the group contrast analysis, the minimum cluster size was 6 voxels in original space at an overall p< 0.05.
Discussion
Event-related fMRI was used to evaluate brain activation patterns associated with pricking and burning pain induced by a diode laser. Only few studies directly compared these pain conditions and the present study is innovative regarding perceptually equalizing pain perception between conditions and assuring that the stimulation of C-fibers was painful. Several brain regions were found to be commonly activated, but differential activation was observed for select brain regions.
Individual variation in brain activation
Pain-evoked activation in the brain was found bilaterally in all predefined ROIs. SI activation upon painful laser-evoked stimulation has been reported previously [4,5], although it was least consistently activated among the subjects in the present study. SI activity is often absent in pain imaging studies [14]. It is hypothesized that in the present study, it was hard to identify significant clusters of activation in SI because the foot representation is relatively small. Both the ipsilateral and contralateral thalamus were only activated in about half of the subjects in the burning pain condition, but in most or all subjects in the pricking pain condition. Along with tissue volume considerations described above, differences in activation in the thalamus could result from time-dispersed arrival of input from the fibers evoked by the laser. In the burning pain condition, the stimulus was of longer duration and signals are likely to arrive at the thalamus more dispersed than in the pricking pain condition.
Shared neural representation
Conjunction analysis indicated overlap in activation of pricking and burning pain in several brain areas including thalamus, insula, mid-ACC, cerebellum, contralateral DLPFC, and the posterior parietal lobe. Activation in these brain areas are typically found in pain imaging studies [2,29,46,53,55,61]. The findings of the present study demonstrate that these brain areas are associated with pain processing of both types of nociceptive input. Common activation to pricking and burning pain is expected since Aδ and C nociceptive signals converge to a great extent at the level of the dorsal horn (e.g., [31,52]). Overlap in the cortical representation of these pain conditions may possibly reflect a role of these regions in representing pain salience [23]. Nevertheless, Aδ- and C-fiber mediated nociceptive signals are neurobiologically distinct which implies some level of differential CNS processing.
Differences in brain activation
Group contrast analyses identified brain areas that were differentially activated in the burning versus pricking pain condition, including the hippocampal formation, cerebellum, fusiform gyrus, cuneus/parieto-occipital sulcus, and DLPFC.
While not typically considered a nociceptive processing region, activation in the hippocampal formation has been previously reported in response to painful heat [21,50] and laser stimulation [3]. The hippocampal formation has been traditionally associated with recent memory consolidation [1]. spatial memory [16,30], and fear-initiated avoidance behavior [35]. Studies using specific conditioning paradigms demonstrated that the hippocampus is also involved in learning associations between pain and predictive cues [49,50]. It is perhaps in its role in learned associations that the differential activation between pain pathways in the hippocampus has relevance. Given the need for precise timing of information for appropriately learned associations, it would be reasonable to propose that nociceptive signals from the Aδ-fibers provide the most useful input. A separate cluster of activation was identified in the fusiform gyrus adjacent to the activated region of the parahippocampal gyrus. Given that there is no prior evidence of nociceptive input to the fusiform gyrus, the most conservative interpretation would be that it represents an extension of parahippocampal gyrus activation.
Activity in the cerebellum is frequently found in pain neuroimaging studies. Cerebellar activation is generally considered to be primarily associated with motor responses [12]. Some studies point to a more direct role of the cerebellum in pain processing by showing release of endogenous opioids upon electrical stimulation of the cerebellum in animals [22]. The need for temporally precise information may also be relevant for brain areas involved in initiating, propagating, and executing defensive motor responses to first pain [12,25,47,56,58].
Differential activation was also found for a multi-sensory integration region: the parieto-occipital sulcus. This area is recognized as a site of integration of innocuous somatosensory signals and visual information [15]. A network of motor, pain, and sensory areas including the posterior parietal cortex was previously found to be activated in response to a prickle sensation evoked by noxious cold stimuli [19]. Because the parieto-occipital sulcus is not routinely found activated in pain neuroimaging studies, it remains to be determined whether preferential Aδ nociceptive activation is a consistent property of this region.
Although the contralateral (right) DLPFC was commonly activated in both the pricking and burning pain condition, the ipsilateral (left) DLPFC was found to be significantly more activated in the burning pain condition. Transcranial magnetic stimulation studies demonstrate that prefrontal regions can modulate pain perception. Stimulation of the DLPFC increased pain tolerance upon hand immersion in cold water, however only when stimulated on the right hemisphere [26]. Significant effects of left prefrontal stimulation were found on decreased patient-controlled analgesia use [6]. It has been postulated that pain modulation in these regions occurs by means of attentional control of pain. Lesion studies, neuronal recordings, and neuroimaging findings demonstrate that a network of frontal and parietal structures is involved in attentional control [13,17,34]. In a previous study, the perceptual illusion of ‘paradoxical heat’, isolated from the perception of pain, was associated with activity in the right anterior-mid insula [20]. The current study specifically focuses on the perception of pain and the findings suggest that the C-fiber mediated nociceptive signals engage the left DLPFC to a greater extent than the Aδ mediated signals.
It should be noted that differential information processing may occur in principle nociceptive processing regions of the cerebrum in a manner that is not easily resolved with fMRI. For instance, the relatively low temporal resolution of fMRI limits its ability to detect differences in the timing of neural processing associated with different nociceptive inputs. Additionally, fMRI is not likely to be able to discern the situation in which small discrete neural modules are differentially active within a given ROI, which may very well be the case for the early nociceptive processing regions [18].
Selective activation of nociceptive fibers
Laser stimulation selectively activates nociceptors while avoiding concomitant activation of mechanoreceptors [48]. Imaging studies show that laser stimulation commonly activates cortical areas including SI, SII, insula, amygdala, ACC, and multiple prefrontal regions (e.g., [5,7]). However, previous studies frequently activated nociceptors simultaneously rather than selectively. Selective activation of Aδ- or C-nociceptors can be achieved by making use of differences in resistance to ischemic pressure between fibers [9–11], differences in heat thresholds between fibers [37], differences in distribution density of the fibers [8,41,42], and differential responses of fibers to the heating rate of the skin and to pharmacological treatment [40,62–66,68]. The present study was designed to separate C- and Aδ-fiber activation by exploiting differences in thermal thresholds, rate of thermal activation sensitivity, and spatial properties of the nociceptor types. Previous studies using contact heat stimulation found that low heating rates (in the range of 1-2°C per second) evoked nociceptive responses that were primarily mediated by C-fibers while high heating rates (in the range of higher than 2°C per second) produced nociceptive responses primarily mediated by Aδ-fibers [63,64,66,68]. With laser stimulation, these same principles apply although heating rates are faster [39].
Conclusion
The principle finding from the present study is that many brain areas are commonly engaged in processing of pricking and burning pain, mediated by Aδ- and C-fiber nociceptors, respectively. Yet, there are some regions that show preferential activation related to Aδ- versus C-fiber input. Stronger activation in the pricking pain condition was found in the hippocampal formation, cerebellum, fusiform gyrus, and cuneus/parieto-occipital sulcus. Stronger activation in the burning pain condition was found in the dorsolateral prefrontal cortex. It remains to be determined whether such differences are related to perception, motor reactions, or other aspects of nociceptive processing.
Acknowledgments
All authors declare that there are no financial interests to disclose. This work was supported by P50-AR49555 to JDG and R44DA016840–03 to MIN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: A simple network model. Proc Natl Acad Sci 1994;91:7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9:463–484. [DOI] [PubMed] [Google Scholar]
- [3].Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain 2002;99:313–321. [DOI] [PubMed] [Google Scholar]
- [4].Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage 2003;18:740–748. [DOI] [PubMed] [Google Scholar]
- [5].Bingel U, Lorenz J, Glauche V, Knab R, Glascher J, Weiller C, Buchel C. Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. Neuroimage 2004;23:224–32. [DOI] [PubMed] [Google Scholar]
- [6].Borckardt JJ, Weinstein M, Reeves S, Kozel A, Nahas Z, Smith AR, Byrne TK, Morgan K, George MS. Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology 2006;105:557–562. [DOI] [PubMed] [Google Scholar]
- [7].Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Biichel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex : a single-trial fMRI study. Brain 2002;125:1326–1336. [DOI] [PubMed] [Google Scholar]
- [8].Bragard D, Chen AC, Plaghki L. Direct isolation of ultra-late (C-fibre) evoked brain potentials by C02 laser stimulation of tiny cutaneous surface areas in man. Neurosci Lett 1996;209:81–84. [DOI] [PubMed] [Google Scholar]
- [9].Bromm B, Neitzel H, Tecklenburg A, Treede RD. Evoked cerebral potential correlates of C-fibre activity in man. Neurosci Lett 1983;43:109–114. [DOI] [PubMed] [Google Scholar]
- [10].Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by C02 laser stimulation. Hum Neurobiol 1984;3:33–40. [PubMed] [Google Scholar]
- [11].Bromm B, Treede RD. Human cerebral potentials evoked by C02 laser stimuli causing pain. Exp Brain Res 1987;67:153–162. [DOI] [PubMed] [Google Scholar]
- [12].Biichel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single-trial laser functional magnetic resonance imaging study. J Neurosci 2002;22:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 2007;315:1860–1862. [DOI] [PubMed] [Google Scholar]
- [14].Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen J-I, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci 1999;96:7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Calvert GA. Crossmodal Processing in the Human Brain: Insights from Functional Neuroimaging Studies. Cereb Cortex 2001;11:1110–1123. [DOI] [PubMed] [Google Scholar]
- [16].Clark RE, Broadbent NJ, Squire LR. The hippocampus and spatial memory: findings with a novel modification of the water maze. J Neurosci 2007;27:6647–6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002;3:201–215. [DOI] [PubMed] [Google Scholar]
- [18].Davis KD. Neurophysiological and anatomical considerations in functional imaging of pain. Pain 2003;105:1–3. [DOI] [PubMed] [Google Scholar]
- [19].Davis KD, Pope GE, Crawley AP, Mikulis DJ. Neural correlates of prickle sensation: a percept-related fMRI study. Nat Neurosci 2002;5:1121–1122. [DOI] [PubMed] [Google Scholar]
- [20].Davis KD, Pope GE, Crawley AP, Mikulis DJ. The perceptual illusion of “paradoxical heat” engages the insular cortex. J Neurophysiol 2004;92:1248–1251. [DOI] [PubMed] [Google Scholar]
- [21].Derbyshire SWG, Jones AKP, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 1997;73:431–445. [DOI] [PubMed] [Google Scholar]
- [22].Dey PK, Ray AK. Anterior cerebellum as a site for morphine analgesia and post-stimulation analgesia. Indian J Physiol Pharmacol 1982;26:3–12. [PubMed] [Google Scholar]
- [23].Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage 2003;20:1540–1551. [DOI] [PubMed] [Google Scholar]
- [24].Forss N, Raij TT, Seppa M, Hari R. Common cortical network for first and second pain. Neuroimage 2005;24:132–142. [DOI] [PubMed] [Google Scholar]
- [25].Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004;127:835–843. [DOI] [PubMed] [Google Scholar]
- [26].Graff-Guerrero A, Gonzalez-Olvera J, Fresan A, Gomez-Martin D, Mendez-Nunez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Cog Brain Res 2005;25:153–160. [DOI] [PubMed] [Google Scholar]
- [27].Granot M, Buskila D, Granovsky Y, Sprecher E, Neumann L, Yarnitsky D. Simultaneous recording of late and ultra-late pain evoked potentials in fibromyalgia. Clin Neurophysiol 2001;112:1881–1887. [DOI] [PubMed] [Google Scholar]
- [28].Greffrath W, Nemenov MI, Schwarz S, Baumgartner U, Vogel H, Arendt-Nielsen L, Treede RD. Inward currents in primary nociceptive neurons of rat and pain sensation in humans elicited by infrared diode laser pulses. Pain 2002;99:145–155. [DOI] [PubMed] [Google Scholar]
- [29].Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol 2001;86:402–411. [DOI] [PubMed] [Google Scholar]
- [30].Hollup SA, Kjelstrup KG, Hoff J, Moser M-B, Moser EI. Impaired recognition of the global location during spatial navigation in rats with hippocampal lesions. J Neurosci 2001;21:4505–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Janig W Neuronal mechanisms of pain with special emphasis on visceral and deep somatic pain. Acta Neurochir Suppl 1987; 38:16–32. [DOI] [PubMed] [Google Scholar]
- [32].Jiang N, Cooper BY, Nemenov MI. Non-invasive diode laser activation of transient receptor potential proteins in nociceptors In: Hamblin MR, Waynant RW, Anders J, editors. Mechanisms for Low-Light Therapy II. Proc SPIE; 2007;Vol. 6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kakigi R, Tran TD, Qiu Y, Wang X, Nguyen TB, Inui K, Watanabe S, Hoshiyama M. Cerebral responses following stimulation of unmyelinated C-fibers in humans: electro- and magneto-encephalographic study. Neurosci Res 2003;45:255–275. [DOI] [PubMed] [Google Scholar]
- [34].Kastner S, Ungerleider L. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 2000;23:315–341. [DOI] [PubMed] [Google Scholar]
- [35].Kjelstrup KG, Tuvnes FA, Steffenach H-A, Munson R, Moser El, Moser M-B Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci 2002;99:10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kou L, Labrie D, Chylek P. Refractive indices of water and ice in the 0.65- to 2.5-μm spectral range. Appl Opt 1993;32:3531–3540. [DOI] [PubMed] [Google Scholar]
- [37].Magerl W, Ali Z, Ellrich J, Meyer RA, Treede RD. C- and A delta-fiber components of heat-evoked cerebral potentials in healthy human subjects. Pain 1999;82:127–137. [DOI] [PubMed] [Google Scholar]
- [38].Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol 2006;291 :R257–R267. [DOI] [PubMed] [Google Scholar]
- [39].Nemenov MI, Zaitsev VG, Mikkelsen J [Abstract]. Limitations of laser application in pain research. International Association for the Study of Pain, 9th World Congress on Pain, 1999, Vienna, Austria. [Google Scholar]
- [40].Nemenov MI. Portable laser and process for producing controlled pain USA PATENT # 7402167, issued 2008, priority date 2002.
- [41].Ochoa J, Mair WGP. The normal sural nerve in man. Ultrastructure and numbers of fibers and cells I. Acta Neuropath 1969;13:197–216. [DOI] [PubMed] [Google Scholar]
- [42].Opsommer E, Masquelier E, Plaghki L. Determination of nerve conduction velocity of C-fibres in humans from thermal thresholds to contact heat (thermode) and from evoked brain potentials to radiant heat (CO2 laser). Neurophysiol Clin 1999;29:411–422. [DOI] [PubMed] [Google Scholar]
- [43].Opsommer E, Weiss T, Plaghki L, Miltner WHR. Dipole analysis of ultralate (C-fibres) evoked potentials after laser stimulation of tiny cutaneous surface areas in humans. Neurosci Lett 2001;298:41–44. [DOI] [PubMed] [Google Scholar]
- [44].Orstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jorum E, Handwerker H, Torebjork E. Pathological C-fibres in patients with a chronic painful condition. Brain 2003;126:567–578. [DOI] [PubMed] [Google Scholar]
- [45].Palmer KF and Williams D. Optical properties of water in the near infrared. J Opt Soc Am 1974;64:1107–1110. [Google Scholar]
- [46].Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 2000;30:263–288. [DOI] [PubMed] [Google Scholar]
- [47].Peyron R, Kupers R, Jehl JL, Garcia-Larrea L, Convers P, Barral FG, Laurent B. Central representation of the RIII flexion reflex associated with overt motor reaction: An fMRI study. Clin Neurophysiol 2007;37:249–259. [DOI] [PubMed] [Google Scholar]
- [48].Plaghki L, Mouraux A. How do we selectively activate skin nociceptors with a high power infrared laser? Physiology and biophysics of laser stimulation. Clin Neurophysiol 2003;33:269–277. [DOI] [PubMed] [Google Scholar]
- [49].Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins NP, Matthews PM. Learning about pain: The neural substrate of the prediction error for aversive events. Proc Natl Acad Sci 2000;97:9281–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 2001;21:9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ploner M, Gross J, Timmermann L, Schnitzler A. Cortical representation of first and second pain sensation in humans. Proc Natl Acad Sci 2002;99:12444–12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Price DD, Wagman IH. Relationships between pre- and postsynaptic effects of A and C fiber inputs to dorsal horn of M. mulatta. Exp Neurol 1973;40:90–103. [DOI] [PubMed] [Google Scholar]
- [53].Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000;288:1769–1772. [DOI] [PubMed] [Google Scholar]
- [54].Qiu Y, Noguchi Y, Honda M, Nakata H, Tamura Y, Tanaka S, Sadato N, Wang X, Inui K, Kakigi R. Brain processing of signals ascending through unmyelinated C fibers in humans: an event-related functional magnetic resonance study. Cereb Cortex 2006;16:1289–1295. [DOI] [PubMed] [Google Scholar]
- [55].Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain Affect Encoded in Human Anterior Cingulate But Not Somatosensory Cortex. Science 1997; 277:968–971. [DOI] [PubMed] [Google Scholar]
- [56].Saab CY, Kawasaki M, Al-Chaer ED, Willis WD. Cerebellar cortical stimulation increases spinal visceral nociceptive responses. J Neurophysiol 2001;85:2359–2363. [DOI] [PubMed] [Google Scholar]
- [57].Spiegel J, Hansen C, Treede R-D. Clinical evaluation for the assessment of impaired pain sensitivity by thulium-laser evoked potentials. Clin Neurophysiol 2000;111:725–735. [DOI] [PubMed] [Google Scholar]
- [58].Sullivan MJL, Thon B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relationship between catastrophizing and pain. Clin J Pain 2001;17:52–64. [DOI] [PubMed] [Google Scholar]
- [59].Tilman DB, Treede RD, Meyer RA, Campbell JN. Response of C fiber nociceptors in the anesthetized monkey to heat stimuli: estimates of receptor depth and threshold. J Physiol 1995;485:753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Treede RD, Meyer RA, Raja SN, Campbell IN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol 1995;483;747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Treede R-D, Kenshalo DR, Gracely RH, Jones AKP. The cortical representation of pain. Pain 1999;79:105–111. [DOI] [PubMed] [Google Scholar]
- [62].Tzabazis A, Klyukinov M, Manering N, Nemenov MI, Schafer SL, Yeomans DC. Differential activation of trigeminal C or Aδ nociceptors by infrared diode laser in rats: behavioral evidence. Brain Res 2005;1037:148–156. [DOI] [PubMed] [Google Scholar]
- [63].Yarnitsky D, Ochoa JL. Studies of heat pain sensation in man: perception thresholds, rate of stimulus rise and reaction time. Pain 1990;40:85–91. [DOI] [PubMed] [Google Scholar]
- [64].Yarnitsky D, Simone DA, Dotson RM, Cline MA, Ochoa JL. Single C nociceptor responses and psychophysical parameters of evoked pain: effect of rate of rise of heat stimuli in humans. J Physiol 1992;450:581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yeomans DC, Cooper BY, Vierck CJ. Effects of systemic morphine on responses of primates to first or second pain sensations. Pain 1996a;66:253–263. [DOI] [PubMed] [Google Scholar]
- [66].Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain 1996b;68:133–140. [DOI] [PubMed] [Google Scholar]
- [67].Yeomans DC, Plaghki L, Herbette S, Klyukinov M, Nemenov MI. [Abstract] Cortical evoked potentials and psychophysical evaluation of pain evoked by infrared diode laser stimulation of skin in humans. International Association for the Study of Pain, 10th World Congress on Pain, 2008, Glasgow, UK. [Google Scholar]
- [68].Zachariou V, Goldstein BD, Yeomans DC. Low but not high rate noxious radiant skin heating evokes a capsaicin-sensitive increase in spinal cord dorsal horn release of substance P. Brain Res 1997;752:143–150. [DOI] [PubMed] [Google Scholar]


