Abstract
Diastereodivergent and enantioselective conversion of isatin ketimines to α-fluoro-β-aminonitriles with vicinal tetrasubstituted stereocenters is achieved by a chiral copper complex/guanidine base catalyzed Mannich reaction with proper choice of the bisphosphine ligand. The reaction is broad in scope, scalable, and provides efficient access to a series of 3-aminoindolinones exhibiting a quaternary carbon-fluorine stereocenter with high yields and stereoselectivities. Selective transformations of the Mannich reaction products into multifunctional 3-aminooxindoles without erosion of enantiomeric and diastereomeric purity highlight the synthetic utility.
Keywords: Enantioselective catalysis, stereodivergence, Mannich reaction, organofluorines, α-fluoro-β-aminonitriles
Graphical Abstract
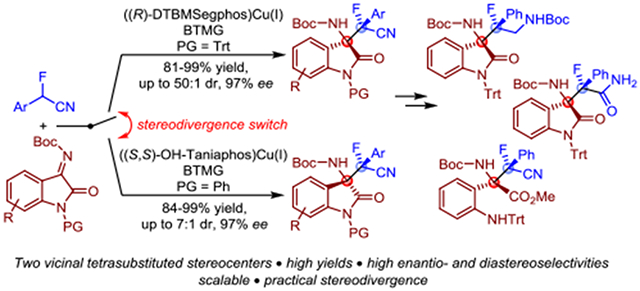
Catalytic enantioselective reactions that produce multifunctional building blocks with carbon-fluorine quaternary stereocenters are of great interest due to the prevalence of this motif in biologically active compounds.1 In many cases, the in situ generation of fluorinated nucleophiles requires the presence of a proximate carbonyl group which substantially limits the pool of substrates that can be applied in catalytic asymmetric synthesis of organofluorines displaying a quaternary chirality center.2 The use of α-fluorinated nitriles devoid of an activating carbonyl moiety remains very challenging because of the inherent fluxionality of α-metalated nitriles and the low C-H acidity, which complicates catalytic formation of α-cyano carbanions under mild reaction conditions.3 To the best of our knowledge, stereodivergent catalytic asymmetric additions with α-fluoro-α-arylnitriles have not been reported to date despite the synthetic potential of these prenucleophiles for the construction of chemically versatile scaffolds around a tetrasubstituted carbon-fluorine stereocenter.
Mannich reactions with fluorinated nucleophiles are particularly attractive because they provide access to pharmaceutically important fluorinated amino compounds.4 Following Shibasaki’s pioneering work on aldol-type reactions with nitriles,5 silyl ketene imines that overcome some of the difficulties mentioned above have been used as reactive nitrile surrogates in asymmetric aldol and Mannich reactions.6 The direct use of nitrile compounds, however, appears more appealing because it obviates the necessity to prepare a silyl ketene imine derivative. To this end, the introduction of Shibasaki’s cooperative soft Lewis acid – hard Brønsted base catalysis strategy has significantly widened the substrate scope.7 In recent years, several groups have achieved enantioselective catalytic Mannich additions to aldimines with nitriles carrying an adjacent carbonyl, sulfonyl or another activating functionality.8 Decarboxylative methods and the catalytic addition of allenylnitriles, benzylnitriles or phenylthioacetonitrile to aldimines have also been reported by Shibasaki, Nakamura and others.9
Enantioselective Mannich reactions with ketimines have been accomplished with activated nitriles (Scheme 1a and b).6j,10 To demonstrate the use of α-fluoro-α-substituted nitriles in asymmetric catalysis, we chose to investigate the possibility of a Mannich reaction with ketimines derived from isatin which affords an important scaffold encountered in many natural products and drugs.11 Because this reaction establishes two chirality centers, we also recognized the importance of providing convenient access to all four stereoisomeric products, preferentially by easily adaptable diastereodivergent protocols.12 We now wish to report a (bisphosphine)copper(I) catalyzed direct asymmetric addition of α-fluoro-α-arylnitriles to isatin ketimines that addresses these challenges (Scheme 1c). Our method provides efficient access to multifunctional α-fluoro β-aminonitriles bearing vicinal tetrasubstituted stereocenters in high yields and with excellent enantio- and diastereoselectivity. Moreover, all four stereoisomers are accessible by suitable selection of the chiral copper catalyst and the isatin protecting group. The practical diastereodivergence, amenability to upscaling and selective functional group manipulation of the fluorinated α,β-aminonitrile moiety toward multifunctional β-fluoro-α,γ-diamines, α-fluoro-β-amino amides and fluorinated α-amino acid derivatives underscore the synthetic utility of this reaction.
Scheme 1.
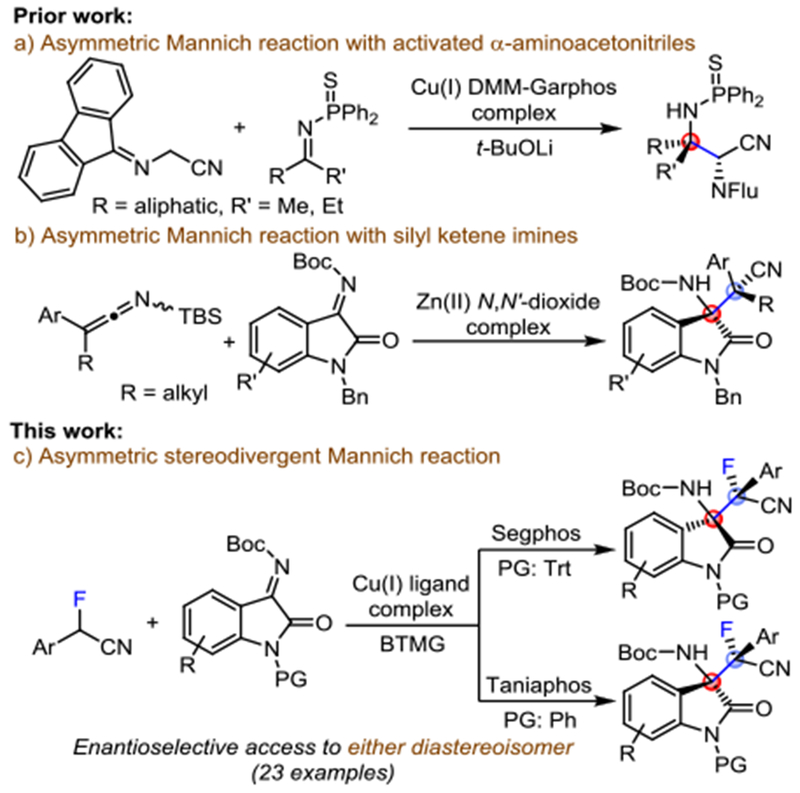
Synthesis of chiral β-aminonitriles bearing vicinal tetrasubstituted stereocenters.
At the beginning of our search for a stereodivergent catalytic asymmetric Mannich reaction, we chose α-fluorobenzylnitrile, 1a, and the isatin derived N-Boc ketimines 2a-c as test compounds and screened various Cu(I) salts, Segphos and Biphep ligands (L1-L4), solvents and base additives (Table 1 and SI). We found that the desired α-fluoro-β-aminonitrile 3aa can be obtained from the N-benzyl isatin derived ketimine 2a in 91% yield using catalytic amounts of copper(I) triflate, DTBM-Segphos (L1) and diisopropylethylamine as base in toluene, albeit with low stereoselectivities (entry 1). The enantio- and diastereoselectivity increased significantly to 80% ee and 5.2:1 dr when the N-trityl ketimine 2b was employed (entry 2). Extensive variation of bisphosphine and phosphinooxazoline ligands (L1-L8) and the introduction of amidine and guanidine bases further improved results (entries 3–13). We were pleased to observe almost quantitative formation of anti-3ab with 83% ee and 6.7:1 dr using 10 mol% of copper hexafluorophosphate, L1 and BTMG in toluene at room temperature (entry 5). A decrease in the reaction temperature finally allowed us to optimize the stereoselectivities and we isolated anti-3ab in 95% yield, 90% ee and 12.3:1 dr at −35 °C (SI and entry 14). Additional investigation of the reaction outcome revealed that the diastereoselectivity can be switched with C1-symmetric bisphosphine ligands L9-L14. Using 5 mol% of CuPF6 and BTMG, the opposite diastereomer was favored when 1,2-ferrocenyl bisphosphines were used as ligands (Table 1, entries 15–19). Poor stereoselectivities were initially observed until we resorted to the N-benzyl or N-phenyl ketimines and copper catalysts carrying either the Taniaphos ligands L9 and L14 or Walphos ligands L11-L13 under otherwise identical reaction conditions. We found that (Walphos)Cu(I) favors high diastereoselectivities while the use of Taniaphos as chiral ligand leads to superior ee’s. For example, the Mannich reaction between 1a and 2a gave syn-3aa in 99% yield with 13.4:1 dr and 70% ee which further increased to 80% when L13 was replaced with Taniaphos L14 (entries 20–22). The introduction of the N-phenyl isatin derived ketimine 2c resulted in excellent enantioselectivity and we obtained syn-3ac in 94% yield with 98% ee and 3.0:1 dr using 5 mol% of L14, CuPF6 and BTMG at −35 °C (entry 23).
Table 1.
Optimization of the stereodivergent asymmetric Mannich reaction.
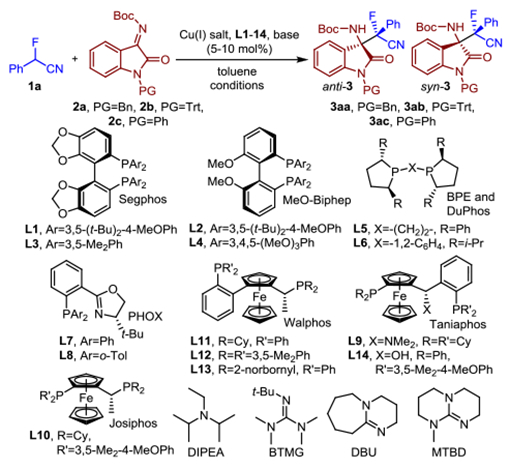 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Cu(I) source | Ligand | 2 | Conditions | Yield (%) | dra (anti/syn) | eeb (%) |
| 1 | Cu(PhMe)0.5OTf | L1 | 2a | DIPEAc, 25 °C | 91 | 1.7:1 | 41 |
| 2 | Cu(PhMe)0.5OTf | L1 | 2b | DIPEAc, 25 °C | 85 | 5.2:1 | 80 |
| 3 | Cu(PhMe)0.5OTf | L2 | 2b | DIPEAc, 25 °C | 74 | 4.6:1 | 75 |
| 4 | Cu(PhMe)0.5OTf | L1 | 2b | BTMG, 25 °C | 98 | 5.8:1 | 83 |
| 5 | Cu(MeCN)4PF6 | L1 | 2b | BTMG, 25 °C | 98 | 6.7:1 | 83 |
| 6 | Cu(MeCN)4PF6 | L1 | 2b | DBU, 25 °C | 99 | 6.6:1 | 73 |
| 7 | Cu(MeCN)4PF6 | L1 | 2b | MTBD, 25 °C | 99 | 5.8:1 | 83 |
| 8 | Cu(MeCN)4PF6 | L3 | 2b | BTMG, 25 °C | 99 | 2.0:1 | 26 |
| 9 | Cu(MeCN)4PF6 | L4 | 2b | BTMG, 25 °C | 99 | 4.0:1 | 79 |
| 10 | Cu(MeCN)4PF6 | L5 | 2b | BTMG, 25 °C | 99 | 1.5:1 | 50 |
| 11 | Cu(MeCN)4PF6 | L6 | 2b | BTMG, 25 °C | 99 | 1.5:1 | 31 |
| 12 | Cu(MeCN)4PF6 | L7 | 2b | BTMG, 25 °C | 99 | 1.3:1 | 40 |
| 13 | Cu(MeCN)4PF6 | L8 | 2b | BTMG, 25 °C | 99 | 1.1:1 | 47 |
| 14 | Cu(MeCN)4PF6 | L1 | 2b | BTMG, −35 °C | 95 | 12.3:1 | 90 |
| 15d | Cu(MeCN)4PF6 | L9 | 2b | BTMG, 25 °C | 97 | 1:1.6 | 3 |
| 16d | Cu(MeCN)4PF6 | L9 | 2a | BTMG, 25 °C | 99 | 1:5.7 | 31 |
| 17d | Cu(MeCN)4PF6 | L10 | 2a | BTMG, −35 °C | 75 | 1:2.5 | 4 |
| 18d | Cu(MeCN)4PF6 | L11 | 2a | BTMG, −35 °C | 99 | 1:7.3 | 19 |
| 19d | Cu(MeCN)4PF6 | L12 | 2a | BTMG, −35 °C | 99 | 1:5.6 | 60 |
| 20d | Cu(MeCN)4PF6 | L13 | 2a | BTMG, −35 °C | 99 | 1:13.4 | 70 |
| 21d | Cu(MeCN)4PF6 | L13 | 2c | BTMG, −35 °C | 98 | 1:8.7 | 75 |
| 22d | Cu(MeCN)4PF6 | L14 | 2a | BTMG, −35 °C | 94 | 1:5.7 | 80 |
| 23d | Cu(MeCN)4PF6 | L14 | 2c | BTMG, −35 °C | 94 | 1:3.0 | 98 |
Reaction condition: 1a (0.055 mmol), 2 (0.050 mmol), Cu(I) source (0.005 mmol), ligand (0.006 mmol) and base in 0.3 mL toluene.
Determined by 19F NMR analysis.
Determined by chiral HPLC analysis.
The base loading was 80 mol%.
The Cu complex and base loading were 5 mol%.
Having optimized the Mannich reaction conditions and with practical stereodivergent protocols in hand, we continued with the evaluation of the substrate scope using a variety of α-fluoro-α-arylacetonitriles (Scheme 2). The (DTBM-Segphos) Cu(I)/BTMG catalyzed reaction with the α-fluoro-arylacetonitriles 1a-1i and ketimine 2b gave quantitative yields and high stereoselectivities demonstrating excellent functional group tolerance of electron-withdrawing and electron-donating substituents in the ortho-, meta-, para-positions of the phenyl ring. It is noteworthy that the reaction with the chloro- and bromo-substituted α-fluorobenzylnitriles 1b and 1c furnished anti-3bb and anti-3cb in 97–98% yield, more than 35:1 dr and 97% ee. Addition of α-fluoro-3,5-dimethoxybenzylnitrile 1i to ketimine 2b produced 83% of anti-3ib with more than 50:1 diastereoselectivity and 91% ee. Excellent results were also obtained with fluoroacetonitriles 1j-l carrying 1,3-benzodioxole-5-yl, 2-naphthyl, or 2-fluorenyl rings. The corresponding α-fluoro-β-aminonitriles anti-3jb-lb were produced in high yield and with up to 45:1 dr and 93% ee.
Scheme 2.

Reaction scope with α-fluoro-α-arylnitriles. The absolute configuration of 3ab and 3lb were determined by crystallographic analysis. The configuration of the other compounds was assigned by analogy.
Slow evaporation of a solution of 3ab in ethanol and of 3lb in a hexane/diethyl ether/dichloromethane (2:2:1) solution led to the formation of single crystals.13 Crystallographic analysis revealed R configuration at the oxindole C3 position and S configuration at the fluorinated carbon atom, and NMR and chiral HPLC measurements proved that these single crystals relate to the major stereoisomer formed using ((R)-DTBM-Segphos)Cu(I) as catalyst. The reactivity substituted N-trityl isatin derived ketimines 2d-2h was also probed (Scheme 3). The presence of methyl, ethyl, and methoxy groups in the 5- or 6-position of the isatin moiety was well tolerated and we obtained high yields and stereoselectivities. All α-fluoro-β-aminonitriles were produced in high yields and with up to 34:1 dr and 96% ee.
Scheme 3.

Reaction scope with N-trityl isatin derived N-Boc ketimines.
We then evaluated the substrate scope for the diastereodivergent protocol using 5 mol% of ((S,Sp)-Taniaphos)Cu(I) as catalyst (Scheme 4). The reaction of five different α-fluoroarylacetonitriles to the N-phenyl isatin ketimine 2c gave syn-3ac-3kc in 84–99% yield and with good to high stereoselectivities ranging from 3:1 to 7:1 dr and 83–97% ee, respectively. We obtained a single crystal of 3ic by slow evaporation of a hexanes/ethanol/chloroform (3:1:1) solution. The crystallographic analysis is in agreement with NMR and chromatographic measurements which confirmed the favored formation of the syn-(S,S)-diastereomer.
Scheme 4.

Diastereodivergent synthesis syn-α-fluoro-β-aminonitriles from N-phenyl isatin N-Boc ketimines using ((S,Sp)-Taniaphos)Cu(I) as catalyst.
Based on NMR analysis and in analogy to previously reported mechanistic studies we propose the catalytic cycle and a plausible transition state shown in Scheme 5 (SI).8d,g,9c Competition binding experiments revealed preferential binding of the α-fluorobenzylnitrile 1a to the (Segphos)Cu(I) complex in the presence of the ketimine 2. We then conducted H/D exchange and titration experiments and observed that the metal coordination of the nitrile significantly accelerates the reversible deprotonation of complex A to the cuprous keteniminate complex B. Irreversible C-C bond formation affords C which undergoes proton transfer and dissociation to 3, regenerating the free Cu(I) complex and BTMG. In the favored transition state, the N-cuprated ketenimine exposes the Si-face for nucleophilic attack by the isatin ketimine which is expected to occupy a tilted orientation to minimize steric repulsion as the large N-trityl group occupies the bottom left axial space and the N-carbamoyl resides in the top right axial space. This exposes the Si-face of the ketimine and gives the (R,S)-diastereomer as observed.
Scheme 5.
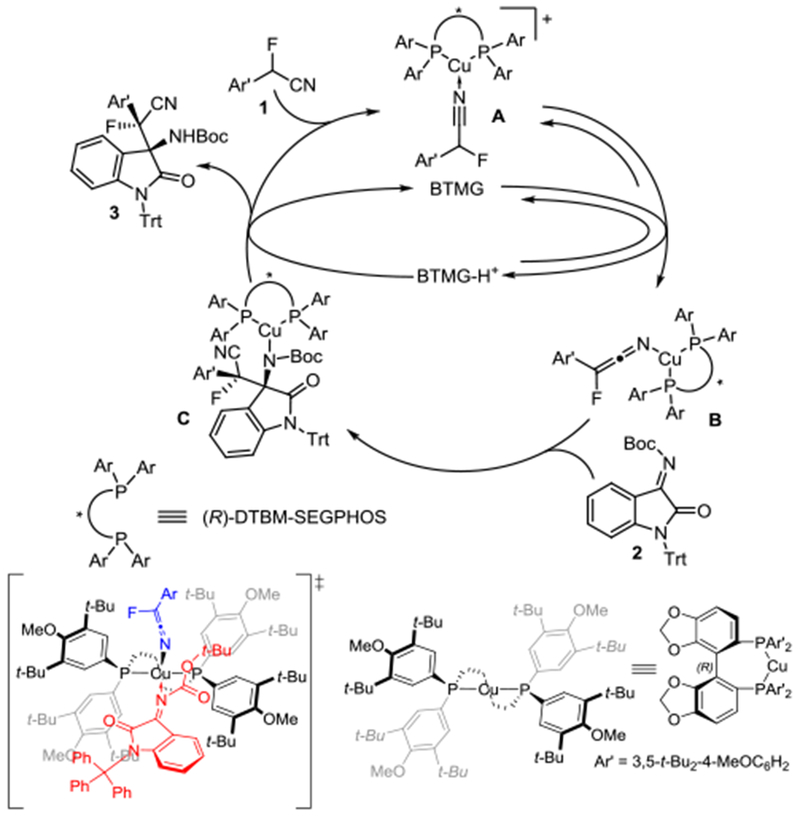
Proposed mechanism.
Finally, the possibility of upscaling and the synthetic utility of the synthesized α-fluoro-β-aminonitriles were investigated (Scheme 6). We were pleased to find that nearly one gram of anti-3ab was produced in quantitative amounts and without compromised stereoselectivities using 5 mol% of the (DTBM-Segphos)Cu(I) catalyst. Reduction of compound 3ab with NaBH4 in the presence of NiCl2 produced β-fluoro-α,γ-diamine 4 in 65% yield.14 Hydrolysis of the cyano group in 3ab using a catalytic amount of Pd(OAc)2 and PPh3 with acetaldoxime in aqueous EtOH gave the α-fluoro-β-amino amide 5, a fluorinated analogue of the cholecystokinin-2 (CCK2)/gastrin receptor antagonist AG-041R,15 in 97% yield.16 Methanolysis with sodium methoxide opened the oxindole lactam ring in 3ab without erosion of the original ee and dr, producing β-fluoro-α-amino acid methyl ester 6, a fluorinated unnatural amino acid derivative.17 Simultaneous deprotection of the trityl and Boc groups in 3ab gave 76% of 7 in 89% ee and 12:1 dr. Our protocol can also be applied to α-alkyl-α-arylnitriles. We obtained 9 from α-methylphenylacetonitrile, 8, in 96% yield and with 85% ee and more than 19:1 dr.
Scheme 6.
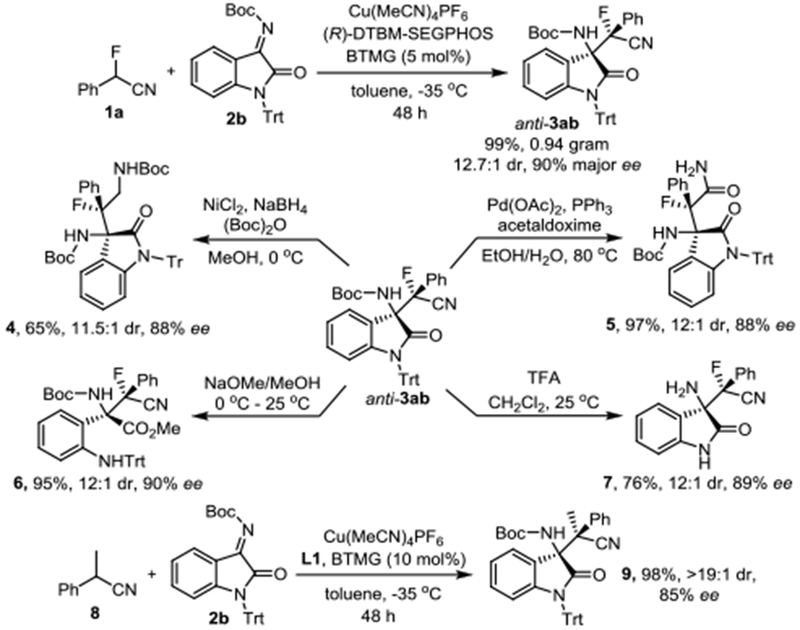
Gram scale synthesis, transformations of α-fluoro-β-aminonitrile 3ab and extension of the substrate scope to α-alkyl-α-arylnitriles.
In summary, we have developed an efficient diastereodivergent catalytic enantioselective Mannich reaction constructing α-fluoro-β-aminonitriles bearing vicinal tetrasubstituted stereocenters via (bisphosphine)copper(I) complex/guanidine catalyzed addition of α-fluoroarylacetonitriles to isatin derived N-Boc ketimines. The switching of diastereoselectivity is very practical and can be conveniently achieved by properly choosing the chiral bisphosphine ligand and the isatin N-protecting group. Using either Segphos or Taniaphos-derived copper(I) complexes and BTMG as base we have prepared a variety of syn- and anti-diastereomers of multifunctionalized 3-aminooxindoles with an adjacent quaternary C-F stereocenter in excellent yields and ee’s. The reaction can be conducted at the gram scale without compromising yield and stereoselectivity and the general utility of α-fluoro β-aminonitriles was demonstrated with selective transformations of the nitrile functionality and oxindole ring opening.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge financial support from NIH (GM106260).
Footnotes
Supporting Information.
Experimental details, characterization data including NMR spectra and HPLC chromatograms. The Supporting Information is available free of charge on the ACS Publications website.
The authors declare no competing financial interest.
REFERENCES
- (1).a) Zhou Y; Wang J; Gu Z; Wang S; Zhu W; J. Acena L; Soloshonok VA; Izawa K; Liu H, Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev 2016, 116, 422–518. [DOI] [PubMed] [Google Scholar]; b) Zhu Y; Han J; Wang J; Shibata N; Sodeoka M; Soloshonok VA; Coelho JAS; Toste FD, Modern Approaches for Asymmetric Construction of Carbon–Fluorine Quaternary Stereogenic Centers: Synthetic Challenges and Pharmaceutical Needs. Chem. Rev 2018, 118, 3887–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).a) Xie C; Wu L; Han J; Soloshonok VA; Pan Y, Assembly of Fluorinated Quaternary Stereogenic Centers Through Catalytic Enantioselective Detrifluoroacetylative Aldol Reactions. Angew. Chem. Int. Ed 2015, 54, 6019–6023. [DOI] [PubMed] [Google Scholar]; b) Xie C; Dai Y; Mei H; Han J; Soloshonok VA; Pan Y, Asymmetric Synthesis of Quaternary α-Fluoro-β-keto-amines via Detrifluoroacetylative Mannich Reactions. Chem. Commun 2015, 51, 9149–9152. [DOI] [PubMed] [Google Scholar]; c) Trost BM; Saget T; Lerchen A; Hung C-I, Catalytic Asymmetric Mannich Reactions with Fluorinated Aromatic Ketones: Efficient Access to Chiral β-Fluoroamines. Angew. Chem. Int. Ed 2016, 55, 781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Balaraman K; Wolf C, Catalytic Enantioselective and Diastereoselective Allylic Alkylation with Fluoroenolates: Efficient Access to C3-Fluorinated and All-Carbon Quaternary Oxindoles. Angew. Chem. Int. Ed 2017, 56, 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Paladhi S; Park SY; Yang JW; Song CE, Asymmetric Synthesis of α-Fluoro-β-Amino-oxindoles with Tetrasubstituted C–F Stereogenic Centers via Cooperative Cation-Binding Catalysis. Org. Lett 2017, 19, 5336–5339. [DOI] [PubMed] [Google Scholar]; f) Ding R; Wolf C, Organocatalytic Asymmetric Synthesis of α-Oxetanyl and α-Azetidinyl Tertiary Alkyl Fluorides and Chlorides. Org. Lett 2018, 20, 892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Balaraman K; Ding R; Wolf C, Stereoselective Synthesis of 3,3’-Bisindolines by Organocatalytic Michael Additions of Fluorooxindole Enolates to Isatylidene Malononitriles in Aqueous Solution. Adv. Synth. Catal 2017, 359, 4165–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Moskowitz M; Balaraman K; Wolf C, Organocatalytic Stereoselective Synthesis of Fluorinated 3,3’-Linked Bisoxindoles. J. Org. Chem 2018, 83, 1661–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Lia B-Y; Du D-M, Chiral Squaramide-Catalyzed Asymmetric Mannich Reactions for Synthesis of Fluorinated 3,3’-Bisoxindoles. Adv. Synth. Catal 2018, 360, 3164–3170. [Google Scholar]
- (3).a) Bordwell FG, Equilibrium Acidities in Dimethyl Sulfoxide Solution. Acc. Chem. Res 1988, 21, 456–463. [Google Scholar]; b) Richard JP; Williams G; Gao J, Experimental and Computational Determination of the Effect of the Cyano Group on Carbon Acidity in Water. J. Am. Chem. Soc 1999, 121, 715–726. [Google Scholar]; c) López R; Palomo C, Cyanoalkylation: Alkylnitriles in Catalytic C-C Bond-Forming Reactions. Angew. Chem. Int. Ed 2015, 54, 13170–13184. [DOI] [PubMed] [Google Scholar]; d) Purzycki M; Liu W; Hilmersson G; Fleming FF, Metalated Nitriles: N- and C-Coordination Preferences of Li, Mg, and Cu Cations. Chem. Commun 2013, 49, 4700–4702. [DOI] [PubMed] [Google Scholar]; For a highly enantio- and diastereoselective cyclopropanation method with diazoacetonitrile see; e) Chandgude AL; Fasan R, Highly Diastereo- and Enantioselective Synthesis of Nitrile-Substituted Cyclopropanes by Myoglobin-Mediated Carbene Transfer Catalysis. Angew. Chem. Int. Ed 2018, 57, 15852–15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).a) Gillis EP; Eastman KJ; Hill MD; Donnelly DJ; Meanwell NA, Applications of Fluorine in Medicinal Chemistry. J. Med. Chem 2015, 58, 8315–8359. [DOI] [PubMed] [Google Scholar]; b) Meanwell NA, Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem 2018, 61, 5822–5880. [DOI] [PubMed] [Google Scholar]
- (5).a) Suto Y; Kumagai N; Matsunaga S; Shibasdaki M, Direct Catalytic Aldol-Type Reactions Using RCH2CN. Org. Lett 2003, 5, 3147–3150. [DOI] [PubMed] [Google Scholar]; b) Suto Y; Tsuji R; Kanai M; Shibasaki M, Cu(I)-Catalyzed Direct Enantioselective Cross Aldol-Type Reaction of Acetonitrile. Org. Lett 2005, 7, 3757–3760. [DOI] [PubMed] [Google Scholar]; c) Kumagai N; Matsunaga S; Shibasaki M, Cooperative Catalysis of a Cationic Ruthenium Complex, Amine Base, and Na Salt: Catalytic Activation of Acetonitrile as a Nucleophile. J. Am. Chem. Soc 2004, 126, 13632–13633. [DOI] [PubMed] [Google Scholar]; d) Sureshkumar D; Ganesh V; Kumagai N; Shibasaki M, Direct Catalytic Addition of Alkylnitriles to Aldehydes by Transition-Metal/NHC Complexes. Chem. Eur. J 2014, 20, 15723–15726. [DOI] [PubMed] [Google Scholar]
- (6).a) Denmark SE; Wilson TW, Silyl Ketene Imines: Highly Versatile Nucleophiles for Catalytic, Asymmetric Synthesis. Angew. Chem. Int. Ed 2012, 51, 9980–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mermerian AH; Fu GC, Nucleophile-Catalyzed Asymmetric Acylations of Silyl Ketene Imines: Application to the Enantioselective Synthesis of Verapamil. Angew. Chem. Int. Ed 2005, 44, 949–952. [DOI] [PubMed] [Google Scholar]; c) Denmark SE; Wilson TW; Burk MT; Heemstra JR Jr., Enantioselective Construction of Quaternary Stereogenic Carbons by the Lewis Base Catalyzed Additions of Silyl Ketene Imines to Aldehydes. J. Am. Chem. Soc 2007, 129, 14864–14865. [DOI] [PubMed] [Google Scholar]; d) Denmark SE; Wilson TW, N-Silyl Oxyketene Imines are Underused yet Highly Versatile Reagents for Catalytic Asymmetric Synthesis. Nat. Chem 2010, 2, 937–943. [DOI] [PubMed] [Google Scholar]; e) Denmark SE; Wilson TW, Lewis Base Catalyzed Enantioselective Additions of an N-Silyl Vinylketene Imine. Angew. Chem. Int. Ed 2012, 51, 3236–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Denmark SE; Wilson TW, Construction of Quaternary Stereogenic Carbon Centers by the Lewis Base Catalyzed Conjugate Addition of Silyl Ketene Imines to α,β-Unsaturated Aldehydes and Ketones. Synlett 2010, 1723–1728. [Google Scholar]; g) Notte GT; Vu JMB; Leighton JL, Highly Enantioselective Mannich Reactions with α-Aryl Silyl Ketene Acetals and Imines. Org. Lett 2011, 13, 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Vu JMB; Leighton JL, A New Synthesis of Pyrrolidines by Way of an Enantioselective Mannich/Diastereoselective Hydroamination Reaction Sequence. Org. Lett 2011, 13, 4056–4059. [DOI] [PubMed] [Google Scholar]; i) Zhao JN; Liu XH; Luo WW; Xie MS; Lin LL; Feng XM, Asymmetric Synthesis of β-Amino Nitriles through a ScIII-Catalyzed Three-Component Mannich Reaction of Silyl Ketene Imines. Angew. Chem. Int. Ed 2013, 52, 3473–3477. [DOI] [PubMed] [Google Scholar]; j) Zhao J; Fang B; Luo W; Hao X; Liu X; Lin L; Feng X, Enantioselective Construction of Vicinal Tetrasubstituted Stereocenters by the Mannich Reaction of Silyl Ketene Imines with Isatin-Derived Ketimines. Angew. Chem. Int. Ed 2015, 54, 241–244. [DOI] [PubMed] [Google Scholar]; k) Zheng J; Lin L; Dai L; Tang Q; Liu X; Feng X, Nickel-Catalyzed Conjugate Addition of Silyl Ketene Imines to In Situ Generated Indol-2-ones: Highly Enantioselective Construction of Vicinal All-Carbon Quaternary Stereocenters. Angew. Chem. Int. Ed 2017, 56, 13107–13111. [DOI] [PubMed] [Google Scholar]
- (7).a) Kumagai N; Shibasaki M, Recent Advances in Direct Catalytic Asymmetric Transformations under Proton-Transfer Conditions. Angew. Chem. Int. Ed 2011, 50, 4760–4772. [DOI] [PubMed] [Google Scholar]; b) Kawato Y; Kumagai N; Shibasaki M, Direct Catalytic Asymmetric Addition of Acetonitrile to N-Thiophosphinoylimines. Chem. Comm 2013, 49, 11227–11229. [DOI] [PubMed] [Google Scholar]
- (8).a) Lee JH; Kim DY, Enantio- and Diastereoselective Mannich-Type Reactions of α-Cyano Ketones with N-Boc Aldimines Catalyzed by Chiral Bifunctional Urea. Adv. Synth. Catal 2009, 351, 1779–1782. [Google Scholar]; b) Monge D; Jensen KL; Franke PT; Lykke L; Jørgensen KA, Asymmetric One-Pot Sequential Organo- and Gold Catalysis for the Enantioselective Synthesis of Dihydropyrrole Derivatives. Chem. Eur. J 2010, 16, 9478–9484. [DOI] [PubMed] [Google Scholar]; c) González PB; Lopez R; Palomo C, Catalytic Enantioselective Mannich-Type Reaction with β-Phenyl Sulfonyl Acetonitrile. J. Org. Chem 2010, 75, 3920–3922. [DOI] [PubMed] [Google Scholar]; d) Yanagida Y; Yazaki R; Kumagai N; Shibasaki M, Asymmetric Synthesis of Isothiazoles Through Cu Catalysis: Direct Catalytic Asymmetric Conjugate Addition of Allyl Cyanide to α,β-Unsaturated Thioamides. Angew. Chem. Int. Ed, 2011, 50, 7910–7914. [DOI] [PubMed] [Google Scholar]; e) Yamashita Y; Matsumoto M; Chen Y-J; Kobayashi S, Catalytic Mannich-Type Reactions of α-Aminoacetonitrile Using Fluorenylidene as a Protecting and Activating Group. Tetrahedron 2012, 68, 7558–7563. [Google Scholar]; f) Ohmatsu K; Goto A; Ooi T, Catalytic Asymmetric Mannich-Type Reactions of α-Cyano α-Sulfonyl Carbanions. Chem. Commun 2012, 48, 7913–7915. [DOI] [PubMed] [Google Scholar]; g) Kondo M; Sugimoto M; Nakamura S, Direct Catalytic Enantioselective Mannich-Type Reaction of Dichloroacetonitrile Using Bis-(imidazoline)-Pd Catalysts. Chem. Commun 2016, 52, 13604–13607. [DOI] [PubMed] [Google Scholar]; h) Kondo M; Nishi T; Hatanaka T; Funahashi Y; Nakamura S, Catalytic Enantioselective Reaction of α-Aminoacetonitriles Using Chiral Bis(imidazoline) Palladium Catalysts. Angew. Chem. Int. Ed 2015, 54, 8198–8202. [DOI] [PubMed] [Google Scholar]; i) Kondo M; Saito H; Nakamura S, Direct Catalytic Enantioselective Mannich-Type Reaction of α,α-Dithioacetonitriles with Imines Using Chiral Bis(imidazoline)-Pd Complexes. Chem. Commun 2017, 53, 6776–6779. [DOI] [PubMed] [Google Scholar]; j) Sun B; Balaji PV; Kumagai N; Shibasaki M, α-Halo Amides as Competent Latent Enolates: Direct Catalytic Asymmetric Mannich-Type Reaction. J. Am. Chem. Soc 2017, 139, 8295–8301. [DOI] [PubMed] [Google Scholar]; k) Balaji PV; Brewitz L; Kumagai N; Shibasaki M, Achiral Trisubstituted Thioureas as Secondary Ligands to CuI Catalysts: Direct Catalytic Asymmetric Addition of α-Fluoronitriles to Imines. Angew. Chem. Int. Ed 10.1002/anie.201812673. [DOI] [PubMed] [Google Scholar]
- (9).a) Yin L; Kanai M; Shibasaki M, Nucleophile Generation via Decarboxylation: Asymmetric Construction of Contiguous Trisubstituted and Quaternary Stereocenters through a Cu(I)-Catalyzed Decarboxylative Mannich-Type Reaction. J. Am. Chem. Soc 2009, 131, 9610–9611. [DOI] [PubMed] [Google Scholar]; b) Hyodo K; Kondo M; Funahashi Y; Nakamura S, Catalytic Enantioselective Decarboxylative Cyanoalkylation of Imines by Using Palladium Pincer Complexes with C2-Symmetric Chiral Bis(imidazoline)s. Chem. Eur. J 2013, 19, 4128–4134. [DOI] [PubMed] [Google Scholar]; c) Hyodo K; Nakamura S; Tsuji K; Ogawa T; Funahashi Y; Shibata N, Enantioselective Reaction of Imines and Benzyl Nitriles Using Palladium Pincer Complexes with C2-Symmetric Chiral Bis(imidazoline)s. Adv. Synth. Catal 2011, 353, 3385–3390. [Google Scholar]; d) Kondo M; Kobayashi N; Hatanaka T; Funahashi Y; Nakamura S, Catalytic Enantioselective Reaction of α-Phenylthioacetonitriles with Imines Using Chiral Bis(imidazoline)-Palladium Catalysts. Chem. Eur. J 2015, 21, 9066–9070. [DOI] [PubMed] [Google Scholar]; e) Kondo M; Omori M; Hatanaka T; Funahashi Y; Nakamura S, Catalytic Enantioselective Reaction of Allenylnitriles with Imines Using Chiral Bis(imidazoline)s Palladium(II) Pincer Complexes. Angew. Chem. Int. Ed 2017, 56, 8677–8680. [DOI] [PubMed] [Google Scholar]
- (10).a) Lin S; Kawato Y; Kumagai N; Shibasaki M, Catalytic Asymmetric Mannich-Type Reaction of N-Alkylidene-α-Aminoacetonitrile with Ketimines. Angew. Chem. Int. Ed 2015, 54, 5183–5186. [DOI] [PubMed] [Google Scholar]; b) Takeda T; Kondoh A; Terada M, Construction of Vicinal Quaternary Stereogenic Centers by Enantioselective Direct Mannich-Type Reaction Using a Chiral Bis(guanidino)imino-phosphorane Catalyst. Angew. Chem. Int. Ed 2016, 55, 4734–4737. [DOI] [PubMed] [Google Scholar]; For a Mannich reaction with an allylic cyanide:; c) Yazaki R; Nitabaru T; Kumagai N; Shibasaki M, Direct Catalytic Asymmetric Addition of Allylic Cyanides to Ketoimines. J. Am. Chem. Soc 2008, 130, 14477–14479. [DOI] [PubMed] [Google Scholar]
- (11).a) Ishimaru T; Shibata N; Horikawa T; Yasuda N; Nakamura S; Toru T; Shiro M, Cinchona Alkaloid Catalyzed Enantioselective Fluorination of Allyl Silanes, Silyl Enol Ethers, and Oxindoles. Angew. Chem., Int. Ed 2008, 47, 4157–4161; [DOI] [PubMed] [Google Scholar]; b) Ma S; Han X; Krishnan S; Virgil SC; Stoltz BM, Catalytic Enantioselective Stereoablative Alkylation of 3-Halooxindoles: Facile Access to Oxindoles with C3 All-Carbon Quaternary Stereocenters. Angew. Chem. Int. Ed 2009, 48, 8037–8041; [DOI] [PubMed] [Google Scholar]; c) Bui T; Syed S; Barbas CF III, Thiourea-Catalyzed Highly Enantio- and Diastereoselective Additions of Oxindoles to Nitroolefins: Application to the Formal Synthesis of (+)-Physostigmine. J. Am. Chem. Soc 2009, 131, 8758–8759; [DOI] [PubMed] [Google Scholar]; d) Antonchick AP; Gerding-Reimers C; Catarinella M; Schürmann M; Preut H; Ziegler S; Rauh D; Waldmann H, Highly Enantioselective Synthesis and Cellular Evaluation of Spirooxindoles Inspired by Natural Products. Nat. Chem 2010, 2, 735–740; [DOI] [PubMed] [Google Scholar]; e) Tan B; Candeias NR; Barbas CF III, Construction of Bispirooxindoles Containing Three Quaternary Stereocentres in A Cascade Using A Single Multifunctional Organocatalyst. Nat. Chem 2011, 3, 473–477; [DOI] [PubMed] [Google Scholar]; f) Guo C; Song J; Huang J-Z; Chen P-H; Luo S-W; Gong L-Z, Core-Structure-Oriented Asymmetric Organocatalytic Substitution of 3-Hydroxyoxindoles: Application in the Enantioselective Total Synthesis of (+)-Folicanthine. Angew. Chem. Int. Ed 2012, 51, 1046–1050; [DOI] [PubMed] [Google Scholar]; g) Wu L; Falivene L; Drinkel E; Grant S; Linden A; Cavallo L; Dorta R, Synthesis of 3-Fluoro-3-aryl Oxindoles: Direct Enantioselective α Arylation of Amides Angew. Chem. Int. Ed 2012, 51, 2870–2873. [DOI] [PubMed] [Google Scholar]; h) Xie W; Jiang G; Liu H; Hu J; Pan X; Zhang H; Wan X; Lai Yi.; Mae D, Highly Enantioselective Bromocyclization of Tryptamines and Its Application in the Synthesis of (−)-Chimonanthine Angew. Chem. Int. Ed 2013, 52, 12924–12927; [DOI] [PubMed] [Google Scholar]; i) Mitsunuma H; Shibasaki M; Kanai M; Matsunaga S, Catalytic Asymmetric Total Synthesis of Chimonanthine, Folicanthine, and Calycanthine through Double Michael Reaction of Bisoxindole. Angew. Chem. Int. Ed 2012, 51, 5217–5221; [DOI] [PubMed] [Google Scholar]; j) Zong L; Du S; Chin KF; Wang C; Tan C-H; Enantioselective Synthesis of Quaternary Carbon Stereocenters: Addition of 3-Substituted Oxindoles to Vinyl Sulfone Catalyzed by Pentanidiums. Angew. Chem. Int. Ed 2015, 54, 9390–9393; [DOI] [PubMed] [Google Scholar]; k) Yu J-S; Liao F-M; Gao W-M; Liao K; Zuo R-L; Zhou J, Michael Addition Catalyzed by Chiral Secondary Amine Phosphoramide Using Fluorinated Silyl Enol Ethers: Formation of Quaternary Carbon Stereocenters. Angew. Chem. Int. Ed 2015, 54, 7381–7385. [DOI] [PubMed] [Google Scholar]; l) Engl OD; Fritz SP; Wennemers H, Stereoselective Organocatalytic Synthesis of Oxindoles with Adjacent Tetrasubstituted Stereocenters. Angew. Chem. Int. Ed 2015, 54, 8193–8197. [DOI] [PubMed] [Google Scholar]; m) Wu M-Y; He W-W; Liu X-Y; Tan B, Asymmetric Construction of Spirooxindoles by Organocatalytic Multicomponent Reactions Using Diazooxindoles. Angew. Chem. Int. Ed 2015, 54, 9409–9413; [DOI] [PubMed] [Google Scholar]; n) Biswas P; Paul S; Guin J, Aerobic Radical-Cascade Alkylation/Cyclization of α,β-Unsaturated Amides: an Efficient Approach to Quaternary Oxindoles. Angew. Chem. Int. Ed 2016, 55, 7756–7760. [DOI] [PubMed] [Google Scholar]; o) Sankar MG; Garcia-Castro M; Golz C; Strohmann C; Kumar K, Engaging Allene-Derived Zwitterions in an Unprecedented Mode of Asymmetric [3+2]-Annulation Reaction. Angew. Chem. Int. Ed 2016, 55, 9709–9713. [DOI] [PubMed] [Google Scholar]; p) Kong W; Wang Q; Zhu J, Synthesis of Diversely Functionalized Oxindoles Enabled by Migratory Insertion of Isocyanide to a Transient σ-Alkylpalladium(II) Complex. Angew. Chem. Int. Ed 2016, 55, 9714–9718. [DOI] [PubMed] [Google Scholar]
- (12).Selected examples:; a) Jiang X; Boehm P; Hartwig JF, Stereodivergent Allylation of Azaaryl Acetamides and Acetates by Synergistic Iridium and Copper Catalysis. J. Am. Chem. Soc 2018, 140, 1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wei L; Zhu Q; Xu S-M; Chang X; Wang C-J, Stereodivergent Synthesis of α,α-Disubstituted α-Amino Acids via Synergistic Cu/Ir Catalysis. J. Am. Chem. Soc 2018, 140, 1508–1513. [DOI] [PubMed] [Google Scholar]; c) Huo X; Zhang J; Fu J; He R; Zhang W, Ir/Cu Dual Catalysis: Enantio- and Diastereodivergent Access to α,α-Disubstituted α-Amino Acids Bearing Vicinal Stereocenters. J. Am. Chem. Soc 2018, 140, 2080–2084. [DOI] [PubMed] [Google Scholar]; d) Itoh T; Kanzaki Y; Shimizu Y; Kanai M, Copper(I)-Catalyzed Enantio- and Diastereodivergent Borylative Coupling of Styrenes and Imines. Angew. Chem. Int. Ed 2018, 57, 8265–8269. [DOI] [PubMed] [Google Scholar]; For a perspective on stereodivergent asymmetric catalysis:; e) Krautwald S; Carreira EM, Stereodivergence in Asymmetric Catalysis. J. Am. Chem. Soc 2017, 139, 5627–5639. [DOI] [PubMed] [Google Scholar]; Diastereodivergent Mannich reactions with carbonyl derived enolates:; f) Yan X-X; Peng Q; Li Q; Zhang K; Yao J; Hou X-L; Wu Y-D, Highly Diastereoselective Switchable Enantioselective Mannich Reaction of Glycine Derivatives with Imines. J. Am. Chem. Soc 2008, 130, 14362–14363. [DOI] [PubMed] [Google Scholar]; g) Nojiri A; Kumagai N; Shibasaki M, Linking Structural Dynamics and Functional Diversity in Asymmetric Catalysis. J. Am. Chem. Soc 2009, 131, 3779–3784. [DOI] [PubMed] [Google Scholar]; h) Lu G; Yoshino T; Morimoto H; Matsunaga S, Stereodivergent Direct Catalytic Asymmetric Mannich-Type Reactions of α-Isothiocyanato Ester with Ketimines. Angew. Chem. Int. Ed 2011, 50, 4382–4385. [DOI] [PubMed] [Google Scholar]; i) Kano T; Song S; Kubota Y; Maruoka K, Highly Diastereo- and Enantioselective Mannich Reactions of Synthetically Flexible Ketimines with Secondary Amine Organocatalysts. Angew. Chem. Int. Ed 2012, 51, 1191–1194. [DOI] [PubMed] [Google Scholar]
- (13).The CCDC numbers for the compounds are 1885326 (3ab), 1885325 (3lb) and 1885980 (3ic). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre.
- (14).a) Caddick S; Haynes AKK; Judd DB; Williams MRV, Convenient Synthesis of Protected Primary Amines from Nitriles. Tetrahedron Lett. 2000, 41, 3513–3516; [Google Scholar]; b) Caddick S; Judd DB; Lewis AKK; Reich MT; Williams MRV, A Generic Approach for the Catalytic Reduction of Nitriles. Tetrahedron 2003, 59, 5417–5423. [Google Scholar]
- (15).a) Kitamura H; Kato A; Esaki T, AG-041R, a Novel Indoline-2-one Derivative, Induces Systemic Cartilage Hyperplasia in Rats. Eur. J. Pharmacol 2001, 418, 225–230. [DOI] [PubMed] [Google Scholar]; b) Kitamura H; Okazaki M, AG-041R, a Novel Indoline-2-one Derivative, Stimulates Chondrogenesis in a Bipotent Chondroprogenitor Cell Line CL-1. Osteoarthr. Cartil 2005, 4, 287–296. [DOI] [PubMed] [Google Scholar]
- (16).a) Kim ES; Kim HS; Kim JN, An Efficient Pd-Catalyzed Hydration of Nitrile with Acetaldoxime. Tetrahedron Lett. 2009, 50, 2973–2975; [Google Scholar]; b) Kim ES; Lee HS; Kim JN, An Efficient Synthesis of Baylis-Hillman Adducts of Acrylamide: Pd-Catalyzed Hydration of Baylis-Hillman Adducts of Acrylonitrile. Tetrahedron Lett. 2009, 50, 6286–6289. [Google Scholar]
- (17).For biological significance of fluorinated amino acids see:; a) Odar C; Winkler M; Wiltschi B, Fluoro Amino Acids: A Rarity in Nature, yet a Prospect for Protein Engineering. Biotechnol. J 2015, 10, 427–446; [DOI] [PubMed] [Google Scholar]; b) Berger AA; Völler J-S; Budisa N; Koksch B, Deciphering the Fluorine Code—The Many Hats Fluorine Wears in a Protein Environment. Acc. Chem. Res 2017, 50, 2093–2103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


