Abstract
Background:
Heart failure (HF) leads to mitochondrial dysfunction and metabolic abnormalities of the failing myocardium coupled with an energy-depleted state and cardiac remodeling. The mitochondrial deacetylase sirtuin 3 (SIRT3) plays a pivotal role in the maintenance of mitochondrial function through regulating the mitochondrial acetylome. Interestingly, unique cardiac and systemic miRNAs have been shown to play an important role in cardiac remodeling by modulating key signaling elements in the myocardium.
Methods:
Cellular signaling was analyzed in human cardiomyocyte-like AC16 cells and acetylation levels in rodent models of SIRT3−/− and transgenic miR-195 overexpression were compared to WT. Luciferase assays, western blotting, immunoprecipitation assays and echocardiographic analysis were performed. Enzymatic activities of pyruvate dehydrogenase (PDH) and ATP synthase were measured.
Results:
In failing human myocardium, we observed induction of miR-195 along with decreased expression of the mitochondrial deacetylase SIRT3 that was associated with increased global protein acetylation. We further investigated the role of miR-195 in SIRT3-mediated metabolic processes and its impact on regulating enzymes involved in deacetylation. Proteomic analysis of the total acetylome showed increased overall acetylation, as well as specific lysine acetylation of two central mitochondrial metabolic enzymes: PDH and ATP synthase. miR-195 downregulates SIRT3 expression through direct 3’UTR targeting. Treatments with either sirtuin inhibitor nicotinamide, siRNA-mediated SIRT3 knockdown or miR-195 overexpression enhanced acetylation of PDH complex and ATP synthase. This effect diminished PDH and ATP synthase activity and impaired mitochondrial respiration. Consistently, SIRT3−/− and miR-195 transgenic mice showed enhanced global protein acetylation, including PDH complex and ATP synthase, associated with decreased enzymatic activity.
Conclusions:
Altogether, these data suggest that increased levels of miR-195 in failing myocardium regulate a novel pathway that involves direct SIRT3 suppression and enzymatic inhibition via increased acetylation of PDH and ATP synthase that are essential for cardiac energy metabolism.
Keywords: miRNA, sirtuin, oxidative phosphorylation, metabolism, heart failure, Myocardial Biology, Metabolism, Heart Failure
Heart failure (HF) is a clinical syndrome with rising incidence and prevalence and high morbidity and mortality rates. Caused by cardiac dysfunction, multiple organ systems are affected resulting in malfunction with devastating consequences1. Besides functional and structural changes in the failing myocardium, cardiac metabolism is impaired resulting in energy-depletion2. Through physiological myocardial energy metabolism, the heart acquires 6 kg of ATP per day in order to sustain its regular functions. A deprivation of cardiac ATP stores is tightly coupled to progressive mechanical failure 2. Mitochondrial metabolic dysfunction plays a central role, as it constitutes the cellular organelle that coordinates multiple metabolic systems and enzymes involved in substrate utilization and oxidative phosphorylation. The primary sources for ATP synthesis in the normal myocardium are fatty acids (around 70%) with glucose, lactate, ketone bodies accounting for the rest3. In the failing myocardium, there is a shift towards enhanced glycolytic flux4 and reduced fatty acid oxidation5–7, oxidative phosphorylation and ATP synthesis8–10.
Protein acetylation is the transfer of an acetyl group from acetyl-CoA to the ɛ-amino group on lysine residues which neutralizes the positive charge of lysine. This molecular mechanism was first discovered as a post-translational modification affecting chromatin remodeling and transcription in histones11, 12. Recent studies revealed that acetylation is also a widespread and evolutionarily conserved posttranslational modification of extranuclear proteins13. This modification regulates several cellular functions such as energy production, oxidative stress, angiogenesis, autophagy and cell death and survival14. High throughput proteomic assessments showed that protein acetylation has profound regulatory consequences in enzymes involved in major metabolic pathways13, 15–17.
In mammalian cells, seven sirtuin (SIRT1–7) homologs of the yeast Sirt2 gene regulate protein translational modifications including acetylation, ADP-ribosylation, malonylation and succinylation18. The dependence of sirtuins on NAD+ makes their enzymatic activity particularly sensitive to dynamic energy fluctuations in metabolism19, 20. The sirtuin inhibitor nicotinamide (NAM) has been involved in the regulation of energetic metabolism by directly regulating sirtuin activity21–25. Of the SIRT family, SIRT3, SIRT4 and SIRT5 are present in mitochondria26–29. SIRT3 is the major mitochondrial deacetylase while SIRT4 functions as an ADP-ribosyltransferase30, 31 and SIRT5 mediates malonylation and succinylation32, 33. Full-length SIRT3 is a 44 kDa protein with a mitochondrial localization sequence which is cleaved to generate a 28 kDa active SIRT3 deacetylase when imported into mitochondria34–36. SIRT3 has been shown to deacetylate and activate the enzymes of key mitochondrial metabolic pathways, such as fatty acid oxidation (long-chain acyl coenzyme A dehydrogenase (LCAD)37), TCA cycle (Succinate dehydrogenase (SDH)38), isocitrate dehydrogenase 2 (IDH2)39), and acetyl-CoA synthetase 2 (AceCS2)40), ETC enzymes (NDUFA9 and NDUFS141), and the antioxidant manganese superoxide dismutase (MnSOD)42, 43. The role of SIRT3 in protein deacetylation and involvement in a wide variety of physiological functions and diseases has indicated this deacetylase as a potential therapeutic application.
MiRNA-mediated control of metabolic processes has recently gained increasing interest. In prior studies, miR-195 has been shown to be induced in response to transverse aortic constriction44, 45 and was shown to be a miR induced in plasma, serum and myocardium of patients with advanced HF46. MicroRNA-195 (miR-195) is a member of the micro-15/16/195/424/497 family, stress-inducible and activated in multiple diseases, such as cancers, heart failure, and schizophrenia47. Overexpression of miR-195 is sufficient to induce a dose-dependent hypertrophy in cultured cardiomyocytes and cardio-specific miR-195 overexpression resulted in pathological cardiac growth with disorganization of cardiomyocytes and the development of HF44. Interestingly, miR-195 regulates SIRT1 by targeting the 3’UTR of SIRT1 mRNA in cardiomyocytes48, 49 through a binding motif that is not unique to SIRT1.
In this paper, we aimed to analyze the function of miR-195 in regulating SIRT3 expression and SIRT3-mediated deacetylation of specific targets and study the impact of acetylation on the cardiac acetylome, myocardial metabolism and function.
Methods
The data, analytic methods, and study materials will be/have been made available to other researchers for purposes of reproducing the results or replicating the procedure. The authors declare that all supporting data are available within the article and its online supplementary files.
Patient cohort
Patients with advanced HF were recruited at Columbia University Medical Center. Myocardial specimens were collected from all patients (n=12) at the time of LVAD implantation for end-stage HF. Control myocardial samples (n=5) were obtained from de-identified specimens collected from non-failing hearts determined to be unusable for cardiac transplantation due to acute recipient issues or donor coronary artery disease, but without evidence of previous cardiac disease (Supplementary Table 1).
The present study was approved by the Institutional Review Board of Columbia University. All patients provided written informed consent before inclusion into the study.
Animal studies
Transverse aortic constriction (TAC) and myocardial infarction (MI) models were used. Transverse aortic banding or ligation of the left descending coronary artery or sham surgery was performed in C57B/L6 mice (Jackson Laboratory, Bar Harbor, Maine, age 10–12 weeks). Animals were anesthetized with a combination of ketamine (80 mg/kg) and xylazine (10 mg/kg) injected intraperitoneally. Pressure controlled ventilation was initiated after intubation at 15 cm H2O. After chest opening, a 9–0 prolene suture was placed around the aortic arch or around the left anterior descending coronary artery 2 mm below the left atrium and ligated. Sham surgery was performed without ligation. Chest closure was performed by suturing together adjacent ribs and the skin. Mice were sacrificed 6 weeks after surgery.
In SIRT3 studies, control mice 129S1/SvlmJ and SIRT3 KO mice 129-Sirt3<tm1.1Fwa>/J were sacrificed at age of 10–12 weeks. In miR-195 studies, a cardiac-specific expression plasmid containing the α-MHC, human growth hormone (GH) poly(A)+ signal and a mouse genomic fragment flanking the miR of interests was used for the generation of transgenic mice (kind donation from the Olson laboratory, UT Southwestern Dallas). Mice were monitored by echocardiography for the development of cardiac failure at age of 8–10 weeks, and sacrificed at age of 10–12 weeks.
The protocol (AAAR3420) was approved by the Columbia University Institutional Animal Care and Use Committee.
Cell culture
AC16 human cardiomyocyte-like cells50 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), and grown in a CO2 incubator maintained at atmospheric oxygen levels and 5% CO2.
AC16 cells were exposed to 100 nM Angiotensin II (Ang II) (Sigma-Aldrich) or vehicle (PBS). Whole cell lysates were prepared after 48 h of treatment. NAM (Sigma-Aldrich) was dissolved in phosphate-buffer saline (PBS). Cells were treated with different concentration of NAM (10, 20, and 40 mM) or vehicle (PBS) as described51 for 20 h.
siRNA and vector transfection
Cells were transfected with siRNA targeting SIRT3 or non-targeting control siRNA (Dharmacon); Firefly/Renilla Duo-Luciferase reporter vector with SIRT3 3’UTR sequences or vector alone; precursor miR-195 clones in non-viral vectors or precursor miR scrambled control clones (GeneCopoeia).
Knockdown of miR-195
Cultures at 30% confluence were transfected with anti-miR miRNA inhibitor of miR-195 or nontargeting control (Ambion). The anti-miR miRNA inhibitor negative control contains a random sequence validated to produce no identifiable effects on known functions of miRNAs.
Mitochondria isolation
Cells were washed and harvested in PBS. Cell pellet collected by centrifugation (2000 rpm, 5 min, 4 °C) were resuspended in RIPA buffer (Thermo Scientific), supplemented with protease inhibitor (cOmplete tablet, Roche) and incubated on ice for 30 min. After centrifugation (13200 rpm, 30 min, and 4 °C) the supernatant was saved as whole cell lysate. Mitochondria isolation kit (Thermo Scientific) was used for mitochondria fraction preparation.
Immunoprecipitation and immunoblot analysis
100 μg of total protein from whole cell lysate or mitochondria fraction was immunoprecipitated. The extract was incubated for 16 h at 4 °C with anti-acetylated-lysine (Cell Signaling) followed by addition of protein G beads and incubated further for 6 h at 4 °C. The beads were centrifuged at 2000 rpm for 2 min and washed three times in PBS buffer. The beads were recovered by centrifugation and aliquots of pellets were analyzed by SDS–PAGE and immunoblotting. For Western blotting, anti-acetylated-lysine (Cell Signaling), anti-SIRT2, anti-SIRT3, anti-SIRT5, anti- SIRT6 (Cell Signaling), anti-COX IV (Cell Signaling), HRP conjugated anti-GAPDH (Cell Signaling), anti-APT5A (Abcam) and anti-PDH cocktail antibodies (Abcam) were used for detection.
Enzymatic activity assay
Pyruvate dehydrogenase enzyme activity microplate assay kit (Abcam) was used. Briefly, samples were prepared through detergent extraction, loaded on a capture antibody pre-coated microplate, and incubated 3 h at room temperature (RT). Then wells were washed, and assay solution was added. Absorbance at 450 nm was measured with 20 sec intervals.
ATP synthase enzyme activity microplate assay kit (Abcam) was used. Briefly, samples were prepared through detergent extraction, loaded on a capture antibody pre-coated microplate, and incubated 3 h at RT. Then wells were washed, and lipid mix was added. Following 45 min incubation at RT, reagent mix was mixed into each well. Absorbance at 340 nm was measured with 1 min intervals.
Measurement of Cellular Respiration
Oxygen consumption rate (OCR) was determined using a Seahorse Bioscience XF24 Extracellular Flux analyzer. AC16 cells were plated on XF24 microplates at 5.0×104 cells/well in low glucose (1 g/L) DMEM supplemented with 1% FBS, penicillin (10 U/mL) and streptomycin (10 U/mL) and 24 h before measurement. Intact cellular respiration was assayed under basal conditions (10 mM D-glucose, 10 mM pyruvate, 0% serum) and after the administration of various drugs as following: mitochondrial inhibitor oligomycin (oligo) (1 μM), mitochondrial uncoupler carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) (1 μM), and respiratory chain inhibitor antimycin A (AA) (1 μM) and rotenone (ROT) (1 μM).
Respiratory parameters were quantified by subtracting respiration rates at times before and after addition of electron transport chain inhibitors according to Seahorse Biosciences; basal respiration: baseline respiration minus AA-dependent respiration; ATP turnover: baseline respiration minus oligo-dependent respiration; H+ leak: oligo-dependent respiration minus AA-dependent respiration; respiratory capacity: FCCP-dependent respiration minus AA-dependent respiration.
qRT-PCR assays
Equivalent amounts (2 μg) of purified RNA were used as a template to synthesize cDNA using oligo-d(T) primers and SuperScript III / RNaseOUT Enzyme Mix (Invitrogen). Relative levels were calculated using ΔCτ method. Primer sequences are provided in Supplemental Material (Supplemental Table 2).
For miR quantification, total RNA was purified using RNeasy Mini Kit (QIAGEN). miRs abundance was assessed by qRT-PCR using All-in-One miR qRT-PCR Reagent Kits and Validated Primers (GeneCopoeia). miR-191 was used as normalization.
Luciferase Assay
Cells were co-transfected with the luciferase constructs and either a scrambled control miR or miR-195 overexpression plasmids. Cells were harvested after 48 h and a dual luciferase assay was performed using a Luc-pair miR Luciferase kit (GeneCopoeia). The expression of Renilla luciferase served as control.
Statistical Analysis
Results were expressed as mean±SEM. Probability values of p<0.05 were considered significant. Comparison among two groups was calculated and statistically compared using 2-tailed Student’s test. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc.).
Results
Increased miR-195 suppresses SIRT3 expression and increases acetylation
Through an unbiased screening of myocardial and circulating miRs in patients with advanced HF and controls, we identified a cluster of miRs differentially regulated in failing myocardium and another set in the circulation through deep-sequencing of non-coding RNAs46. miR-195 was found to be induced in the failing myocardium of patients with HF (3.04-fold increase) and in animal models of pressure-overload cardiomyopathy following transaortic banding and ischemic myocardium(2.01-fold after TAC and 2.09-fold after MI, Figure 1A). It showed high abundance suggesting that this miR may represent a molecular signature of failing myocardium.
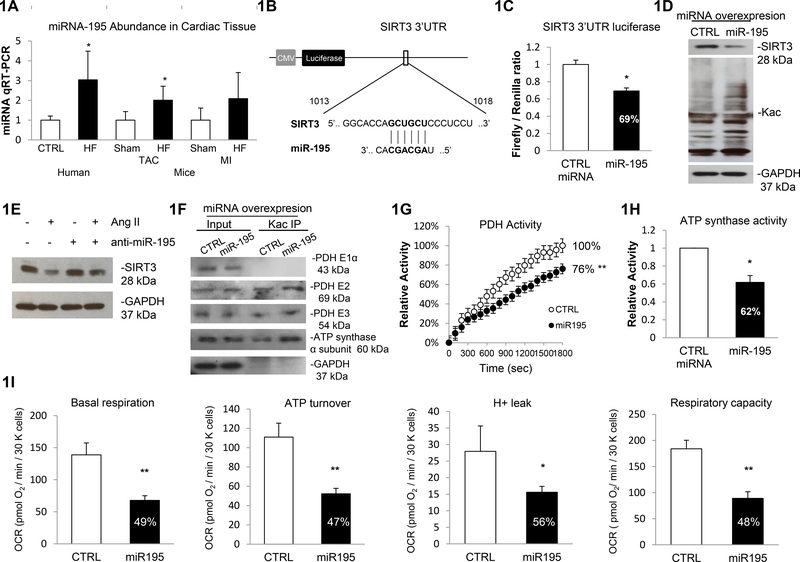
Figure 1. Increased miR-195 suppresses SIRT3 expression and increases acetylation.
1A. miR-195 abundance in human cardiac tissue was assessed by qRT-PCR, which detected a 3.04-fold increase (p<0.05) in patients with HF. Control n=5, HF n=11. Cardiac miR-195 abundance in mouse model was assessed by qRT-PCR, which detected a 2.01-fold (p<0.05) and 2.09-fold elevation of the mature miR-195 in TAC HF mice and MI HF mice, compared to TAC Sham and MI Sham mice respectively. miR-191 was used as normalization. 1B. Prediction of SIRT3 as a target of miR-195. The prediction was conducted by using data from microRNA target prediction Systems (www.microrna.org). 1C. Luciferase assay confirmed miR-195 association at SIRT3 mRNA 3’UTR. Constructs carrying the SIRT3 3’ UTR or not (vector) were co-transfected with scramble miR control or miR-195 precursor in AC16 cells. The ratios of the firefly/Renilla values for the SIRT3 construct relative to the vector construct were shown. The firefly/Renilla values were calculated from three independent samples. Errors represent the SD derived from three independent experiments and p<0.05. 1D. miR-195 overexpression induced global protein acetylation. Cells were transfected with overexpression vector containing miR-195 precursor or scramble miR control, and the whole cell lysates were prepared. Global acetylation level and SIRT3 expression were analyzed by western blot and GAPDH was used as loading control. 1E. Silencing of miR-195 rescues SIRT3 expression in response to Ang II stimulation. AC16 cells were treated with Ang II or/and anti-miR-195 or miRNA inhibitor negative control. Whole cell lysates were prepared and the SIRT3 expression was analyzed by western blot and GAPDH was used as loading control. 1F. PDH complex and ATP synthase α subunit were more acetylation in cells overexpressing miR-195. A representative Kac immunoprecipitation (IP) reaction from three independent assays was shown. Cell lysates prepared from miR-195/scramble control miR transfected cell were subjected to IP assay using anti-Acetylated-Lysine (anti-Kac). Equivalent amounts of the pellets (IP) were analyzed by western blotting. 10% of the cell lysate used in the IP reaction was shown as input. 1G. miR-195 overexpression resulted in 24% decrease in PDH activity. Errors represent the SD derived from three independent experiments and p<0.01. 1H. miR-195 overexpression resulted in 38% decrease in ATP synthase activity. Errors represent the SD derived from three independent experiments and p<0.05. 1I. Basal Raspiration, ATP turnover, H+ leak and respiratory capacity were all significantly decreased in AC16 cells overexpression miR-195. The OCR was measured as described before. Measurements were made in triplicate (mean ans s.d.), and results were indicative of three independent experiments (p<0.05 or p<0.01). * represents p<0.05, ** represents p<0.01.
Computational prediction of targets of miR-195 identified a putative-binding site of miR-195 in the SIRT3 mRNA 3’UTR (www.microRNA.org, as shown in Figure 1B). We, therefore, performed luciferase reporter activity assays to validate this prediction. In cardiomyocyte-like AC16 cells, miR-195 or scrambled miR overexpression vectors were co-transfected with reporter constructs containing a luciferase gene under the control of either the SIRT3 3’UTR or control vector 3’UTR. Luciferase activity assays showed a significant repression (−31%) in firefly/renilla ratio of SIRT3 3’UTR vector normalized with control vector in cells overexpressing miR-195 relative to the control miR (Figure 1C). This result indicates that miR-195 directly targets at SIRT3 mRNA 3’UTR and negatively regulates SIRT3 expression. In agreement with these data, overexpression of miR-195 resulted in a pronounced decrease in SIRT3 protein levels (−54%) accompanied by increased total protein acetylation (+53%) (Figure 1D, Supplementary Figure 1). miR-195 expression was induced in cells transfected with miR-195 overexpression vectors as determined by qRT-PCR (14.2-fold, Supplementary Figure 2).
It has been reported that the expression of SIRT3 was reduced in Ang II treated cardiomyocytes and in hearts of Ang II-induced cardiac hypertrophic mice52, 53. Interestingly, other groups have shown that Ang II significantly promoted the expression of miR-195 level in mice cardia tissues54, 55. In order to investigate whether Ang II induced miR-195 stimulation has critical impact on the downregulation of SIRT3, we assessed SIRT3 expression in cultured cells transfected with miRNA inhibitor of miR-195 in response to Ang II stimulation. Consistent with previous reports, we have confirmed the elevated miR-195 abundance in AC16 cells treated with Ang II (Supplementary Figure 3). We also showed SIRT3 expression level decreased with Ang II induction (−32%). Interestingly, silencing of miR-195 alleviated the decrease of SIRT3 expression induced by Ang II stimulation (−12%), thereby indicating a causative role of miR-195 in modulating SIRT3 expression (Figure 1E and Supplementary Figure 4).
Specific hyperacetylation of pyruvate dehydrogenase (PDH) complex and ATP synthase, key enzymes of myocardial energy metabolism, were confirmed by immunoprecipitation using an antibody against acetylated lysine (Kac) followed by Western blotting, showing an increase in acetylation levels of dihydrolipoyl transacetylase (PDH E2, +75%), dihydrolipoyl dehydrogenase (PDH E3, +38%) and ATP synthase α subunit (+77%) in response to miR-195 overexpression (Figure 1F, Supplementary Figure 5). The enhanced acetylation resulted in a 24% decrease in PDH activity (Figure 1G) and a 38% decrease in ATP synthase activity (Figure 1H). By using a Seahorse XF24 analyzer, we quantified the respiration parameters which indicated that basal respiration (−51%), ATP production (−53%), H+ leak (−44%) and respiratory capacity (−52%) were all decreased in miR-195 overexpressed cells (Figure 1I).
Hyperacetylation and reduced enzymatic activities in miR-195 overexpressing mice
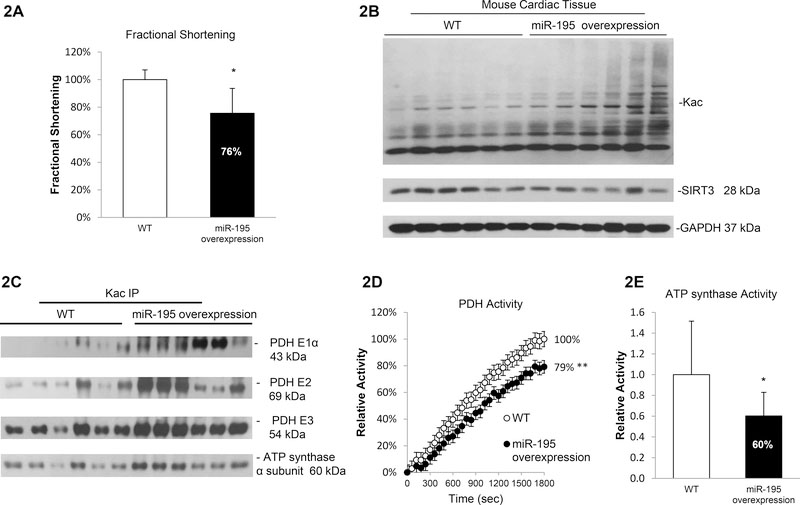
To further investigate the role of miR-195 in vivo, we analyzed the regulation of acetylation in a cardiac specific miR-195 overexpression mouse model. Echocardiography on animals at age of 8–10 weeks showed that miR-195 transgenic mice displayed decreased fractional shortening (−24%), indicating impaired cardiac function and the potential of heart failure during development (Figure 2A, Supplementary Table 3). We also observed a trend towards an increase in lung weight in miR-195 overexpression mice compared to wild type, suggesting the miR-195 transgenic mice have cardiomyopathy and failure (Supplementary Figure 6). Myocardium of miR-195 transgenic mice showed reduced SIRT3 expression (−34%) and a global increase in protein acetylation (+57%) (Figure 2B, Supplementary Figure 7) along with a 7.8-fold elevation in miR-195 levels (Supplementary Figure 8). Immunoprecipitation assays showed increased protein acetylation of pyruvate dehydrogenase E1α (PDH E1α, +289%), PDH E2 (+151%), PDH E3 (+51%) and ATP synthase α subunit (+53%) (Figure 2C and Supplementary Figure 9 and 10) in myocardium of miR-195 overexpression mice. PDH activity in miR-195 transgenic mice showed a 21% decrease (Figure 2D and Supplementary Figure 11) while ATP synthase activity showed a 40% decrease (Figure 2E and Supplementary Figure 12). Taken together, these data demonstrate a crucial role for miR-195 in cardiomyocytes and identify SIRT3 as a direct target of miR-195 leading to hyperacetylation of key metabolic proteins.
Figure 2. Hyperacetylation and reduced enzymatic activities in miR-195 overexpressing mice.
2A. Echocardiographic analysis showed decreased fractional shortening (p<0.05) in miR-195 transgenic mice compared with WT littermates. Mice were monitored by echocardiography for development of failure at age of 8–10 weeks. 2B. Increased protein acetylation and decreased SIRT3 expression in miR-195 transgenic mice. Whole cell lysates were prepared from heart tissue of WT or miR-195 overexpression mice. Global acetylation level and SIRT3 expression were analyzed by western blotting. GAPDH was used as loading control. 2C. Induced acetylation in PDH complex and ATP synthase α subunit in miR-195 transgenic mice. Whole cell lysates were prepared from heart tissue of WT or miR-195 overexpression mice and subjected to IP assay using anti-Kac. Equivalent amounts of the pellets (IP) were resolved by SDS/PAGE and proteins were detected by immunoblotting. 2D. miR-195 overexpression mice showed a 21% decrease in PDH activity. Errors represent the SD derived from three independent experiments and p<0.01. 2E. miR-195 overexpression mice showed a 40% decrease in ATP synthase activity. Errors represent the SD derived from three independent experiments and p<0.05. * represents p<0.05, ** represents p<0.01.
Increased protein acetylation and decreased SIRT3 expression in the failing myocardium
We next hypothesized that the miR-195 regulated expression of the mitochondrial deacetylase SIRT3 alters acetylation levels of key mitochondrial enzymes contributing to abnormal cardiac metabolism and function. To test our hypothesis, we analyzed SIRT3 expression and global protein acetylation in both animal models of cardiomyopathies and human failing myocardium. In myocardium of mice following TAC and MI, we detected elevated total protein acetylation (+41% after TAC and +51% after MI) that was accompanied by reduced SIRT3 levels (−19% after TAC and –39% after MI, Figure 3A and Supplementary Figure 13). Interestingly, the myocardium of heart failure mice induced by TAC or MI both showed decreased SIRT3 expression levels and broad protein acetylation enhancement, indicating the decreased SIRT3 expression and increased protein acetylation are general responses to the development of heart failure. Similarly, failing human myocardium showed broad protein acetylation levels compared to control myocardium (+272%, Figure 3B). Testing expression levels of various members of the sirtuin deacetylase family revealed decreased expression of SIRT3 in failing human myocardium (−46%, Figure 3B and Supplementary Figure 14 and 15).
Figure 3. Increased protein acetylation and decreased SIRT3 expression in failing myocardium.
3A. Increased protein acetylation and decreased SIRT3 expression in heart failure animal model. Whole cell lysates were prepared from heart tissue of sham or TAC or MI mice. Global acetylation level and SIRT3 expression were analyzed by western blotting. GAPDH was used as loading control. 3B. Increased protein acetylation and decreased SIRT3 expression in human samples. Whole cell lysates were prepared from heart tissue of s normal or HF patients. Global acetylation level and SIRTs expression were analyzed by western blotting. GAPDH was used as loading control. 3C. Pathway analysis of top 5 acetylation-related pathway networks in failing myocardium compared to normal. Normal and failing heart tissues were homogenized and the acetylated proteins were enriched by anti-Kac antibody. Statistical analysis of the total spectra counts showed networks with at least 1.5 fold increased in acetylation (p <0.01).
We next performed a global analysis of lysine acetylation profile of mitochondrial proteins in human failing (n=4) and non-failing myocardium (n=4). Proteomic analysis by mass spectrometry showed lysine acetylation of several key enzymes involved in fatty acid and glucose metabolism (Table 1). Pathway network analysis for proteins with at least 1.5-fold increase in acetylation in the failing myocardium showed that oxidative phosphorylation proteins were the most significantly acetylated (Figure 3C). Taken together, these data suggest that mitochondrial hyperacetylation in human HF is associated with changes in levels of the deacetylase SIRT3.
Table 1.
Proteomic analysis of acetylated mitochondrial enzymes involved in fatty acid and glucose metabolism.
| Proteomic Analysis Mitochondrial Enzymes with Increased Acetylation | |
|---|---|
| ECHS1 | Enoyl-CoA hydratase, mitochondrial ECHS1 |
| PDH | Pyruvate dehydrogenase E1 component subunit alpha |
| Pyruvate dehydrogenase E1 component subunit beta | |
| Dihydrolipoyl dehydrogenase, Pyruvate dehydrogenase E3 | |
| ATP synthase | ATP synthase subunit alpha, mitochondrial ATP5A1 |
| ATP synthase subunit beta, mitochondrial ATP5B | |
| ATP synthase subunit d, mitochondrial ATP5H | |
| ATP synthase subunit e, mitochondrial ATP5I | |
| ATP synthase subunit g, mitochondrial ATP5L | |
| ATP synthase subunit O, mitochondrial ATP5O | |
| ATP synthase lipid-binding protein, mitochondrial ATP5G1 | |
| CPT1 | Carnitine O-palmitoyltransferase 1 |
| MDH | Malate dehydrogenase, mitochondrial MDH2 |
Inhibition of sirtuin deacetylase function increases acetylation of multiple mitochondrial proteins and reduces specific enzymatic activity of PDH and ATP synthase
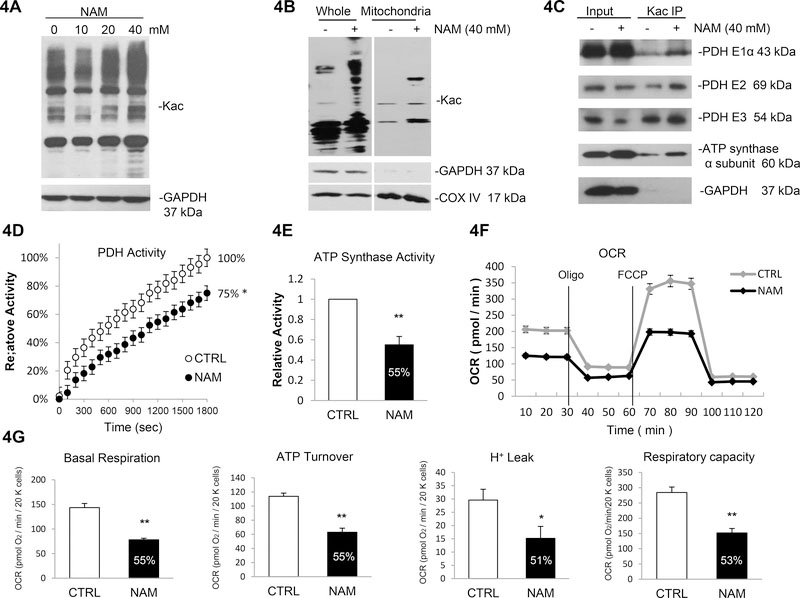
To further investigate the targets of sirtuin deacetylase, we used the potent sirtuin deacetylase inhibitor NAM in the following study. NAM may be considered a general inhibitor of the sirtuin family and its inhibitory effects have also been demonstrated for SIRT1, SIRT2 and SIRT356, 57. NAM treatment (40 mM) induced protein acetylation51 in a dose-dependent manner in both total cell lysates (+67%) and the mitochondrial fraction (+174%) (Figure 4A, Supplementary Figure 16, 4B and Supplementary Figure 17).
Figure 4. Sirtuin inhibitor Nicotinamide (NAM) induces acetylation of multiple mitochondrial proteins and reduces enzymatic activity.
4A. NAM induced acetylation in AC16 cell line. Cells were treated with increasing concentration of NAM or vehicle (PBS). Global acetylation level was analyzed by western blot. 4B. NAM induces acetylation of mitochondrial proteins. Whole cell lysates and mitochondrial fraction were isolated for western blotting analysis. 4C. Induced acetylation in PDH complex and ATP synthase α subunit in response to NAM treatment. AC16 cells were treated with NAM or vehicle; cell lysates were prepared and subjected to IP assay using anti-Kac. Equivalent amounts of the pellets (IP) were resolved by SDS/PAGE and proteins were detected by immunoblotting. 10% of the cell lysate used in the IP reaction was shown as input. 4D. 25% decrease in PDH activity in response to NAM treatment. PDH activity was measured following 20 h of NAM treatment or vehicle. Errors represent the SD derived from three independent experiments and p<0.05. 4E. 45% decrease in ATP synthase activity in response to NAM treatment. ATP synthase activity was measured following 20h of NAM treatment. Errors represent the SD derived from three independent experiments and p<0.01. 4F. Oxygen consumption rate (OCR) was decreased in NAM treated cells. The AC16 cells were seeded 24 h before analyzed by a Seahorse XF24 Analyzer. The OCR was measured continuously throughout the experimental period at baseline and in the presence of the indicated drugs. Oligo indicates oligomycin (1 μM); FCCP indicates carbonylcyanide p-trifluoromethoxyphenylhydrazone (1 μM); ROT + AA indicate mixture of rotenone (1 μM) and antimycin A (1 μM). 4G. Basal Raspiration, ATP turnover, H+ leak and respiratory capacity were all significantly decreased in NAM treated AC16 cells compared to control. Measurements were made in triplicate (mean ans s.d.), and results are indicative of three independent experiments (p<0.05 or p<0.01). * represents p<0.05, ** represents p<0.01.
In the current study, we specifically focused on the two rate limiting metabolic enzymes in acetyl-coA generation and ATP production identified in the prior screening: pyruvate dehydrogenase complex (PDC) and ATP synthase. Further, these enzymes were shown to be hyperacetylated in miR-195 transgenic mice (Figure 2C). Immunoprecipitation assays showed that NAM treatment inhibited deacetylase activity, which increased protein acetylation of PDH E1α (+76%), PDH E2 (+117%), PDH E3 (+145%) and ATP synthase α subunit (+50%) (Figure 4C and Supplementary Figure 18).
Consistent with these findings, acetylation of several subunits of PDH complex was associated with decreased PDH activity (−25%, Figure 4D). Given that ATP synthase is essential for mitochondrial energy generation, we tested ATP synthase activity, which was decreased in response to NAM (−45%, Figure 4E). We further measured lower oxygen consumption rate (OCR) and impaired mitochondrial respiration (Figure 4F), with significant reduction in basal respiration (−45%), ATP production (−45%), H+ leak (−49%) and respiratory capacity (−47%) following NAM treatment (Figure 4G).
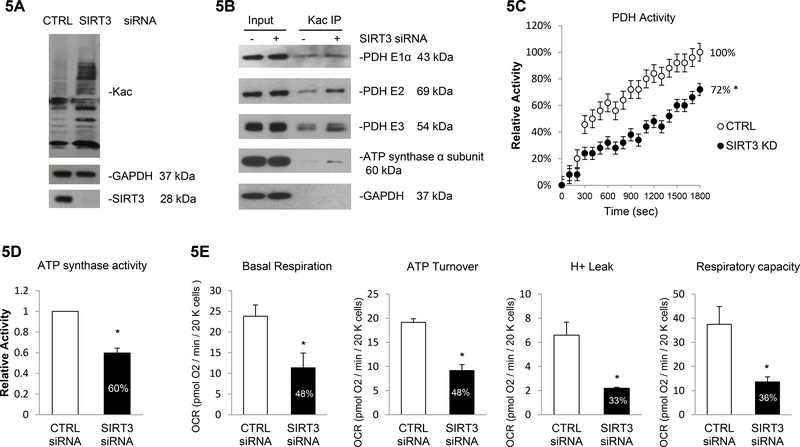
As more than one SIRT proteins exist in the heart, we next analyzed the specific impact of SIRT3 inhibition. Transfection with siRNA reduced SIRT3 protein expression (−93%, Figure 5A) without affecting SIRT2, SIRT5 and SIRT6 expression (Supplementary Figure 19), confirming a successful specific knockdown of mitochondrial deacetylase SIRT3. siRNA-mediated SIRT3 knockdown induced total protein acetylation (+127%, Figure 5A and Supplementary Figure 20). Immunopecipitation assay showed that siRNA-mediated deletion of SIRT3 enhanced acetylation of PDH E1α (+29%), E2 (+124%), E3 (+46%) and ATP synthase α subunit (+216%) (Figure 5B and Supplementary Figure 21), which was further associated with 28% decrease in PDH activity (Figure 5C) and 40% decrease in ATP synthase activity (Figure 5D). Correlated with the enhanced acetylation state of ATP synthase, the SIRT3 knockdown resulted in lower mitochondrial respiration with reduction in basal respiration (−52%), ATP production (−52%), H+ leak (−67%) and respiratory capacity (−64%) (Figure 5E).
Figure 5. SIRT3 deacetylates multiple mitochondrial proteins and regulates mitochondrial metabolism.
5A. SIRT3 knockdown induced global protein acetylation. Cells were transfected with siRNAs targeting SIRT3 or non-specific sequence, and the whole cell lysates were prepared. Global acetylation level and SIRT3 expression were analyzed by western blot and GAPDH was used as loading control. 5B. PDH complex and ATP synthase α subunit were more acetylation in SIRT3 depleted cells. A representative IP reaction from three independent assays was shown. Cell lysates prepared from SIRT3/CTRL siRNA treated cell were subjected to IP assay using anti-Kac. Equivalent amounts of the pellets (IP) were analyzed by western blotting as describe above. 10% of the cell lysate used in the IP reaction was shown as input. 5C. SIRT3 knockdown resulted in 28% decrease in PDH activity. Errors represent the SD derived from three independent experiments and p<0.05. 5D. SIRT3 knockdown resulted in 40% decrease in in ATP synthase activity. Errors represent the SD derived from three independent experiments and p<0.05. 5E. Basal Respiration, ATP turnover, H+ leak and respiratory capacity were all significantly decreased in AC16 cells depleted with SIRT3. The OCR was measured as described before. Measurements were made in triplicate (mean ans s.d.), and results were indicative of three independent experiments (p<0.05). * represents p<0.05.
These data suggest that inhibition of sirtuin induces hyperacetylation of PDH complex and ATP synthase α subunit leading to altered enzymatic activity and consequent decrease in mitochondrial function.
SIRT3 gene deletion mice showed increased protein acetylation and decreased enzymatic activity
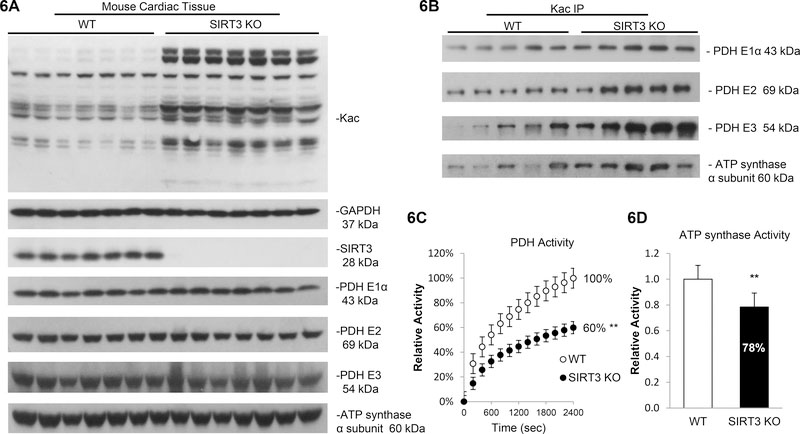
To further investigate the role of SIRT3 deacetylase in vivo, heart tissue from both WT and SIRT3 gene deletion mice were tested for protein acetylation levels. We first confirmed that the SIRT3 expression was completely abolished without affecting SIRT2, SIRT5 and SIRT6 expression (Figure 6A and Supplementary Figure 22). After detecting the global increase in total protein acetylation in SIRT3−/− mice (+174%, Figure 6A and Supplementary Figure 23), the acetylation level of SIRT3 targets, PDH complex and ATP synthase, were measured. Consistent with the results obtained using human cardiomyocyte-like AC16 cell line, PDH E1α (+59%), E2 (+65%), E3 (+279%) and ATP synthase α subunit (+88%) were more acetylated in SIRT3−/− mice (Figure 6B and Supplementary Figure 24), while their basal expression levels remained unchanged (Figure 6A and Supplementary Figure 23). Finally, we found that acetylation of PDH complex and ATP synthase induced by SIRT3 deletion was associated with a decrease in PDH (−40%, Figure 6C) and ATP synthase activity (−22%, Figure 6D). Together, these data suggest that SIRT3 may play a protective role in the myocardium by preventing key mitochondrial enzymes from acetylation and alteration in their activity in maintaining mitochondrial function.
Figure 6. SIRT3 knockout (KO) mice showed increased protein acetylation and decreased enzymatic activity.
6A. Total protein acetylation level strikingly increased in SIRT3 KO mice. Whole cell lysates were prepared from heart tissue of WT or SIRT3 KO mice. Global acetylation level, expression of SIRT3, PDH complex subunits and ATP synthase α subunit were analyzed by western blotting. GAPDH was used as loading control. 6B. PDH complex and ATP synthase α subunit were more acetylation in SIRT3 KO mice. Cell lysates prepared from WT or SIRT3 KO mice were subjected to IP assay using anti-Kac. Equivalent amounts of the pellets (IP) were analyzed by western blotting as describe above. 10% of the cell lysate used in the IP reaction was shown as input. 6C. SIRT3 KO mice showed 40% decrease in PDH activity. Errors represent the SD derived from three independent experiments and p<0.01. 6D. SIRT3 KO mice showed 22% decrease in in ATP synthase activity. Errors represent the SD derived from three independent experiments and p<0.01. ** represents p<0.01.
Increased acetylation and impaired metabolism in failing myocardium
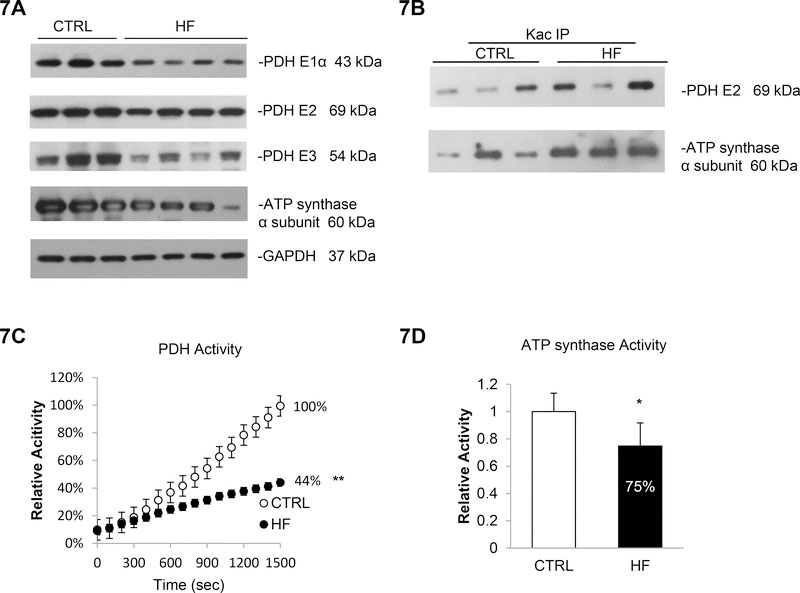
We expanded this study and investigated whether PDH complex and ATP synthase have altered acetylation status and function in human failing myocardium (n=4). The expression of PDH E1α (−53%), E2 (−14%) and E3 subunit (−41%) showed a mild decrease in heart tissue of HF patients compared to normal hearts (Figure 7A and Supplementary Figure 25). Moreover, an increase in the acetylation level of PDH E2 (+79%) was detected (Figure 7B top and Supplementary Figure 26). Both, the lower expression level and the increased acetylation contribute to a reduced PDH activity in HF patients (−56% compared to non-diseased myocardium; Figure 7C). In line with these changes in PDH complex, ATP synthase α subunit also showed reduced basal expression (−39%, Figure 7A and Supplementary Figure 25) and increased acetylation levels (+107%, Figure 7B bottom and Supplementary Figure 26) as well as a 25% decrease in ATP synthase activity (Figure 7D) in HF patients compared to normal hearts. Together, these results indicate that the alteration in PDH complex and ATP synthase function correlates with changes in both basal expression level and acetylation status contributing to mitochondrial dysfunction and cardiomyopathy.
Figure 7. Increased acetylation and impaired metabolism in failing myocardium.
7A. PDH complex subunits and ATP synthase α subunit were less expressed in failing myocardium. Whole cell lysate prepared from normal or HF patients’ heart tissues were analyzed by western blotting. GAPDH was used as loading control. 7B. PDH E2 and ATP synthase α subunit were more acetylation in failing myocardium. Cell lysates prepared from cardiac tissues were subjected to IP assay using anti-Kac. Equivalent amounts of the pellets (IP) were analyzed by western blotting as describe above. 10% of the cell lysate used in the IP reaction was shown as input. 7C. PDH activity was 56% reduced in HF patients. Errors represent the SD derived from three independent experiments and p<0.01. 7D. ATP synthase activity was 25% reduced in HF patients. Errors represent the SD derived from three independent experiments and p<0.05. * represents p<0.05, ** represents p<0.01.
Discussion
Mitochondrial dysfunction including metabolic abnormalities and energy depletion develop in the failing myocardium and contribute to cardiac remodeling. The major finding of our study is that miR-195 controlled suppression of SIRT3 in failing myocardium results in hyperacetylation of several key mitochondrial proteins. We demonstrated that miR-195 regulates myocardial SIRT3 expression through direct targeting at the SIRT3 mRNA 3’UTR. This effect leads to hyperacetylation of PDH and ATP synthase in failing myocardium and contributes to altered mitochondrial energy metabolism which is associated with cardiac dysfunction in humans.
We investigated the underlying mechanisms using several translational methods. First, via unbiased miR screening, we detected increased levels of miR-195 in the heart and plasma of HF patients. Bioinformatic analysis followed by in vitro experiments in human cardiomyocyte-like AC16 cells showed that miR-195 is a direct inhibitor of SIRT3 (Fig 1). Moreover, by treating cells with the SIRT3 inhibitor NAM, SIRT3 siRNA and miR-195, we demonstrated that SIRT3-mediates deacetylation of PDH and ATP synthase compromises their activity and impairs mitochondrial function (Fig 1, 4 and 5). Consistently, SIRT3 expression was reduced in human failing myocardium, in which we also observed increased acetylation and decreased activities of PDH and ATP synthase (Fig 3 and 7). We also showed that miR-195 transgenic mice exhibit reduced SIRT3 expression levels associated with a global increase in total protein acetylation compared to WT mice (Fig 2). This findings link the previously described cardiomyopathic phenotype of these mice44 to suppression of SIRT3, hyperacetylation of key metabolic enzymes and energy depletion. Finally, SIRT3 KO mice showed increased acetylation levels of PDH and ATP synthase which contributed to a decrease in enzymatic activity (Fig 6). Taken together, our data suggest a novel pathway of combined epigenetic, transcriptional and post-translational regulation of proteins that are involved in cardiac energy metabolism during HF development and progression. This pathway involves miR-195-mediated SIRT3 suppression and increased protein acetylation of PDH and ATP synthase. These changes inhibit PDH and ATP synthase activity leading to impaired energy metabolism and ATP deprivation.
Mitochondria are the main cellular organelles responsible for energy balance and metabolism homeostasis, and are implicated in several human diseases. The mitochondrial deacetylase SIRT3 plays a pivotal role in the maintenance of mitochondrial function. Several recent studies revealed SIRT3 as a major regulator of the mitochondrial acetylome controlling dynamics of metabolic reprogramming and antioxidant defense mechanisms by targeting a series of key modulators and their relevant pathways. For example, acetyl-CoA synthase 2 (AceCS2), 3-hydroxy-3-methylglutaryl CoA synthase 2 (HMGCS2), long-chain acyl CoA dehydrogenase (LCAD), Isocitrate dehydrogenase 2 (IDH2), glutamate dehydrogenase (GDH), NDUFA9 subunit of complex I of ETC, succinate dehydrogenase (SDH, complex II), oligomycin sensitivity conferring protein (OSCP), ornithine transcarbamoylase (OTC), manganese superoxide dismutase (Mn-SOD) and cyclophilin D (CyPD) have all been identified as targets of SIRT3, which modulates their enzymatic activity by deacetylation37–43, 58–61. The absence of SIRT3 contributes to a striking hyperacetylation of mitochondrial proteins with metabolic perturbations, and ultimately increases the susceptibility and progression of metabolic syndrome58, 59.
Most studies have shown that SIRT3-mediated deacetylation contributes to the activation of metabolic enzymes and pathways. Our data demonstrate that loss of SIRT3 leads to hyperacetylation of PDH and ATP synthase which is associated with decreased enzymatic activity and impaired mitochondrial respiration. However, conflicting results suggesting a positive correlation between acetylation and enzymatic activity have also been observed. SIRT3 has an important role in mitochondria function as it deacetylates and inhibits CypD activity60, 61. Moreover, Zhao et al62 showed that acetylation of enoyl-CoA hydratase / 3-hydroxyacyl-CoA dehydrogenase, a fatty acid β-oxidation associated enzyme, contributes to its enzymatic activity enhancement in cardiac myocytes. LCAD acetylation has also been demonstrated to increase its activity and accelerate the fatty acid β-oxidation in the heart63, while another group reported that the hyperacetylation of LCAD reduces its enzymatic activity37. It remains to be explored whether the opposite effect of SIRT3 mediated metabolic alterations is associated with heart disease type-specificity, cellular conditions or microenvironment. Further studies are necessary to examine the dual regulatory roles of SIRT3 on its targets in different models of heart disease, and discuss its potential translation into therapy of heart failure.
The failing myocardium is characterized by changes in energy metabolism, a shift from fatty acid to glucose utilization for ATP production and overall energy depletion4, 64. As part of these changes, we have also observed altered expression of human genes participating in mitochondrial metabolic flux in response to miR-195 overexpression (Supplementary Figure 27), which is in line with previous reports on myocardial lipotoxicity in advanced HF and the corrective impact of mechanical unloading64. During heart failure progression, myocardial metabolism is characterized by a reduction of overall oxidative capacity, reduced FA and glucose oxidation rates and an increased rate of glycolysis with resulting impairments in ATP production2, 65–66. Alternative substrates such as ketone bodies and lactate have been postulated as additional sources of ATP production in the failing myocardium65, 67–68. The majority of cardiac ATP production originates from mitochondrial metabolic flux through various pathways, in which SIRT3 plays a central regulatory role. Interestingly, the dependence of sirtuin activity on NAD+ suggests that mitochondrial SIRT3, the metabolic sensor of changes in the energy status, might coordinate global shifts in metabolic pathways and mediate the beneficial effects in energy homeostasis accordingly, especially during energetic deficiency stages, such as caloric restriction69. Reduced SIRT3 expression has also been observed in obesity and diabetes70, 71, in post-infarction HF72, TAC-induced HF73 and in Dahl salt-sensitive and spontaneously hypertensive rats74. Loss of SIRT3 in cardiomyocytes contributes to the development of cardiac hypertrophy and HF accompanied by hyperacetylation of numerous key metabolic enzymes and proteins participating in oxidative stress75–77. Conversely, SIRT3-overexpressing mice are protected from hypertrophy43. During caloric restriction, SIRT3 mediates reprogramming in cardiac energy metabolism to allow respiration, by promoting glucose utilization, fat acid oxidation and amino acid metabolism which fuels the TCA cycle and facilitates ETC mediated ATP production to overcome the low energy input59, 78. Since the derangement of these metabolic pathways and energy perturbations are associated with the progression of HF, pharmacologic activators of SIRT3 might potentially ameliorate disease-specific patterns.
We have demonstrated that SIRT3 mediated deacetylation of PDH complex and ATP synthase α subunit. This post-translational modification alters their enzymatic activity individually and the mitochondrial respiration as the functional consequence. However, the critical lysine residues targeted for acetylation have not been determined yet. Recent studies have suggested that Lys321 of PDH E1α subunit79, Lys259 and Lys480 of ATP synthase β subunit80, and Lys139 of OSCP of ATP synthase81 are potential regulatory targets. Future studies will aim at elucidating target Lys residues on PDH complex E2/E3 and ATP synthase α, the acetylation status of which is important for their enzymatic activity.
The stress-responsive miR-195 has been suggested to associate with the cardiac remodeling and the development of heart diseases. It has been shown that miR-195 expression is induced in cardiac tissue from mice in response to TAC44 and miR-195 is up-regulated both during the early hypertrophic growth phase and late-stage of HF45. Consistently, we also observed that TAC and MI down-regulates SIRT3 expression and results in global protein hyperacetylation (Fig 3A and 3B) which might be due to enhanced miR-195 abundance. These studies suggested unique cardiac and systemic miRs are important in cardiac remodeling and miR-195 is an excellent biomarker and genetic signature of cardiac stress profiles. Furthermore, overexpression of miR-195 is sufficient to induce cardiac hypertrophy and HF in cultured cardiomyocytes and transgenic mice44, indicating the specific functional effect of this miR in hypertrophic growth. While miR-195 mediated regulation of SIRT1 has been demonstrated48, 49, SIRT1 and SIRT3 might coordinate well-orchestrated networks to regulate cardiac stress response.
In conclusion, we here show a novel regulatory pathway in the failing myocardium that involves miR-195 controlled suppression of SIRT3 leading to hyperacetylation and mitochondrial dysfunction. These findings might provide novel targets for selective therapeutic interventions in the syndrome of HF.
Supplementary Material
Clinical Perspective.
What is new?
Acetylome analysis of failing human myocardium revealed hyperacetylation of mitochondrial enzymes including PDH and ATP synthase.
This was accompanied by induction of miR-195, decreased mitochondrial deacetylase SIRT3 and global protein hyperacetylation.
miR-195 suppresses SIRT3 expression through direct 3’UTR targeting.
Pharmacologic inhibition of SIRT3, SIRT3 knockdown and miR-195 overexpression all enhanced PDH and ATP synthase acetylation, diminished enzymatic activity and impaired mitochondrial respiration.
SIRT3−/− and miR-195 transgenic mice showed hyperacetylation and decreased PDH and ATP synthase enzymatic activity.
Thus, a novel pathway of cardiac energy metabolism controls SIRT3 suppression and hyperacetylation of PDH and ATP synthase through miR-195 in failing myocardium.
What are the clinical implications?
Out data reveal a novel molecular pathway controlling cardiac metabolism in failing myocardium.
We demonstrate an important mechanistic role of a specific miRNA (miR-195) controlling cardiac energy metabolism through induction of hyperactylation of key mitochondrial enzymes in human heart failure.
This suggests miR-195 as potential therapeutic target in heart failure.
Further, we show that protein hyperacetylation resulting from dysregulated sirtuin deacetylase levels is a distinct molecular regulator of mitochondrial function.
Altogether, this study identifies several new targets contributing to impaired energy metabolism in the failing myocardium.
Acknowledgments:
We thank Dr. Eric N. Olson for the cardiac-specific miR-195 overexpression plasmid.
X.Z. and R.J. performed experiments; X.Z., R.J., X.L. and E.M. analyzed data; X.Z., R.J., X.L., E.C., P.K., D.B., K.D, I.G., E.M. P.C.C. and P.C.S. interpreted results of experiments; X.Z. prepared figures; X.Z. drafted manuscript; M.F., S.M-W., K.D., P.C.C. and P.C.S. edited and revised manuscript; P.C.S. conception and design of research; all authors approved the final version of manuscript.
Funding Sources:
This work was supported by the National Heart, Lung and Blood Institute, National Institutes of Health, through Grant Number HL095742, HL101272, HL114813, and HL112853 (Pathway to Independence - K.D.). Further support was provided by the Irving Institute of Clinical and Translational Research at Columbia University (UL1 RR024156), the Else Kröner Fresenius Foundation (P.C.S.) and FDA Postdoctoral Fellowship (AHA 16POST27700029).
Footnotes
Disclosures:
The authors have declared that no conflict of interest exists.
References:
- 1.Kapiloff MS, Emter CA. The cardiac enigma: current conundrums in heart failure research. F1000Res. 2016; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neubauer S The failing heart--an engine out of fuel. N Engl J Med.. 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 3.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–1733. [DOI] [PubMed] [Google Scholar]
- 4.Taegtmeyer H Genetics of energetics: transcriptional responses in cardiac metabolism. Ann Biomed Eng. 2000;28:871–876. [DOI] [PubMed] [Google Scholar]
- 5.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. [DOI] [PubMed] [Google Scholar]
- 6.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. [DOI] [PubMed] [Google Scholar]
- 7.Chandler MP, Kerner J, Huang H, Vazquez E, Reszko A, Martini WZ, Hoppel CL, Imai M, Rastoqi S, Sabbah HN, Stanley WC. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2004;287:H1538–543. [DOI] [PubMed] [Google Scholar]
- 8.Marin-Garcia J, Goldenthal MJ, Moe GW. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc Res. 2001;52:103–110. [DOI] [PubMed] [Google Scholar]
- 9.Quigley AF, Kapsa RM, Esmore D, Hale G, Byrne E. Mitochondrial respiratory chain activity in idiopathic dilated cardiomyopathy. J Card Fail. 2000;6:47–55. [DOI] [PubMed] [Google Scholar]
- 10.Casademont J, Miro O. Electron transport chain defects in heart failure. Heart Fail Rev. 2002;7:131–139. [DOI] [PubMed] [Google Scholar]
- 11.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and Methylation of Histones and Their Possible Role in the Regulation of Rna Synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunstein M Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. [DOI] [PubMed] [Google Scholar]
- 13.Weinert BT, Wagner SA, Horn H, Henriksen P, Liu WR, Olsen JV, Jensen LJ, Choudhary C. Proteome-wide mapping of the Drosophila acetylome demonstrates a high degree of conservation of lysine acetylation. Sci Signal. 2011;4:ra48. [DOI] [PubMed] [Google Scholar]
- 14.Tanno M, Kuno A, Horio Y, Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic Res Cardiol. 2012;107:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriksen P, Wagner SA, Weinert BT, Sharma S, Bacinskaja G, Rehman M, Juffer AH, Walther TC, Lisby M, Choudhary C. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol Cell Proteomics. 2012;11:1510–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinert BT, Iesmantavicius V, Moustafa T, Scholz C, Wagner SA, Magnes C, Zechner R, Choudhary C. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol Syst Biol. 2014;10:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C. Lundby C, Olsen JV. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. [DOI] [PubMed] [Google Scholar]
- 20.Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A.. 2000;97:5807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo CM, Smith DL Jr., Smith JS. Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol Cell Biol. 2004;24:1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. [DOI] [PubMed] [Google Scholar]
- 25.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. [DOI] [PubMed] [Google Scholar]
- 26.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99:13653–13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. [DOI] [PubMed] [Google Scholar]
- 28.Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158:647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. [DOI] [PubMed] [Google Scholar]
- 31.Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E.. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. [DOI] [PubMed] [Google Scholar]
- 31.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai VB, Sundaresan NR, Jeevanandam V, Gupta MP. Mitochondrial SIRT3 and heart disease. Cardiovasc Res. 2010;88:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper HM, Spelbrink JN. The human SIRT3 protein deacetylase is exclusively mitochondrial. Biochem J. 2008;411:279–285. [DOI] [PubMed] [Google Scholar]
- 36.Bao J, Lu Z, Joseph JJ, Carabenciov D, Dimond CC, Pang L, Samsel L, McCoy JP Jr, Leclerc J, Nguyen P, Gius D, Sack MN. Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. J Cell Biochem 2010;110:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A. 2006;103:10224–10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busk PK, Cirera S. MicroRNA profiling in early hypertrophic growth of the left ventricle in rats. Biochem Biophys Res Commun. 2010;396:989–993. [DOI] [PubMed] [Google Scholar]
- 46.Akat KM, Moore-McGriff D, Morozov P, Brown M, Gogakos T, Correa Da Rosa J, Mihailovic A, Sauer M, Ji R, Ramarathnam A, Totary-Jain H, Williams Z, Tuschl T, Schulze PC. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. PProc Natl Acad Sci U S A. 2014;111:11151–11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He JF, Luo YM, Wan XH, Jiang D. Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle, and apoptosis. J Biochem Mol Toxicol. 2011;25:404–408. [DOI] [PubMed] [Google Scholar]
- 48.Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92:75–84. [DOI] [PubMed] [Google Scholar]
- 49.Mortuza R, Feng B, Chakrabarti S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia. 2014;57:1037–1046. [DOI] [PubMed] [Google Scholar]
- 50.Davidson MM1, Nesti C, Palenzuela L, Walker WF, Hernandez E, Protas L, Hirano M, Isaac ND. Novel cell lines derived from adult human ventricular cardiomyocytes. J Mol Cell Cardiol. 2005;39:133–147. [DOI] [PubMed] [Google Scholar]
- 51.Zhou R, Yang F, Chen DF, Sun YX, Yang JS, Yang WJ. Acetylation of Chromatin-Associated Histone H3 Lysine 56 Inhibits the Development of Encysted Artemia Embryos. PloS One. 2013;8:e68374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol. 2013;304:H1103–1113. [DOI] [PubMed] [Google Scholar]
- 53.Yue Z, Ma Y, You J, Li Z, Ding Y, He P, Lu X, Jiang J, Chen S, Liu P. NMNAT3 is involved in the protective effect of SIRT3 in Ang II-induced cardiac hypertrophy. Exp Cell Res. 2016;347:261–273. [DOI] [PubMed] [Google Scholar]
- 54.Zampetaki A, Attia R, Mayr U, Gomes RS, Phinikaridou A, Yin X, Langley SR, Willeit P, Lu R, Fanshawe B, Fava M, Barallobre-Barreiro J, Molenaar C, So PW, Abbas A, Jahangiri M, Waltham M, Botnar R, Smith A, Mayr M. Role of miR-195 in aortic aneurysmal disease. Circ Res. 2014;115:857–866. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Liu Y, Han Q. Puerarin Attenuates Cardiac Hypertrophy Partly Through Increasing Mir-15b/195 Expression and Suppressing Non-Canonical Transforming Growth Factor Beta (Tgfbeta) Signal Pathway. Med Sci Monit. 2016;22:1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang ES, Song SB. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell Mol Life Sci. 2017;74(18):3347–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Ma X, He Y, Yuan C, Chen Q, Li G, Chen X. Sirtuin 5: a review of structure, known inhibitors and clues for developing new inhibitors. Sci China Life Sci. 2017;60:249–256. [DOI] [PubMed] [Google Scholar]
- 58.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stančáková A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV Jr, Kahn CR, Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci. 2010;123:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging. 2010;2:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res. 2014;103:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knöll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Jong KA, Lopaschuk GD. Complex Energy Metabolic Changes in Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Can J Cardiol. 2017;33:860–871. [DOI] [PubMed] [Google Scholar]
- 66.Dong Z, Zhao P, Xu M, Zhang C, Guo W, Chen H, Tian J, Wei H, Lu R, Cao T. Astragaloside IV alleviates heart failure via activating PPARalpha to switch glycolysis to fatty acid beta-oxidation. Sci Rep. 2017;7:2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation. 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bedi KC Jr., Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108:14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pougovkina O, te Brinke H, Ofman R, van Cruchten AG, Kulik W, Wanders RJ, Houten SM, de Boer VC. Mitochondrial protein acetylation is driven by acetyl-CoA from fatty acid oxidation. Hum Mol Genet. 2014;23:3513–3522. [DOI] [PubMed] [Google Scholar]
- 72.Parodi-Rullan R, Barreto-Torres G, Ruiz L, Casasnovas J, Javadov S. Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cell Physiol Biochem. 2012;29:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen T, Liu J, Li N, Wang S, Liu H, Li J, Zhang Y, Bu P. Mouse SIRT3 attenuates hypertrophy-related lipid accumulation in the heart through the deacetylation of LCAD. PloS One. 2015;10:e0118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grillon JM, Johnson KR, Kotlo K, Danziger RS. Non-histone lysine acetylated proteins in heart failure. Biochim Biophys Acta. 2012;1822:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng H, He X, Hou X, Li L, Chen JX. Apelin gene therapy increases myocardial vascular density and ameliorates diabetic cardiomyopathy via upregulation of sirtuin 3. Am J Physiol Heart Circ Physiol. 2014;306:H585–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285:3133–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng H, Vaka VR, He X, Booz GW, Chen JX. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med. 2015;19:1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. [DOI] [PubMed] [Google Scholar]
- 79.Fan J, Shan C, Kang HB, Elf S, Xie J, Tucker M, Gu TL, Aguiar M, Lonning S, Chen H, Mohammadi M, Britton LM, Garcia BA, Alečković M, Kang Y, Kaluz S, Devi N, Van Meir EG, Hitosugi T, Seo JH, Lonial S, Gaddh M, Arellano M, Khoury HJ, Khuri FR, Boggon TJ, Kang S, Chen J. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell. 2014;53:534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahman M, Nirala NK, Singh A, Zhu LJ, Taguchi K, Bamba T, Fukusaki E, Shaw LM, Lambright DG, Acharya JK, Acharya UR. Drosophila Sirt2/mammalian SIRT3 deacetylates ATP synthase beta and regulates complex V activity. J Cell Biol. 2014;206:289–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vassilopoulos A, Pennington JD, Andresson T, Rees DM, Bosley AD, Fearnley IM, Ham A, Flynn CR, Hill S, Rose KL, Kim HS, Deng CX, Walker JE, Gius D. SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid Redox Signal. 2014;21:551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.