Abstract
PURPOSE
Head and neck cancer is the sixth most common cancer in the world, and the largest burden occurs in developing countries. Although the primary risk factors have been well characterized, little is known about temporal trends in head and neck cancer across Thailand.
METHODS
Head and neck squamous cell carcinoma (HNSCC) occurrences diagnosed between 1990 and 2014 were selected by International Classification of Diseases (10th revision; ICD10) code from the Songkhla, Lampang, Chiang Mai, and Khon Kaen cancer registries and the US SEER program for oral cavity (ICD10 codes 00, 03-06), tongue (ICD10 codes 01-02), pharynx (ICD10 codes 09-10, 12-14), and larynx (ICD10 code 32). The data were analyzed using R and Joinpoint regression software to determine age-standardized incidence rates and trends of annual percent change (APC). Incidence rates were standardized using the Segi (1960) population. Stratified linear regression models were conducted to assess temporal trends in early-onset HNSCC across 20-year age groups.
RESULTS
Although overall HNSCC rates are decreasing across all registries, subsite analyses demonstrate consistent decreases in both larynx and oral cavity cancers but suggest increases in tongue cancers among both sexes in the United States (APCmen, 2.36; APCwomen, 0.77) and in pharyngeal cancer in Khon Kaen and US men (APC, 2.1 and 2.23, respectively). Age-stratified APC analyses to assess young-onset (< 60 years old) trends demonstrated increased incidence in tongue cancer in Thailand and the United States as well as in pharyngeal cancers in Khon Kaen men age 40 to 59 years and US men age 50 to 59 years.
CONCLUSION
Although overall trends in HNSCC are decreasing across both Thailand and the United States, there is reason to believe that the etiologic shift to oropharyngeal cancers in the United States may be occurring in Thailand.
INTRODUCTION
Worldwide, head and neck cancer (HNC) accounts for more than 550,000 occurrences and 300,000 deaths each year,1,2 and the 5-year overall survival rate is a dismal 40% to 50%.3 HNC is the sixth most common cancer in the world,4 and the highest incidence is observed in South and Southeast Asia.5,6 Many of these occurrences are attributed to use of betel quid, although there have been no systematic studies of risk factors for this set of cancers within these regions of the world. In the Western world, decreased combustible tobacco use and increased human papillomavirus (HPV) prevalence have allowed epidemiologic shift that has highlighted the role of high-risk HPV strains in head and neck squamous cell carcinoma (HNSCC) pathology.7,8 This subset of the disease is seen more often in younger patients and most commonly presents in the oral cavity, tongue, and oropharynx.9,10 However, most of these data are from Western countries, and little knowledge exists about the presentation and prevalence of this important etiologic factor in other high-incidence areas, such as Thailand.
A first step to address the burden of HNC in Southeast Asia is to analyze surveillance data for trends in the incidence of HNSCC. In the past few decades, rapid socioeconomic development has allowed improved control of communicable diseases, which resulted in the emergence of cancer as the leading cause of death.11 Thailand has an efficient universal health care system that, among many other benefits, facilitates the collection of health statistics.12 Subsequently, Thailand’s high-quality cancer statistics are obtained and managed by 16 province- and regional-based registries that build on the health care infrastructure.13 In addition, the sociocultural factors in Thailand are similar to those in surrounding countries; thus, incidence trends may reflect regional changes in HNSCC.
Here, we take advantage of data available from the Thai Cancer Information Network to evaluate incidence trends in four areas of Thailand. We identified evidence for increases in HPV-associated oropharyngeal cancer similar to those seen in the United States and noted overall decreases in cancers historically associated with smoking and alcohol across all study sites.
METHODS
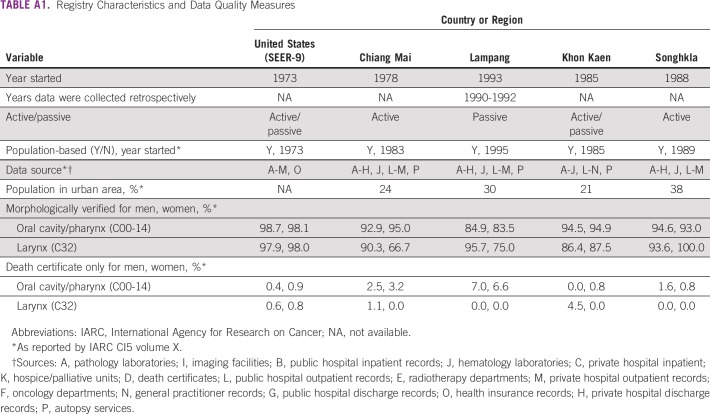
HNSCC incidence data available from 1990 to 2014 were obtained from the Songkhla, Khon Kaen, Lampang, and Chiang Mai cancer registries and were compared with those found in the US SEER program (Fig 1).14-18 Each Thai registry has case ascertainment of approximately 90% within the catchment area.19 Chiang Mai, which was the first cancer registry established in Thailand, has actively obtained cancer occurrences from all provincial hospitals within the province since 1983.20 Lampang passively collects data from cancer centers and all public and private hospitals within the province.21 Khon Kaen, which represents an estimated third of the overall population of Thailand, collects data from all care facilities using both active and passive methods.22 The province of Songkhla has a population-based registry that was established in 1989 and that is managed by the Prince of Songkla University.23 Finally, the US SEER database, which was established in 1973, uses both passive and active data collection (Appendix Table A1). All registries provide data for the Cancer Incidence in Five Continents repository of the International Agency for Research on Cancer.24
FIG 1.
Map of Thailand: Inclusion of four cancer registries.
Inclusion criteria for case selection was based upon International Classification of Disease, (10th edition; ICD10) codes. Site-specific cancers were defined by the following codes: oral cavity (C00, C03-C06), tongue (C01-C02), pharynx (C09-C10, C12-C14), and larynx (C32). Overall HNSCC incidence was calculated as the sum across all subsites. In addition to diagnosis, patient case information included the following: age, sex, date of diagnosis, stage, histology, and morphology. For the Thai data, population denominators by registry, year, age, and sex were based upon decennial census data from 1990, 2000, and 2010, which were obtained by the Thai National Statistical Office.25,26 Annual intercensal population structures for the various provinces were estimated by 5-year sex-specific age groups using a log-linear function between successive censuses. Population counts beyond 2010 were estimated by the Office of the National Economic and Social Development Board.27,28 Population denominators for the SEER data were obtained from SEER*Stat 8.3.5 and were based upon the US Census Bureau Population Estimates Program.18,29
Data were analyzed using R statistical software, version 3.3.3.30 To assess sex-specific HNSCC trends, annual percent change (APC) was calculated using age-adjusted incidence rates in the Joinpoint regression model, version 4.5.0.1.31 This software uses a Monte Carlo permutation method to assess the number of joinpoints, slope in the trends, and their significance.32 When no patient cases were present within a given year, a half-case was added to the age strata with the largest population to enable computation on the log-linear scale.14,32 This method was repeated for all subsites, standardized to the Segi (1960) world population for comparability.33,34 Additional exploratory analyses using stratified linear regression models were conducted to assess temporal trends in earlier-onset HNSCC across 20-year age groupings.
RESULTS
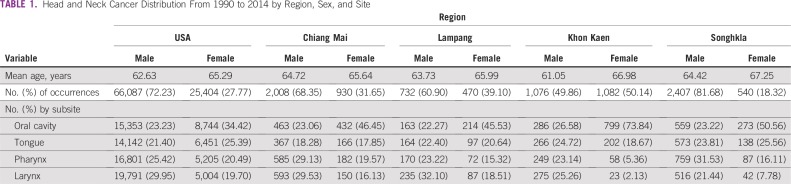
Between 1990 and 2014, 91,491 occurrences of HNSCC were reported in the United States; 2,938, in Chiang Mai; 2,158, in Khon Kaen; 2,947, in Songkhla; and 1,202, in Lampang. The distribution of age at diagnosis was relatively similar across all study sites, and means ranged from 61 to 67 years old. HNSCC incidence was higher among men than women in all registries except Khon Kaen, where women represented 50.14% of the observed cancer occurrences. In the United States, Chiang Mai, and Lampang, laryngeal cancer accounted for the highest proportion of HNSCC among men, whereas oral cavity and pharynx cancers were predominant in Khon Kaen and Songkhla, respectively. Among women, oral cavity cancers contributed the greatest case burden across all registries (Table 1).
TABLE 1.
Head and Neck Cancer Distribution From 1990 to 2014 by Region, Sex, and Site
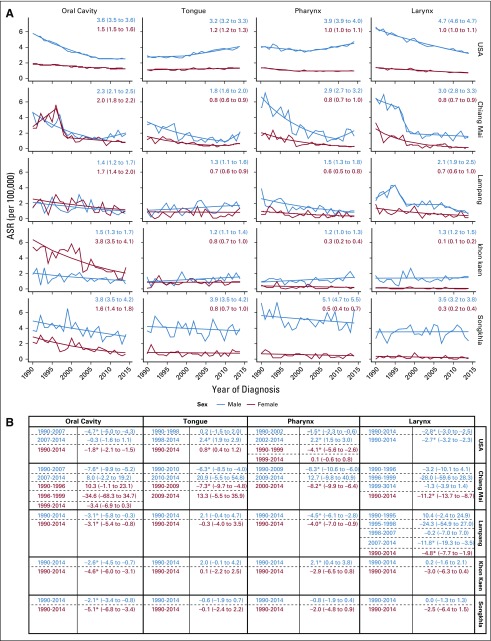
Songkhla had the highest overall HNSCC incidence among men: the expected age-standardized rate (EASR) was 14.68 per 100,000 in 2014. Although much lower among men, the United States stood out as having the highest HNSCC burden among women: the 2014 EASR was 4.32 per 100,000. Overall, these rates are decreasing across all registries except Khon Kaen and Chiang Mai, where there is an observed, nonstatistically significant increase in incidence among men (APC, 0.5 [95% CI, −0.6 to 1.6] and 8.1 [95% CI, −3.3 to 20.9], respectively). Despite these general decreases, analyses to assess subsite incidence trends demonstrated great variability in slope, and corresponding significance, across registries and anatomic locations (Fig 2).
FIG 2.
Subsite Joinpoint analyses of head and neck squamous cell carcinoma (HNSCC) trends by sex and registry. (A) Expected age-standardized rate from 1990 to 2014 (EASR1990-2014) and 95% CIs. (B) Annual percent change (APC) and 95% CIs. (*) denotes statistical significance at alpha = .05.
When compared with other registries, laryngeal cancer rates were highest among US women and men (EASR1990-2014, 1.0 and 4.7 per 100,000, respectively). Laryngeal cancer among women is significantly decreasing in Lampang (APC, −4.8; 95% CI, −7.7 to −1.9), Chiang Mai (APC, −11.2; 95% CI, −13.7 to −8.7), and the United States (APC, −2.7; 95% CI, −3.2 to −2.3). Among men, these trends have been more variable, and the most recent significant decreases were observed in Lampang from 2007 to 2014 (APC, −11.8; 95% CI, −19.3 to −3.5) and the United States (APC, −2.8; 95% CI, −3.0 to −2.5; Fig 2).
Khon Kaen women and Songkhla men had the highest oral cavity cancer rates: the EASR1990-2014 was 3.8 per 100,000 for both. Although oral cavity cancers contributed the greatest proportion of HNSCC occurrences among women, the incidence of these cancers has declined across all registries; significant decreases were observed in Songkhla (APC, −5.1; 95% CI, -6.8 to −3.4), Khon Kaen (APC, −4.6; 95% CI, −6.0 to −3.1), Lampang (APC, −3.1; 95% CI, −5.4 to −0.8), and the United States (APC, −1.8; 95% CI, −2.1 to −1.5). Similarly, trends among men also noted reductions in oral cavity cancer incidence, particularly in Songkhla (APC, −2.1; 95% CI, −3.4 to −0.8), Khon Kaen (APC, −2.6; 95% CI, −4.5 to −0.7), and Lampang (APC, −3.1; 95% CI, −5.8 to −0.3; Fig 2).
Pharyngeal cancer rates were highest among US women (EASR1990-2014, 1.0 per 100,000) and Songkhla men (EASR1990-2014, 5.1 per 100,000). Decreased incidence rates were noted among Chiang Mai women (APC, −8.2; 95% CI, −9.9 to −6.4) as well as both women (APC, −4.0; 95% CI, −7.0 to −0.9) and men (APC, −4.5; 95% CI, −6.1 to −2.8) in Lampang. Conversely, increases in pharyngeal cancer incidence were found among men in Khon Kaen (APC, 2.1; 95% CI, 0.4 to 3.8) and the United States from 2002 to 2014 (APC, 2.2; 95% CI, 1.5 to 3.0). Tongue cancer rates were highest among US women (EASR1990-2014, 1.2 per 100,000) and Songkhla men (EASR1990-2014, 3.9 per 100,000). These rates were significantly increased among US women (APC, 0.8; 95% CI, 0.4 to 1.2) and men from 1998 to 2014 (APC, 2.4; 95% CI, 1.9 to 2.9). A suggested increase in incidence of tongue cancer was observed among Lampang men and among both sexes in Khon Kaen and Chiang Mai, but these trends did not reach statistical significance (Fig 2).
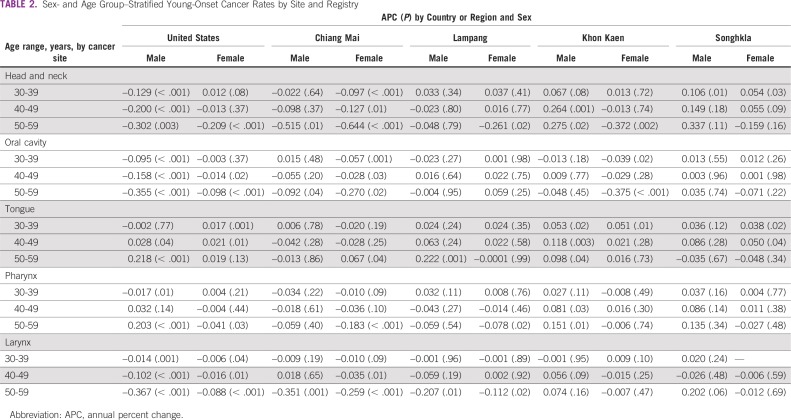
Exploratory analyses into younger-onset (< 60 years old) trends demonstrated significant increases in tongue cancer among Chiang Mai women age 50 to 59 years, Khon Kaen women age 30 to 39 years, Khon Kaen men age 30 to 59 years, Lampang men age 50 to 59 years, Songkhla women age 30 to 49 years, US women age 30 to 49 years, and US men age 40 to 59 years. Pharyngeal cancers also increased in Khon Kaen men age 40 to 59 years and in US men age 50 to 59 years (Table 2; Fig 2).
TABLE 2.
Sex- and Age Group–Stratified Young-Onset Cancer Rates by Site and Registry
DISCUSSION
In this study, we found that HNSCC occurrences decreased across four registries in Thailand as well as the United States, and we noted distinct variability across anatomic subsite, sex, and geographic location. Although the vast majority of these changes may be attributed to antismoking campaigns, changes in policy, and consequent shifts in risk behaviors, evidence also seems to suggest the growing impact of HPV-related etiology on increasing oropharyngeal cancer incidence in Thailand and the United States.
HNC comprises highly heterogeneous cancers, with global incidence reflective of trends in tobacco and alcohol use35,36 and sexual norms.37,38 As these risk behaviors change over time, the site-specific incidence that contributes to overall HNC trends reflects these changes in a population- and sex-specific manner. In 1990, the largest proportion of HNSCC among men was observed in the larynx in the United States and Lampang (EASR1990, 6.40 and 1.89 per 100,000, respectively), oral cavity in Khon Kaen and Songkhla (EASR1990, 2.09 and 4.97 per 100,000, respectively), and pharynx in Chiang Mai (EASR1990, 6.67 per 100,000). In contrast, in 2014, the largest drivers of HNSCC among men shifted to pharyngeal cancer in the United States, Songkhla, and Chiang Mai (EASR2014, 4.54, 4.70, and 2.33 per 100,000, respectively) and tongue cancer in Khon Kaen and Lampang (EASR2014, 1.62 and 1.83 per 100,000, respectively). Among women, oral cavity cancers seem to have historically predominated HNSCC incidence across all registries and, though to a lesser degree, continue to do so in Khon Kaen, Chiang Mai, and Lampang. Conversely, in the United States and Songkhla, these trends seem to have shifted in recent years as a result of a decrease in oral cavity incidence and an increase in tongue cancer incidence. Nevertheless, HNSCCs among women have greatly decreased across all Thai registries, whereas such observations are less apparent among men.
Although an estimated 7% of oral cavity cancers in the United States are attributed to chewing tobacco,39 the practice of chewing betel quid—which includes areca nut, slaked lime, betel leaf, and often tobacco—is widely prevalent throughout Thailand. A study conducted in Northeastern Thailand found that ever chewers of betel quid had a 9.01-fold increased risk of oral cancer compared with never chewers in multivariable analyses40; numerous other studies also have implicated betel quid use in the etiology of oral cavity cancer.40-45 Several studies from Northeastern Thailand have demonstrated the high prevalence of betel use among women, often as a social activity and particularly among older generations.42,45,46 Conversely, chewing tobacco use among women in the United States has historically been low; according to the National Survey on Drug Use and Health, an estimated 0.5% of adult women used smokeless tobacco in the United States in 2016.47 After years of education and political campaigns to decrease betel quid use in Thailand, recent data show declines in this risk behavior, especially among younger populations.13,42 The impact of this cultural shift is apparent in the consistent decreases in oral cavity cancers, particularly among women—most notably in Khon Kaen, where the incidence reduced by one third, from an EASR1990 of 6.37 per 100,000 to an EASR2014 of 2.08 per 100,000 (APC, −4.6; 95% CI, −6.0 to −3.1).
Tobacco use has long been considered the leading cause of HNSCC, often working synergistically with alcohol consumption.48,49 A study in the International Head and Neck Cancer Epidemiology Consortium estimated that approximately 90% of patients with HNSCC have a history of tobacco use,50 which confers a 10-fold increase in laryngeal cancer risk and a four- to five-fold increase in hypopharynx, oropharynx, and oral cavity cancer risk.51 Risk is dose dependent in nature,50,52 so several studies have suggested smoking cessation to reduce the risk of HNSCC.53,54 In the United States, increased societal awareness and support for combustible tobacco control has led to a decrease in cigarette consumption55,56 and, consequently, laryngeal cancer rates; the APC is −2.8% (95% CI, −3.0 to −2.5) among men and −2.7% (95% CI, −3.2 to −2.3) among women. Beginning in 1991, Thailand was the first nation in Asia to implement strong tobacco control policies.57,58 Since then, numerous regulations, including bans on advertisement and smoking in certain public places, health warnings, and taxation, have been put in place.59,60 As a result of these aggressive control methods, the overall smoking rate in Thailand has decreases from 30% in 1976% to 19.9% in 201361; lung cancer rates, particularly in Northern Thailand, have also decreased.62 As demonstrated in Figure 2, the EASR of laryngeal cancer among women in Thailand has been, and remains, relatively low, and observed decreases are noted across all registries. In addition to the aforementioned control methods, cultural norms in Thailand have historically resulted in women abstaining from smoking and drinking.13,61 Interestingly, a comparison of reported alcohol consumption rates in Thailand between 2003 to 2004 and those in 2008 to 2009 demonstrated decreased drinking rates among young men and increasing rates among young women, which points to a potential change in cultural practices.63,64 Although inconsistent across time, significant decreases in laryngeal cancer incidence can be observed among men in Lampang; the APC was −11.8% (95% CI, −19.3 to −3.5) from 2007 to 2014. Chiang Mai also shows decreased incidence of laryngeal cancer among men, but these trends did not reach statistical significance; trends in both Khon Kaen and Songkhla remained stagnant.
Historically, tongue and pharyngeal tumors have been associated with risk factors similar to other HNSCC sites, such as combustible tobacco and alcohol.52,65 An etiologic shift in oropharyngeal tumors in the United States has led to increased global awareness of high-risk HPV as a causal factor in carcinogenesis of these sites, and current prevalence estimates in the United States are near 80%.8,66-72 Although pharyngeal cancer incidence rates varied widely across the different registries, tongue cancer rates seemed to increase across all registries except in both men and women in Songkhla and women in Lampang. Studies to assess the differences in HPV-positive and -negative HNSCC demonstrated an increase in HPV-positive tumors among younger cohorts who had a lower smoking prevalence and multiple sexual partners.72 In an exploratory analysis to assess the change in incidence of tongue and pharynx cancer among younger individuals (< 60 years old), we found notable increases in tongue cancer across all registries as well as in pharyngeal cancer in Khon Kaen men (Table 2). A sensitivity analysis to assess these trends in the oropharynx, as defined by ICD10 codes C01, C02.4, C09.0-09.9, and C10.0-10.9, demonstrated large increases in incidence rates among Khon Kaen men age 30 to 39 and 50 to 59 years, Khon Kaen women age 40 to 49 years, Songkhla women age 50 to 59 years, and Lampang women age 30 to 39 years (data not shown).8 To date, there have been only two studies conducted in the Thai population to assess HPV prevalence in HNSCC—both in the oral cavity.73,74 The more recent of the two studies used 146 archival tissue samples of oral squamous cell carcinoma and found that 56.2% were positive for high-risk HPV and that 43.8% expressed HPV E6/E7 mRNA.73
This study was the first, to our knowledge, to assess HNSCC trends across multiple registries in Thailand compared with the United States. Although data obtained from population-based registries allow extrapolation of results to the pertinent provinces, it is difficult to approximate the number of unreported occurrences of cancer, particularly among those living in rural villages. The introduction of universal health coverage by the Thai National Health Security Office in 2002 allowed improved access to care, which led to a presumed increase in case ascertainment and, consequently, increased incidence trends. In this study, however, we did not observe any notable changes in incidence between the periods before and after the introduction of this policy, and we primarily observed decreases in overall HNSCC incidence.
The comparison of trends across different registries poses challenges because of the variability not only in data collection methods but also in population composition. Although Thailand registries boast high-quality data collection16,19 with methods that vary from passive to active to a combination of the two,20-23 analyses of incidence near the inception of a given registry may lead to concerns about data reliability; this is particularly relevant in Lampang, where registry data before 1993 were collected retrospectively and may be biased toward cases of patients who were alive when the registry was foundation. Nevertheless, this study assessed HNSCC trends across 24 years to result in comprehensive temporal analyses. Finally, changes in population structure have become increasingly apparent across Thailand, with rural to urban migration of individuals within Thailand as well as immigration into the country.75,76
Although HNSCC seems to be decreasing across Thailand and the United States, there is evidence to suggest that the increase in high-risk HPV-associated oropharyngeal cancers previously reported in the United States may also be relevant in Thailand. HPV-positive tumors have been more responsive to treatment, which ultimately led to suggested restaging and, consequently, less aggressive treatment of these lesions by the American Joint Committee on Cancer.77 Because of the younger-onset nature of these lesions, de-escalation of treatment may lead to improvements in morbidity and mortality of patients. With the potential increase in HPV-positive tumors in Thailand, additional molecular studies are necessary to evaluate oropharyngeal etiology and improve public health practice and treatment protocols across Thailand.
Appendix
TABLE A1.
Registry Characteristics and Data Quality Measures
Footnotes
Presented at the American Association of Cancer Research Annual Meeting, Chicago, IL, April 14-18, 2018; the University of Michigan Rogel Cancer Center Spring Symposium, Ann Arbor, MI, June 14, 2018; and the International Head and Neck Cancer Epidemiology Consortium Annual Meeting, Milan, Italy, June 29, 2018.
Supported by the National Institutes of Health Grant No. T32AG027708, the University of Michigan Office of Global Public Health, the University of Michigan Center for Southeast Asian Studies, the Thailand Government through Khon Kaen University, Prince of Songkla University, Chiang Mai University, and the Ministry of Public Health Lampang Cancer Hospital.
AUTHOR CONTRIBUTIONS
Conception and design: Ilona Argirion, Hutcha Sriplung, Patravoot Vatanasapt
Collection and assembly of data: Ilona Argirion, Krittika Suwanrungruang, Donsuk Pongnikorn, Imjai Chitapanarux, Hutcha Sriplung, Patravoot Vatanasapt, Laura S. Rozek
Data analysis and interpretation: Ilona Argirion, Katie R. Zarins, Kali Defever, Joanne T. Chang, Hutcha Sriplung, Patravoot Vatanasapt, Laura S. Rozek
Provision of study material or patients: Imjai Chitapanarux
Administrative support: Patravoot Vatanasapt, Laura S. Rozek
Manuscript writing: All authors
Final approval of manuscript: All authors
Agree to be accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Katie R. Zarins
Research Funding: Brooklyn Therapeutics (Inst)
Laura S. Rozek
Research Funding: Brooklyn Therapeutics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Head and Neck Cancer: Review of Cancer Medicines on the WHO List of Essential Medicines. 2014. Geneva, Switzerland, WHO. [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Gupta B, Johnson NW, Kumar N. Global epidemiology of head and neck cancers: A continuing challenge. Oncology. 2016;91:13–23. doi: 10.1159/000446117. [DOI] [PubMed] [Google Scholar]
- 6.Mehanna H, Paleri V, West CM, et al. Head and neck cancer: Part 1. Epidemiology, presentation, and prevention. BMJ. 2010;341:c4684. doi: 10.1136/bmj.c4684. [DOI] [PubMed] [Google Scholar]
- 7.Stein AP, Saha S, Yu M, et al. Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the United States across time. Chem Res Toxicol. 2014;27:462–469. doi: 10.1021/tx500034c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kansy K, Thiele O, Freier K. The role of human papillomavirus in oral squamous cell carcinoma: mMyth and reality. Oral Maxillofac Surg. 2014;18:165–172. doi: 10.1007/s10006-012-0383-0. [DOI] [PubMed] [Google Scholar]
- 10.Marur S, D’Souza G, Westra WH, et al. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vatanasapt V, Martin N, Sriplung H, et al. Cancer incidence in Thailand, 1988-1991. Cancer Epidemiol Biomarkers Prev. 1995;4:475–483. [PubMed] [Google Scholar]
- 12.Tangcharoensathien V, Witthayapipopsakul W, Panichkriangkrai W, et al. Health systems development in Thailand: A solid platform for successful implementation of universal health coverage. Lancet. 2018;391:1205–1223. doi: 10.1016/S0140-6736(18)30198-3. [DOI] [PubMed] [Google Scholar]
- 13.Tangjaturonrasme N, Vatanasapt P, Bychkov A. Epidemiology of head and neck cancer in Thailand. Asia Pac J Clin Oncol. 2018;14:16–22. doi: 10.1111/ajco.12757. [DOI] [PubMed] [Google Scholar]
- 14.Demanelis K, Sriplung H, Meza R, et al. Differences in childhood leukemia incidence and survival between Southern Thailand and the United States: A population-based analysis. Pediatr Blood Cancer. 2015;62:1790–1798. doi: 10.1002/pbc.25571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez CS, Virani S, Meza R, et al. Current and future burden of prostate cancer in Songkhla, Thailand: Analysis of incidence and mortality trends from 1990 to 2030. J Glob Oncol. doi: 10.1200/JGO.17.00128. doi: 10.1200/JGO.17.00128 [epub on January 30, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virani S, Sriplung H, Rozek LS, et al. Escalating burden of breast cancer in southern Thailand: Analysis of 1990-2010 incidence and prediction of future trends. Cancer Epidemiol. 2014;38:235–243. doi: 10.1016/j.canep.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Mitro SD, Rozek LS, Vatanasapt P, et al. Iodine deficiency and thyroid cancer trends in three regions of Thailand, 1990-2009. Cancer Epidemiol. 2016;43:92–99. doi: 10.1016/j.canep.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 18.SEER SEER*Stat Database: Cancer incidence: Surveillance Research Program, National Cancer Institute SEER*Stat software ( seer.cancer.gov/seerstat) version 8.3.5.
- 19.Suwanrungruang K, Sriplung H, Attasara P, et al. Quality of case ascertainment in cancer registries: A proposal for a virtual three-source capture-recapture technique. Asian Pac J Cancer Prev. 2011;12:173–178. [PubMed] [Google Scholar]
- 20.International Association of Cancer Registries . Chiang Mai cancer registry. http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=990 [Google Scholar]
- 21.International Association of Cancer Registries . Lampang cancer registry. http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=992 [Google Scholar]
- 22.International Association of Cancer Registries . Khon Kaen provincial registry. http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=991 [Google Scholar]
- 23.International Association of Cancer Registries . Songkhla cancer registry. http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=994 [Google Scholar]
- 24.Forman D, Bray F, Brewster D, et al. 2013. Cancer Incidence in Five Continents (vol. X). Lyon, France, International Agency for Research on Cancer, [Google Scholar]
- 25.National Statistics Office of Thailand . Preliminary report of the 2010 population and housing census (Whole Kingdom) http://popcensus.nso.go.th/upload/popcensus-08-08-55-E.pdf [Google Scholar]
- 26.National Statistics Office of Thailand . Report: Older persons in Thailand. http://web.nso.go.th/en/survey/age/tables_older_2014.pdf [Google Scholar]
- 27.Sriplung H, Bilheem S, Kuntipundee T, et al. Differences in cancer incidence among predominantly Muslim and Buddhist subpopulations in Songkhla. Asian Pac J Cancer Prev. 2014;15:9979–9983. doi: 10.7314/apjcp.2014.15.22.9979. [DOI] [PubMed] [Google Scholar]
- 28.Sriplung H, Singkham P, Iamsirithaworn S, et al. Success of a cervical cancer screening program: Trends in incidence in songkhla, southern Thailand, 1989-2010, and prediction of future incidences to 2030. Asian Pac J Cancer Prev. 2014;15:10003–10008. doi: 10.7314/apjcp.2014.15.22.10003. [DOI] [PubMed] [Google Scholar]
- 29.SEER . SEER Population File: Total U.S. (1969-2016) <Katrina/Rita Adjustment> - Linked To County Attributes - Total U.S., 1969-2016 Counties. National Cancer Institute; Bethesda, MD: 2017. [Google Scholar]
- 30.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 31.SEER Joinpoint regression program. https://surveillance.cancer.gov/joinpoint/
- 32.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 33.Bray F, Guilloux A, Sankila R, et al. Practical implications of imposing a new world standard population. Cancer Causes Control. 2002;13:175–182. doi: 10.1023/a:1014344519276. [DOI] [PubMed] [Google Scholar]
- 34.Segi M, Kyōkai NT, Igakubu TD. Cancer mortality for selected sites in 24 countries. Japan Cancer Society; Nagoya, Japan: 1960. https://www.who.int/healthinfo/paper31.pdf [Google Scholar]
- 35.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: An emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 36.Sankaranarayanan R, Masuyer E, Swaminathan R, et al. Head and neck cancer: A global perspective on epidemiology and prognosis. Anticancer Res. 1998;18(6B):4779–4786. [PubMed] [Google Scholar]
- 37.Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers: A pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39:166–181. doi: 10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Souza G, Agrawal Y, Halpern J, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol. 2008;9:667–675. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 40.Loyha K, Vatanasapt P, Promthet S, et al. Risk factors for oral cancer in northeast Thailand. Asian Pac J Cancer Prev. 2012;13:5087–5090. doi: 10.7314/apjcp.2012.13.10.5087. [DOI] [PubMed] [Google Scholar]
- 41.International Agency for Research on Cancer (IARC) Working Group on the Evaluation of Carcinogenic Risks to Humans . 2004. Betel-Quid and Areca-Nut Chewing and Some Areca-Nut-Derived Nitrosamines. IARC Monogr Eval Carcinog Risks Hum No. 85, Lyon, France, IARC, [PMC free article] [PubMed] [Google Scholar]
- 42.Sriamporn S, Parkin DM, Pisani P, et al. A prospective study of diet, lifestyle, and genetic factors and the risk of cancer in Khon Kaen Province, northeast Thailand: Description of the cohort. Asian Pac J Cancer Prev. 2005;6:295–303. [PubMed] [Google Scholar]
- 43.Vatanasapt P, Suwanrungruang K, Kamsa-Ard S, et al. Epidemiology of oral and pharyngeal cancers in Khon Kaen, Thailand: A high incidence in females. Asian Pac J Cancer Prev. 2011;12:2505–2508. [PubMed] [Google Scholar]
- 44.Kampangsri W, Vatanasapt P, Kamsa-Ard S, et al. Betel quid chewing and upper aerodigestive tract cancers: A prospective cohort study in khon kaen, Thailand. Asian Pac J Cancer Prev. 2013;14:4335–4338. doi: 10.7314/apjcp.2013.14.7.4335. [DOI] [PubMed] [Google Scholar]
- 45.Simarak S, de Jong UW, Breslow N, et al. Cancer of the oral cavity, pharynx/larynx and lung in North Thailand: Case-control study and analysis of cigar smoke. Br J Cancer. 1977;36:130–140. doi: 10.1038/bjc.1977.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vatanasapt V, Sriamporn S, MacLennan R. Contrasts in risk factors for cancers of the oral cavity and hypopharynx and larynx in Khon Kaen, Thailand. Oral Oncol. 1991;2:39–42. [Google Scholar]
- 47.Substance Abuse and Mental Health Services Administration 2016 National Survey on Drug Use and Health: Detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf.
- 48.Wynder EL, Bross IJ, Feldman RM. A study of the etiological factors in cancer of the mouth. Cancer. 1957;10:1300–1323. doi: 10.1002/1097-0142(195711/12)10:6<1300::aid-cncr2820100628>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 49.Keller AZ, Terris M. The association of alcohol and tobacco with cancer of the mouth and pharynx. Am J Public Health Nations Health. 1965;55:1578–1585. doi: 10.2105/ajph.55.10.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 51.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: Recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 52.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 53.Franceschi S, Talamini R, Barra S, et al. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990;50:6502–6507. [PubMed] [Google Scholar]
- 54.Schlecht NF, Franco EL, Pintos J, et al. Effect of smoking cessation and tobacco type on the risk of cancers of the upper aero-digestive tract in Brazil. Epidemiology. 1999;10:412–418. doi: 10.1097/00001648-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention Tobacco use: United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:986–993. [PubMed] [Google Scholar]
- 56.Alamar B, Glantz SA. Effect of increased social unacceptability of cigarette smoking on reduction in cigarette consumption. Am J Public Health. 2006;96:1359–1363. doi: 10.2105/AJPH.2005.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chantornvong S, McCargo D. Political economy of tobacco control in Thailand. Tob Control. 2001;10:48–54. doi: 10.1136/tc.10.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chantornvong S, Collin J, Dodgson R, et al. Political economy of tobacco control in low-income and middle-income countries: Lessons from Thailand and Zimbabwe. Bull World Health Organ. 2000;78:913–919. [PMC free article] [PubMed] [Google Scholar]
- 59.Vateesatokit P, Hughes B, Ritthphakdee B. Thailand: Winning battles, but the war’s far from over. Tob Control. 2000;9:122–127. doi: 10.1136/tc.9.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haman S. Thailand: Victories and defeats in the long war. Tob Control. 2003;12:8–8. doi: 10.1136/tc.12.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smoking and Drinking Behavior in Thailand. National Statistical Office; Bangkok, Thailand: National Statistical Office; 2015. [Google Scholar]
- 62.Pongnikorn D, Daoprasert K, Waisri N, et al. Cancer incidence in northern Thailand: Results from six population-based cancer registries 1993-2012. Int J Cancer. 2018;142:1767–1775. doi: 10.1002/ijc.31203. [DOI] [PubMed] [Google Scholar]
- 63.Porapakkham Y, Bunyarattapan P. Thai National Health Examination Survey III, 2003-2004. Health System Research Institute; Bangkok, Thailand: 2006. http://www.surveillance.cphs.chula.ac.th/index.php/knowledge-of-health/bkk-health-watch/122-3-2546-2547 [Google Scholar]
- 64.Aekplakorn W. Thai National Health Examination Survey V, 2014. Health System Research Institute; Bangkok, Thailand: 2016. http://thaitgri.org/?p=37869 [Google Scholar]
- 65.Zheng T, Holford T, Chen Y, et al. Risk of tongue cancer associated with tobacco smoking and alcohol consumption: A case-control study. Oral Oncol. 1997;33:82–85. doi: 10.1016/s0964-1955(96)00056-5. [DOI] [PubMed] [Google Scholar]
- 66.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 67.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park K, Cho KJ, Lee M, et al. p16 immunohistochemistry alone is a better prognosticator in tonsil cancer than human papillomavirus in situ hybridization with or without p16 immunohistochemistry. Acta Otolaryngol. 2013;133:297–304. doi: 10.3109/00016489.2012.741327. [DOI] [PubMed] [Google Scholar]
- 69.Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer: Systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 70.Lewis JS, Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma: An entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 72.Rettig EM, D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am. 2015;24:379–396. doi: 10.1016/j.soc.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Phusingha P, Ekalaksananan T, Vatanasapt P, et al. Human papillomavirus (HPV) infection in a case-control study of oral squamous cell carcinoma and its increasing trend in northeastern Thailand. J Med Virol. 2017;89:1096–1101. doi: 10.1002/jmv.24744. [DOI] [PubMed] [Google Scholar]
- 74.Khovidhunkit SO, Buajeeb W, Sanguansin S, et al. Detection of human papillomavirus in oral squamous cell carcinoma, leukoplakia and lichen planus in Thai patients. Asian Pac J Cancer Prev. 2008;9:771–775. [PubMed] [Google Scholar]
- 75.Singhanetra-Renard A. Population mobility and the transformation of a village community in northern Thailand. Asia Pac Viewp. 1999;40:69–87. doi: 10.1111/1467-8373.00082. [DOI] [PubMed] [Google Scholar]
- 76.Kulsrisombat N. De Facto Urban Regeneration: A Case Study of Chiang Mai City, Thailand, Sustainable City Regions. Springer; Tokyo, Japan: 2008. https://link.springer.com/content/pdf/10.1007/978-4-431-78147-9.pdf [Google Scholar]
- 77.Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers: Major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]