One hundred and fifty years of marine wildlife trafficking records precede and predict modern IUU fishing patterns.
Abstract
The complexity of trade networks is a major challenge to controlling wildlife trafficking and illegal, unreported, and unregulated (IUU) fishing. These networks may not be modern inventions, but have developed over centuries, from integrated global markets that preceded modern regulatory policies. To understand these linkages, we curated 150 years of tortoiseshell transactions and derived biologically informed harvest models to estimate the trade in critically endangered hawksbill sea turtles (Eretmochelys imbricata). We find that trade networks concentrated in Southeast Asia harvested 9 million turtles, over six times previous estimates. These networks spread from within the Pacific, to the Indian and Atlantic basins, and became markedly more complex after 1950. Our results further indicate that the magnitude and extent of the coastally restricted hawksbill exploitation parallel current patterns of IUU fishing. Policies to combat these interlinked illegal practices should assimilate the important role of small-scale, coastal fisheries in these increasingly complex global networks.
INTRODUCTION
The proliferation of markets for illicit wildlife products has generated severe ecological and social consequences (1). In the ocean, this effect is best illustrated by illegal, unreported, and unregulated (IUU) fishing, which can be associated with human rights abuses, wildlife trafficking, drug, weapon, and other illegal trade (2–4). Valued at up to $23.5 billion annually, IUU fishing can mask the magnitude of declining global marine fisheries catches, is inversely correlated with governance capacity, and can undermine introduced management initiatives (5, 6). IUU fishing relies on complex human networks with transshipments connecting even the smallest-scale fisheries in remote island nations to distant water fleets and associated transport networks with little governance (7). The complexity of IUU fisheries networks decreases transparency, in part by obscuring the volumes traded, and compounds the inability of local management to keep pace with global exploitation (8).
Along with social and economic impacts, IUU fishing directly threatens imperiled marine wildlife, such as sea turtles (9). Therefore, understanding the development and structure of trade networks may help address both IUU fishing and wildlife trafficking. The development of globalized trade in marine wildlife involves geographic expansion, increasing the distances between the fishery and destination market (8). While research has focused on serial depletion of target species and the resulting expansion of fishing effort (8, 10), the complexity of networks supporting this global expansion deserves further study. Global trade networks are particularly opaque for species hunted for preserved parts, such as turtle shell or shark fins, as these commodities are disaggregated, which obscures identification and can be stockpiled for years by globally distributed buyer networks before arriving at their final destination (11). These networks may be especially concealed for high-value products, threatened species, and certain markets, such as those within China (12, 13).
The endangered hawksbill sea turtle (Eretmochelys imbricata) has been traded internationally perhaps longer and more intensively than any other marine species. Tortoiseshell has been valued by many cultures for millennia and is derived from the carapace scutes of the hawksbill sea turtle that are then carved into decorative and functional objects (14). The modern trade can be traced to 17th century Japan and Europe, when tortoiseshell carving was popularized and trade facilitated by expanding colonial networks (15). Since the 19th century, the tortoiseshell industry in Japan has been the major producer of carved tortoiseshell (16, 17). Trade was unregulated until 1977, when the Convention on International Trade of Endangered Species of Wild Fauna and Flora (CITES) banned the international trade of hawksbill turtles for all signatories by listing the global population as an Appendix I species. Many countries with notable hawksbill populations joined in the 1970s–1980s (e.g., Indonesia, the Philippines, and Malaysia). Japan took an exception to the CITES trade ban until 1992. Several other countries (e.g., Fiji, Vietnam, and Solomon Islands) did not sign onto CITES until years later. While nearly all CITES signatories have now agreed to an international trade ban, legal domestic exploitation in several countries (18) and tortoiseshell trafficking exist (12, 13, 19).
Tortoiseshell trade networks remain poorly described. Hawksbill sea turtles are one of the least abundant sea turtle species (16), with population estimates of fewer than 25,000 nesting females across their circumtropical range (20). Hawksbills are not threatened by incidental bycatch by high-seas commercial fisheries as they do not occupy pelagic waters for pronounced periods (21). Instead, hawksbill turtles have been directly targeted and may be the most heavily exploited sea turtle species (16). Direct exploitation is thought to be the major driver of their decline (16). Previous estimates of trade impact suggested that 1.4 million adult turtles were killed from 1950 to 1992 (16). However, these estimates have been limited by the underlying data and their interpretation.
Here, we compile the largest dataset of hawksbill tortoiseshell trade, which we analyze using morphometric models that we derived from stranding and seizure specimens to develop a range of size-based population depletion scenarios. The result is an estimate of the total number of turtles killed over 150 years, along with a spatially explicit analysis of the increasing complexity of global trade networks. These findings provide a more data-rich and, therefore, relatively realistic estimate of the total magnitude of global trade and serve as a reference point to assess the status of current hawksbill populations (22). Bringing transparency to these trade pathways is key to preventing further exploitation of this critically endangered species and to crafting solutions to the interdependent and seemingly intractable problems of IUU fishing and wildlife trafficking.
RESULTS
Biologically informed models increase the estimated magnitude of global tortoiseshell trade
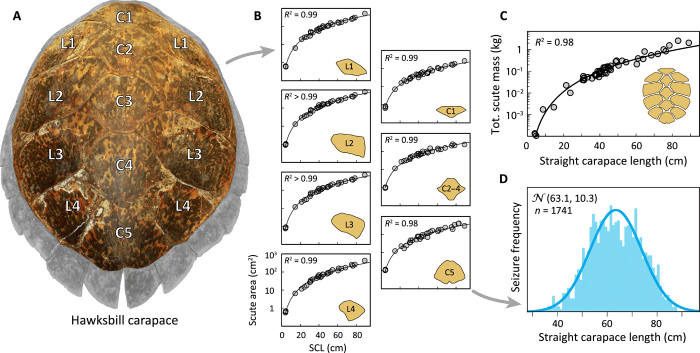
Previous estimates of hawksbill turtle exploitation relied on a simple assumption that all traded turtles were adults (16, 17). This assumption is unrealistic because juvenile and subadult turtles were also targeted and the age structure of exploited populations is known to change over time (23). In contrast, our derived morphometric relationships of length to tortoiseshell mass (Fig. 1C), based on measurements of tortoiseshell specimens (n = 1741 pieces) from a single seized shipment, indicate that modern harvests were likely composed primarily of juvenile hawksbills [average = 63.1-cm straight carapace length (SCL), SD = 10.3; Fig. 1D and see Materials and Methods]. Further, as this seizure of stacked scutes measured 0.22 m3 and contained 134 complete sets of carapace scutes, we estimate 1 m3 of stacked scutes with this age structure contains 609 individual sea turtles, indicative of the high number of individual turtles contained within seized shipments of scutes. These relationships inform our size-based scenarios we use to interpret historical trade records of tortoiseshell mass.
Fig. 1. Derived tortoiseshell morphometrics show modern trade was dominated by juvenile turtles.
(A) Lateral and central scutes of the hawksbill carapace, widely marketable in the tortoiseshell trade, are shown in color. (B) The relationship between SCL of an individual and the area (square centimeter) of each scute. (C) The relationship between individual length and total tortoiseshell mass. (D) On the basis of these relationships, the frequency of hawksbill turtle sizes recorded in one-seized shipment from the 1980s contained 1741 individuals and consisted of mostly large juveniles (average = 63.1-cm, SD = 10.3 SCL). See table S2 for model parameters.
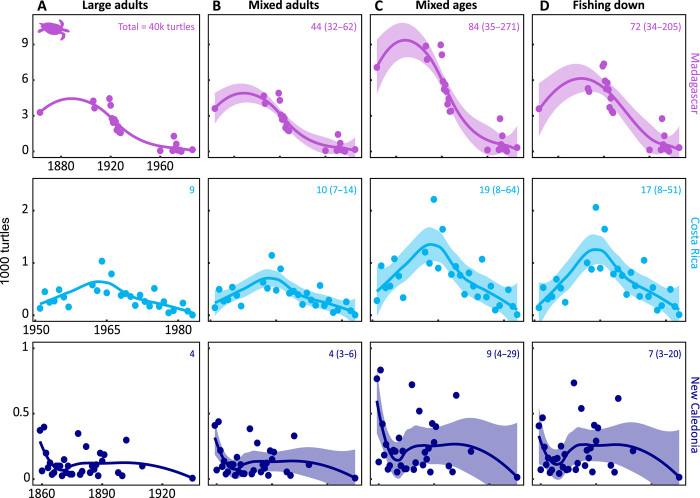
Our morphometric analyses inform four demographic scenarios to estimate the number of hawksbill sea turtles traded from 1844 to 1992 (Fig. 2 and see Materials and Methods). Globally, at least 4,640,062 individuals were exploited under the large adults scenario, 5,122,951 individuals under the mixed adults scenario, 9,834,837 individuals under the mixed ages scenario, and 8,976,503 under the fishing down scenario. Of these, the fishing down scenario is the most probable given the evidence of how fishing structures populations (23).
Fig. 2. Demographically explicit exploitation scenarios roughly double estimates of population impact.
Understanding the biological impact of tortoiseshell trade requires converting trade records (listed in mass of tortoiseshell) into number of turtles. We do this in four ways, left to right: (A) only large adults taken (size fixed at 80-cm SCL), (B) mixed adults taken (average = 80-cm SCL, SD = 4.3), (C) mixed-age classes dominated by juvenile turtles (reflects Fig. 1D; average = 63.1-cm SCL, SD = 10.3), and (D) fishing down the population [demographic depletion from scenarios (A) to (C) over the time series]. Median estimates (points) and locally estimated smoothing scatterplot (LOESS) models (curves) are shown with 95% confidence intervals for annual exports, total individuals exported (in 1000 turtles) under each scenario listed (with confidence interval) for three countries representative of each ocean basin—Indian (Madagascar), Atlantic (Costa Rica), and Pacific (New Caledonia).
Historical trade networks expanded spatially over time
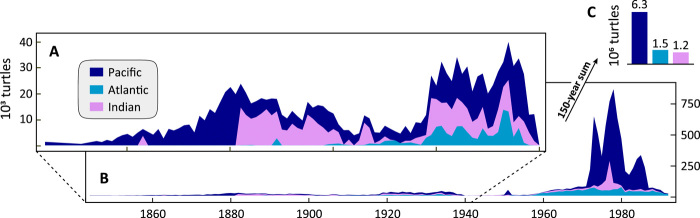
The modern global tortoiseshell trade expanded outward from Japan beginning in the Pacific basin in 1844, followed by the Indian basin in 1863 and the Atlantic basin in 1882 (Fig. 3). Records from the Japanese Customs archive began in 1868, the first year of the Meiji Era. World War II (WWII) imparted a clear signal in the cessation of international trade in the early 1940s, continuing through U.S. occupation (Fig. 3). The international trade of tortoiseshell spiked markedly in the 1970s through the 1980s. This peak consisted largely of exports from the Pacific basin (>100,000 kg year−1 for 10 years within 1970–1990), from one large shipment from India in 1977 of 108,705 kg, as well as consistent exports from the Atlantic basin of 12,000 to 34,000 kg year−1. Under the fishing down harvest scenario, 6,250,969 individual hawksbill sea turtles were harvested from the Pacific basin, 1,498,196 from the Atlantic basin, and 1,227,338 from the Indian basin over the entire period (Fig. 3).
Fig. 3. Over 150 years, approximately 9 million hawksbill turtles were traded globally.
Stacked area curves of the estimated number of individuals harvested under the fishing down scenario (Fig. 2D) are shown for (A) pre-WWII and (B) over the entire time series for each ocean basin. (C) Basin-wide harvested totals over the time series. This shows the geographic expansion of the global trade in tortoiseshell and the marked increase in trade coinciding with the establishment of international wildlife protections.
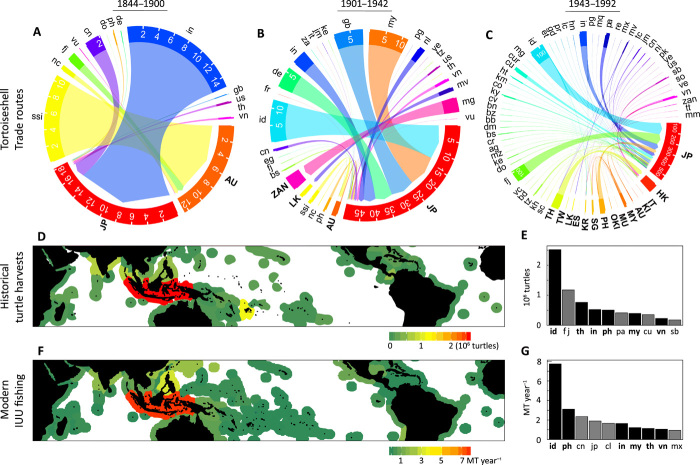
Country-specific patterns of imports and exports identified major historical operators. There were 26 importing and 73 exporting countries/territories from 1844 to 1992. The top importer was Japan (80.2%), followed by Hong Kong (11.4%), Singapore (2.2%), Australia (1.8%), South Korea (1.0%), and all other countries/territories (3.4%; see Materials and Methods and fig. S1). The top exporter was Indonesia (28.1%), followed by Fiji (13.1%), Thailand (8.5%), India (5.8%), the Philippines (5.7%), and all other countries/territories (38.8%; Fig. 4E).
Fig. 4. Historical patterns of tortoiseshell trade networks reflect modern IUU fishing.
Chord diagrams show the increasing complexity of tortoiseshell trade routes from (A) 19th century, (B) before WWII 20th century, and (C) after WWII. Arrows are from source to destination country, arrow width is proportionate to the number of hawksbill sea turtles traded (10,000 turtles). Export-only countries are labeled in lower case, and all other countries are labeled in bold and are capitalized (two-letter United Nations country codes; full list provided in table S3). (D) Total number of hawksbill sea turtles exported under the fishing down scenario, shown for each country’s EEZ, and constrained to the species’ geographic range (30°N to 30°S). (E) Top 10 tortoiseshell-exporting countries, listed by descending rank, in millions of turtles. (F) Modern IUU fishing in million metric tons (MT) per year per EEZ, derived from published assessments (60). (G) The top 10 countries with the highest estimates of IUU fishing in their EEZs (from 2005 to 2010), ranked in descending order, in million metric tons per year. Countries in the top 10 for both (D) and (F) are bold in (E) and (G).
CITES ratification by exporting countries influenced export volume
During the timeframe of this dataset, most of the countries banned international trade of hawksbill sea turtles via CITES accession in the late 1970s to early 1980s (fig. S2). Twenty-two exporting countries continued to export after their bans (fig. S2). Some countries increased their exports in the years immediately before their ban including Indonesia, Malaysia, the Philippines, Belize, and Honduras. Other countries had stopped exporting tortoiseshell well before their trade ban including Sri Lanka, the Maldives, New Caledonia, as well as several colonial powers such as the Netherlands, Italy, France, and the United Kingdom.
Trade networks developed complexity over time
Patterns of historical trade pathways demonstrated the increasing number of global trade partners over time, particularly after WWII (Fig. 4, A to C). From 1844 to 1900, there were 13 known connections (see Materials and Methods) between 13 export and 2 import countries (Fig. 4A). From 1901 to 1942, there were 30 connections between 29 export and 4 import countries (Fig. 4B). From 1943 to 1992, there were 109 connections between 61 export and 16 import countries (Fig. 4C). Using the number of turtles traded across source-destination connections, we calculated a Shannon index value of 1.3 before 1901, 2.2 from 1901 to 1942, and 3.0 from 1943 to 1992, a basic indication that large numbers of turtles moved through numerous channels as the trade networks increased in complexity (see Materials and Methods).
Historical trade reflects current trafficking patterns
Historically important tortoiseshell exporters had some of the highest rates of contemporary IUU fishing in their exclusive economic zones (EEZs), with Southeast Asia emerging as a clear hot spot for trade (Fig. 4, D to F). Indonesia was the top historical exporter and the country with the highest rate of modern IUU fishing. Six countries (Indonesia, Thailand, India, the Philippines, Malaysia, and Vietnam) were in both the top 10 lists of turtle exporters and IUU fishing rates within their EEZs. This overlap cannot fully be explained by geographic or biological factors. EEZ area does not predict IUU rate per country (5), which we would expect if larger areas are more difficult to enforce. Hawksbill sea turtle habitat (here defined as coral reef area) does not predict turtle exports per country (Kendall’s rank correlation coefficient tau-b = 0.158, P = 0.32), which we would expect if exploitation only reflected the turtle population size supported. The historical economic and political aspects of trade, therefore, influence the relative rankings of countries with the highest IUU rates and turtle exports.
Seizure records at U.S. ports of entry revealed trafficked hawksbill sea turtles or parts arrived from 72 countries (fig. S3) on 352 occasions from 1999 to 2018. Of the 20 countries with the most hawksbill sea turtle seizures, 14 were also historical turtle exporters (fig. S3). The most frequently used transportation mode was air cargo, followed by personal accompanying baggage and mail (fig. S4). Seventeen of 352 seizures arrived by ocean cargo, indicating that much of the modern global trade has shifted from sea to air transportation. The majority (>65%) of hawksbill sea turtle products seized entering the United States was raw, unprocessed whole turtle, carapace, or scutes rather than carved tortoiseshell (fig. S4). Of the unprocessed records of varying sizes, 41% was from the Caribbean, Central America, and Mexico, indicating that the United States may be a node for hawksbills transported from the western hemisphere to Asia.
DISCUSSION
Our estimate of the total magnitude of global trade demonstrates that the impact of the historical tortoiseshell trade on the hawksbill sea turtle population has been markedly underestimated. Previous estimates of this trade suggest that 1,374,242 turtles were traded for tortoiseshell (16, 17). This calculation assumed only that adults were harvested and incorporated 42 years of records (1950–1992). Using those same demographic assumptions and curating an additional 108 years of trade data, we estimate that at least 4,640,062 individuals were harvested. However, considering tortoiseshell morphometrics (Fig. 1, B and C) and the demographics of recent seizures (Fig. 1D), we estimate that the global tortoiseshell trade killed 8,976,503 turtles, over six times more than this previous estimate (16). This difference underscores how biologically relevant and demographically informed assumptions improve population assessments.
While our dataset extends the baseline for hawksbill turtles back a century, it still may not capture the full magnitude of global trade (24, 25). Nineteenth century exports from the Bahamas comprised as many as 7000 turtles annually (26, 27), and previous extrapolations of these trade data yielded a precolonial baseline population estimate of 11 million turtles in the Caribbean (28). Therefore, it is likely that our relatively low estimate of trade for the Atlantic and Indian oceans is biased by the lack of consistent trade data for these regions. Nonetheless, our global estimate of nearly 9 million hawksbill sea turtles traded over 150 years, or an average of just under 60,000 turtles traded annually, is significant when we consider recent estimates of their global population size. In 2004, between 21,000 and 24,000 females were estimated to nest annually (20). This historical harvest has a marked impact on the population, given that the age at maturity for hawksbill has been estimated at 20 to 40 years with a generation time of 35 years (29).
Sea turtles are important grazers, maintaining the health of sea grass and coral reef ecosystems. Their population decline has likely altered ecosystem dynamics. Historical declines of sea turtles have reduced grazing rates by up to 800 times relative to today (28). At these scales, their removals have likely altered the structure and function of these important ecosystems across the world.
Our results provide a unique window into the development of global trade networks for marine wildlife. Trade connections increased by 8.4 times from the 18th century to the second half of the 20th century. This increase, in part, reflects rapid globalization and advances in transportation technology that increased connectivity following WWII. These patterns also reflect the importance of reexport, particularly for the trade in preserved marine wildlife parts. Taiwan, Singapore, South Korea, and Hong Kong were known trade network nodes, aggregating and then exporting tortoiseshell to Japan (13, 16, 30). We removed exports from these countries from our analysis because they would likely duplicate harvest estimates. However, their important role as aggregators is evident in their import volumes (fig. S1). Similarly, many exporting countries did not have sufficient hawksbill populations to supply the volume of tortoiseshell they exported. For example, hawksbill sea turtles do not nest on Mainland India in significant numbers and only nest in the Andaman and Nicobar islands (administered by India) at low levels (30), yet India was the fourth greatest exporter from our records. India, therefore, also likely functioned as an aggregating node in a larger network of wildlife trade.
Despite this complexity, two nations emerged as key players in the global tortoiseshell trade. Japan was the major importer of tortoiseshell, with 76% of total volume sent to Japan as a single destination and 80% when we included multidestination shipments (fig. S1). Although Japan’s comprehensive record-keeping biases this result, Japan is widely described as the top importer during this period (16, 17). In addition, the top five importing countries were all located in the western Pacific; several of these countries were likely reexporting to Japan. Indonesia was the major exporter, exporting by itself an order of magnitude more tortoiseshell than a majority of other source countries, with an estimated 2,521,569 turtles exported over 85 years. Indonesia, the heart of the Coral Triangle, is the global epicenter of marine biodiversity (31). Indonesia’s extensive coastline and coral reefs supported substantial hawksbill populations.
The dominant feature of the 150-year record of tortoiseshell trade is the marked peak in volume during 1970–1985 in which 74% of all turtles were traded (Fig. 3). Throughout the 20th century, tortoiseshell markets were sensitive to international political conditions. WWII paused international trade and the subsequent U.S. occupation closed Japanese borders to most importation (32). U.S. policy subsequently shifted from demilitarization to strengthening commerce during the Korean War, and Japan began sustained economic growth in the mid-1950s (32), which coincided with the resumption of tortoiseshell trade (Fig. 3). Favorable economic and political conditions in Japan and Indonesia, the two major tortoiseshell traders, may explain the late century increase in trade. This peak closely followed reduced trade barriers and rapid growth in Japan, where from 1946 to 1976, the economy increased 55-fold (32). This growth expanded the Japanese middle class and may have fueled an increased demand for luxury products such as tortoiseshell. Likewise, 71% of Indonesia’s turtle exports occurred from 1976 to 1980 following “New Order” policies geared to increase foreign investment and strengthen trade with Japan and Western nations. Policies during this period also encouraged redistributing populations from Java to smaller islands, partly to exploit Indonesia’s wealth of natural resources (33). An increase in Japanese demand, local hawksbill population depletions, and the increasing connectivity following globalization may have led to increased trade complexity and volume after WWII.
While the CITES trade ban was the major driver of the decline in tortoiseshell trade, it likely contributed to the trade peak. Within the timeframe of our dataset, most countries signed onto CITES from 1975 to 1985. The marked decline in trade through the end of Japan’s reservation can be largely explained by the hawksbill’s listing as an Appendix I species in 1977 (fig. S2). Many of the top exporting countries increased their exports immediately before signing onto CITES (fig. S2). Others, including some former colonial powers, had ceased well before (fig. S2). As 1979 was the year Indonesia entered CITES into force, Indonesia’s export peak (1976–1980) may indicate stockpiling or increased harvest in anticipation of enforcement of the ban. This anticipation trend has been reported for CITES listings previously and generally reflects the unloading of stockpiles or increased harvest (34).
Substantial hawksbill exploitation continues. China has emerged as a major consumer of hawksbill sea turtles (12, 35), with vessel trafficking routes from Indonesia, Malaysia, and the Philippines to China’s Hainan province. A top exporting country historically, Vietnam has greatly reduced domestic hawksbill exploitation (19). However, seizures on Vietnamese vessels fishing in the Philippines indicate expanded foreign poaching (35). In Indonesia, mislabeled hawksbill harvests within the legal green turtle (Chelonia mydas) fishery, local tortoiseshell processing operations, and foreign fleet poaching have all been reported (12, 13). While vessel transport of hawksbill products is the major mode from the Coral Triangle to China, trafficking to Japan has shifted to air transport. Seizure records indicate small-scale shipments of scutes smuggled by air or mail have replaced large vessel shipments and mainly originate from Singapore (12, 13). However, two large shipments from Indonesia (+400 shell pieces and products in 2000 and 89 kg of scutes in 2003) were seized in Japan, indicating that the major historical import route remains (12). This shift to air transport may reflect effective vessel inspection and tortoiseshell’s high value in Japan, which reduce the risk relative to reward for small air shipments. U.S. seizure records similarly emphasized air transportation’s contribution to the global trafficking of hawksbill sea turtle (fig. S4).
The rapid economic growth of the middle class in China has fueled the global trade of luxury marine wildlife products (36, 37). The shark fin market is concentrated in a few Asian trade network nodes including Singapore, Taiwan, Japan, mainland China, and the largest, Hong Kong (36, 37). Like the tortoiseshell trade, Southeast Asia’s luxury seafood trade flowed to these same East Asian markets over a long history, expanded geographically, and intensified over time (36). Several of these trading centers are major hawksbill sea turtle harvesting and trade destinations. Like these marine luxury wildlife products, tortoiseshell is nonperishable and therefore stockpiled and traded globally, distancing consumers from exploiters, reducing traceability, and decoupling the feedback of reduced demand with increasing cost over value (11).
Our results also underscore the degree to which these global markets rely on the mobilization of local networks of small-scale artisanal fishers. Hawksbill sea turtles are associated with coral reefs and other nearshore habitats and, therefore, typically caught in coastal artisanal fisheries and not in pelagic commercial fisheries (21). Artisanal vessels are known to participate in transshipment, including hand line vessels targeting tuna in western Indonesia that transfer catch to large vessels for overseas trade (7). Furthermore, Hainan fisheries enforcement agency data suggest that marine turtles in the market originate as bycatch and targeted catch from local fishermen in the Philippines, Malaysia, Indonesia, and Vietnam (12). These small-scale fisheries can have marked population impacts (38, 39), particularly when they are serving global luxury markets.
Bringing transparency to these trade pathways is key to beginning to craft solutions to the seemingly intractable, broad problem of IUU fishing. IUU operators exploit gaps in jurisdiction and authority (40) related to vessel size, vessel origin, inspection authority limits, proximity to shore, transit direction, and region. Transshipment is a noted enforcement gap. IUU practices are especially associated with transshipping on the high seas and can connect artisanal coastal fisheries with distant-water fleets (2, 3, 7). Banning all transshipments has been proposed previously to reduce IUU fishing and is currently in place in the South East Atlantic Fisheries Organization [a Regional Fishery Management Organization (RFMO) governance treaty] with partial bans in several other RFMOs (2). Improved regulation of transshipments might also benefit hawksbills in RFMOs covering Southeast Asia, where rates of IUU fishing (Fig. 4) (41), wildlife trafficking (42), and hawksbill nesting populations (16) are relatively high.
Given that geographic and biological factors do not fully explain turtle export or IUU fishing patterns at the country level, governance may play a critical role in shaping trafficking networks. Effective enforcement requires independent monitoring programs, patrol surveillance in territorial waters, transparent systems of vessel flags identities, complete reporting to local and flag countries, universal vessel tracking, matching monitoring technology to capacity, and data sharing, for which many developing countries have limited capacity (41, 43). In addition, closing IUU fishing enforcement loopholes requires interagency coordination, training law enforcement agencies that conduct inspections related to other illicit activities and cross-deputization agreements (44, 45). To complicate matters further, nations and RFMOs will also need to adaptively manage across boundaries to keep pace with shifting distributions of fisheries due to climate change (46). Beyond governance, campaigns to reduce consumer demand for tortoiseshell products may achieve similar success as shark fin campaigns (47).
The historical pattern of tortoiseshell trade provides a map for current trafficking, although some network paths have shifted, strengthened, or faded. The strong links between IUU fishing and marine wildlife poaching and trafficking underscore the need for integrated monitoring and management of small-scale coastal fisheries and high-seas commercial fleets. Success here may benefit the continued persistence of endangered marine wildlife and reduce human rights abuses, and drug, weapon, and other illegal trade also associated with these networks.
MATERIALS AND METHODS
Specimens and trade data
U.S. Fish and Wildlife Service (USFWS) Office of Law Enforcement provided seized carapaces that had been traded illegally, and National Oceanic and Atmospheric Administration Pacific Islands Fisheries Science Center (USFWS permit TE-72088A-0) collected stranded hawksbill sea turtle carapaces. Whole carapace specimens (n = 58) ranged from 4- to 89-cm SCL, across all demographics from emergent hatchlings to breeding adults. USFWS provided one additional single seizure of ~65 kg of stacked disaggregated scutes confiscated in 1988 in a shipment from the Caribbean to the United States. Nine published accounts (30, 48–55) and Japanese Customs archives provided records of tortoiseshell shipments from 1844 to 1992, when Japan ended their exemption to the CITES ban on international trade (dataset S1). Records contained the year, mass of tortoiseshell shipped, source country or region, destination country or countries, ocean basin, and citation source. We curated records to remove duplicate import and export records that often occurs as a result of reexport (table S1).
To ensure that data were not double-counted, if import and export data existed in same year, the smaller value was discarded. If there were multiple values noted as import or export in the same year, then the smaller value was assumed to be a subset of the larger value and discarded. To account for reexport, we (i) discarded all export data from the following known reexporters: Singapore, Hong Kong, Taiwan, and South Korea; (ii) discarded data from European countries, USA, and Canada after 1950, with the assumption that these data were recorded at the original point of export; (iii) discarded data from countries with freshwater turtles (Zambia, Laos). For European countries before 1950, we reattributed turtles to source basins based on their colonial holdings (table S1). From 1882 to 1887, Japanese Customs data recorded imports from the India, Thailand, Vietnam, Indonesia, and Malaysia as the “East Indies.” Beginning in 1888, Thailand was categorized separately from the East Indies but contained no records. Starting in 1889, each country’s exports were recorded separately, and the recorded values from India were comparable to those from the East Indies before 1888, while the other East Indies countries contributed insignificantly. This pattern indicated the records from the East Indies before 1888 largely originated from India and attributed as such.
Specimen preparation
Carapace specimen preparation followed published methods (29). Each carapace was placed in a labeled, research-grade plastic bag that was perforated to allow gases to escape and submerged in seawater for one to three weeks. After this process, we separated the 13 carapace scutes used primarily in the tortoiseshell industry from all other tissues, removing epibionts and other adherents. We scrubbed scutes with a mildly abrasive sponge using soap and warm water, soaked in 90% ethanol for 5 min, and air-dried in a fume hood. For all scutes, we identified their position (Fig. 1A), weighed, measured, and kept them in dry archive (dataset S2).
Calculating turtle demographics from scute size
We related scute metrics to turtle morphometrics to derive relationships useful for understanding trade demographics. To calculate the demographics of seized tortoiseshell shipments (consisting only of scutes), we first developed length-to-area relationships for each individual scute (Fig. 1B). As scutes are curved and irregularly shaped, we calculated their precise area using ArcGIS 9.3. For 32 of the total 58 specimens, we traced scute outlines on paper, imaged the tracings on a 1-cm grid surface, scanned and georeferenced the images (constraining root mean square error to <0.10), created polygon shapefiles for each scute, and calculated their area. To rapidly assess large seizures, we developed a basic scaling rule relating the crude rectangular area (longest length × widest width) to the precise area calculated above (fig. S5). In these relationships, we paired symmetrical (left and right) lateral scutes, as well as central scutes 2, 3, and 4 (as these are not later distinguishable). We plotted the precise area against SCL and the crude area against the precise area, fitting power law models to the data (fig. S6). Next, for all 58 specimens, we derived the length-to–tortoiseshell mass relationship, again fitting a power law to the data (Fig. 1C). Last, we measured the crude area of 1741 scutes from a law enforcement seizure, used the above-derived relationships to calculate the lengths of turtles from which the scutes were taken, and used normal statistics to summarize the shipment demographics (Fig. 1D).
Estimation methods of sea turtles harvested
Each export record (kilograms of shell) was converted to the number of individuals harvested under four harvest scenarios (see fig. S7) using the relationship between scute mass produced by a hawksbill of a given SCL. The four harvest scenarios included the following: “large adults,” where all turtles were assumed to be an average adult length [80-cm SCL (29)], or the traditional harvest assumption (16, 17); “mixed adults,” where all turtles were from a normally distributed range of adults (72- to 90-cm SCL; average = 80 cm ± 4 SD); “mixed ages,” where turtles included a range of juveniles and adults based on a normal distribution of the seized shipment (average = 63.1 cm ± 10.3 SD); and “fishing down,” where turtles harvested were initially from a normally distributed range of adults (mixed adults); but over the time series for each source country, the harvest demographic incrementally shifted linearly to a mixed-sizes distribution (mixed ages), which it reached at the 75th quantile of harvested biomass for the time series. The concept of fishing down marine food webs refers to the sequential depletion of higher trophic levels affecting community function or, as in this case, larger size classes altering population structure and growth (23, 56). This population depletion reflected opportunistic harvesting in which the easier to obtain nesting females were harvested first and fishers spatially expanded to capture a range of juveniles and adults.
We reported total estimates of harvest under each scenario by summing the median estimates of each export shipment per source country across all years globally as well as for representative countries from each basin. Upper and lower confidence intervals were summed to provide ranges. We fit locally estimated scatterplot smoothing (LOESS) smoothing models to the estimates with 95% confidence intervals for representative country time series using the R package ggplot2.
Spatial components of historical trade and current IUU patterns
We reported estimates using the fishing down scenario, the most biologically realistic scenario, for ocean basin-wide and country scales. Export records that were not assignable to an ocean basin source were categorized as “Pacific or Indian,” “Atlantic or Pacific,” or “unknown” basin origin. For basin-wide analyses, these unknown shipment masses were redistributed among the three known basins (Pacific, Indian, or Atlantic) according to the relative proportions of known basin exports per year.
Country-specific pathways of importing and exporting were visualized as chord diagrams using migest and circlize R packages (57, 58). A connection between two countries was defined as at least one record of direct tortoiseshell trade from an exporting country to an importing country. Each chord pathway represented a connection between two countries and was proportionate in width to the total number of estimated turtles traded. To examine import patterns, we redistributed export records with multiple destination countries among each individual destination country according to the relative proportions imported over the time series. Imports from nine of these destination countries/regions were not redistributed because they did not appear in our records as a single destination. These included Canada, New Caledonia, Germany, China, Great Britain, Switzerland, Mexico, the Netherlands, and the United States. These redistributed volumes were used in chord pathway visualization (Fig. 4) and in examining top importing countries (see Results and fig. S1). To describe increasing trade network complexity, Shannon index values were calculated using each source-destination pair as an entity in the “diverse” R package (59).
To compare spatial patterns of historical hawksbill harvest with current IUU fishing, estimates of turtles were totaled for each exporting country and visualized within each country’s EEZ. The IUU fishing data presented were obtained from Watson (60). These data were IUU catch estimates in commercial fishing based on rates of IUU fishing calculated by Agnew et al. (5). The data were average estimates per 30-min spatial cell in metric tons per square kilometer per year from 2015 to 2018. Estimates were averaged across country’s EEZs and multiplied by square kilometers to obtain the total produced within each EEZ per year. To calculate the relationship between hawksbill sea turtle habitat and turtle exports per country, we used coral reef area data from the World Atlas of Coral Reefs (61) and measured the association between the two parameters by calculating Kendall’s rank coefficient, tau-b. All analyses were performed using R version 3.4.3 and RStudio 1.1.419.
Seizure records of hawksbill sea turtle parts and products at U.S. ports of entry were obtained from USFWS Office of Law Enforcement via Freedom of Information Act request no. FWS-2018-00548 (dataset S3). The 352 records spanned the time period 1999 to 2018.
Supplementary Material
Acknowledgments
G. Balazs, S. Green, B. Baker, and A. M. Lauritsen provided hawksbill carapaces. T. Jones, S. Murakawa, T. Iwamura, S. Brunson, S. Imamura, D. Francke, and A. Niccum assisted with necropsies, carapace preparation, and data collection. Data collection from Japanese Customs archives was previously granted by Nippon Life Insurance Foundation. A. Lawrence and A. McCracken assisted with database management. A. Boustany, S. Jorgensen, T. Gagne, J. Moxley, T. Nicholson, A. Norton, S. Becker, and several anonymous reviewers improved earlier versions of this manuscript. Funding: There are no funding sources for this research. Author contributions: L.M. and K.S.V.H. designed the study. E.A.M. and K.S.V.H. analyzed the data and drafted figures. E.A.M., L.M., and K.S.V.H. wrote the manuscript. All authors contributed data and edited the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Data files are in the Supplementary Materials and in the Open Science Framework (OSF.IO/J2NT6). Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav5948/DC1
Fig. S1. Japan was the major importer of tortoiseshell from 1844 to 1992.
Fig. S2. Turtle exports per country varied in relation to CITES trade ban.
Fig. S3. Illegally trafficked hawksbill sea turtle parts and products are seized entering the United States from more than 70 countries.
Fig. S4. Seized hawksbill sea turtles are trafficked to the United States largely by air transportation and in raw forms more frequently than processed tortoiseshell.
Fig. S5. Power law models show the relationship between calculated precise and crude areas for individual scutes and scute groupings from hawksbill sea turtles.
Fig. S6. Hawksbill sea turtle specimens used in this study were a range of sizes.
Fig. S7. Demographically explicit scenarios produce different estimates of the number of hawksbill sea turtles harvested.
Table S1. Hawksbill sea turtle export data curation methods.
Table S2. Model parameters for tortoiseshell morphometric relationships in Fig. 1.
Table S3. Complete list of United Nations country abbreviations (alpha 2) used in Fig. 4 and figs. S1 and S3.
Dataset S1. Hawksbill sea turtle historical trade records.
Dataset S2. Hawksbill sea turtle scute morphometrics.
Dataset S3. U.S. seizure records of hawksbill sea turtles.
REFERENCES AND NOTES
- 1.Brashares J. S., Abrahms B., Fiorella K. J., Golden C. D., Hojnowski C. E., Marsh R. A., McCauley D. J., Nunez T. A., Seto K., Withey L., Wildlife decline and social conflict. Science 345, 376–378 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Ewell C., Cullis-Suzuki S., Ediger M., Hocevar J., Miller D., Jacquet J., Potential ecological and social benefits of a moratorium on transshipment on the high seas. Mar. Policy 81, 293–300 (2017). [Google Scholar]
- 3.Chapsos I., Hamilton S., Illegal fishing and fisheries crime as a transnational organized crime in Indonesia. Trends Organ. Crime, 1–19 (2018). [Google Scholar]
- 4.L. S. Wyler, P. A. Sheikh, “International illegal trade in wildlife: Threats and U.S. Policy” (no. ADA486486, Congressional Research Service, The Library of Congress, 2008).
- 5.Agnew D. J., Pearce J., Pramod G., Peatman T., Watson R., Beddington J. R., Pitcher T. J., Estimating the worldwide extent of illegal fishing. PLOS ONE 4, e4570 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauly D., Zeller D., Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 7, 10244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.M. A. McCoy, “Regulation of transshipment by the Western and Central Pacific Fisheries Commission: Issues and considerations for FFA member countries (FFA report no. 2007/26, Gillet, Preston and Associates Inc., 2007).
- 8.Anderson S. C., Flemming J. M., Watson R., Lotze H. K., Serial exploitation of global sea cucumber fisheries. Fish Fish. 12, 317–339 (2010). [Google Scholar]
- 9.Riskas K. A., Tobin R. C., Fuentes M. M. P. B., Hamann M., Evaluating the threat of IUU fishing to sea turtles in the Indian Ocean and Southeast Asia using expert elicitation. Biol. Conserv. 217, 232–239 (2018). [Google Scholar]
- 10.Berkes F., Hughes T. P., Steneck R. S., Wilson J. A., Bellwood D. R., Crona B., Folke C., Gunderson L. H., Leslie H. M., Norberg J., Nyström M., Olsson P., Österblom H., Scheffer M., Worm B., Globalization, roving bandits, and marine resources. Science 311, 1557–1558 (2006). [DOI] [PubMed] [Google Scholar]
- 11.McClenachan L., Cooper A. B., Dulvy N. K., Rethinking trade-driven extinction risk in marine and terrestrial megafauna. Curr. Biol. 26, 1640–1646 (2016). [DOI] [PubMed] [Google Scholar]
- 12.T. Lam, X. Ling, S. Takahashi, E. A. Burgess, Market Forces: An Examination of Marine Turtle Trade in China and Japan (TRAFFIC East Asia, Hong Kong, 2011). [Google Scholar]
- 13.P. P. van Dijk, C. R. Shepherd, Shelled Out? A Snapshot of Bekko Trade in Selected Locations in South-East Asia (TRAFFIC Southeast Asia, 2004). [Google Scholar]
- 14.J. Frazier, Prehistoric and ancient historic interactions between humans and marine turtle, in The Biology of Sea Turtles, P. L. Lutz, J. A. Musick, J. Wyneken, Eds. (CRC Press, 2003), vol. II, chap. 1, pp. 1–38. [Google Scholar]
- 15.M. Chaiklin, Imports and autarky: Tortoiseshell in early modern Japan, in Luxury in Global Perspective: Objects and Practices, 1600–2000, B.-S. Grewe, K. Hofmeester, Eds. (Cambridge Univ. Press, 2016), pp. 218–241. [Google Scholar]
- 16.J. A. Mortimer, M. Donnelly (IUCN SSC Marine Turtle Specialist Group), Eretmochelys imbricata. The IUCN Red List of Threatened Species 2008, e.T8005A12881238 (2008).
- 17.T. Milliken, H. Tokunaga, The Japanese Sea Turtle Trade 1970–1986 [TRAFFIC(Japan), 1987].
- 18.Humber F., Godley B. J., Broderick A. C., So excellent a fishe: A global overview of legal marine turtle fisheries. Divers. Distrib. 20, 579–590 (2014). [Google Scholar]
- 19.D. Stiles, An Assessment of the Marine Turtle Products Trade in Viet Nam (TRAFFIC Southeast Asia, 2008). [Google Scholar]
- 20.J. R. Spotila, Sea Turtles: A Complete Guide to their Biology, Behavior, and Conservation (The Johns Hopkins Univ. Press, 2004), 212 pp. [Google Scholar]
- 21.Van Houtan K. S., Francke D. L., Alessi S., Jones T. T., Martin S. L., Kurpita L., King C. S., Baird R. W., The developmental biogeography of hawksbill sea turtles in the North Pacific. Ecol. Evol. 6, 2378–2389 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kittinger J. N., Van Houtan K. S., McClenachan L., Lawrence A. L., Using historical data to assess the biogeography of population recovery. Ecography 36, 868–872 (2013). [Google Scholar]
- 23.Hsieh C.-h., Reiss C. S., Hunter J. R., Beddington J. R., May R. M., Sugihara G., Fishing elevates variability in the abundance of exploited species. Nature 443, 859–862 (2006). [DOI] [PubMed] [Google Scholar]
- 24.W. Williams, Mr Penrose: The Journal of Penrose, Seaman (Indiana Univ. Press, 1969). [Google Scholar]
- 25.M. Craton, G. Saunders, Islanders in the Stream: A History of the Bahamian People: Volume One: From Aboriginal Times to the End of Slavery (University of Georgia Press, 1992). [Google Scholar]
- 26.G. Northcroft, Sketches of Summerland, Giving Some Account of Nassau and the Bahama Islands (Nassau Guardian, 1900). [Google Scholar]
- 27.R. A. Naylor, Influencia británica en el comercio centroamericano durante las primeras décadas de la Independencia (1821–1851). [Antigua, Guatemala: Centro de Investigaciones Regionales de Mesoamerica, and South Woodstock (Vermont): Plumsock Mesoamerican Studies, 1988].
- 28.McClenachan L., Jackson J. B. C., Newman M. J. H., Conservation implications of historic sea turtle nesting beach loss. Front. Ecol. Environ. 4, 290–296 (2006). [Google Scholar]
- 29.Van Houtan K. S., Andrews A. H., Jones T. T., Murakawa S. K. K., Hagemann M. E., Time in tortoiseshell: A radiocarbon-validated chronology in sea turtle scutes. Proc. R. Soc. 283, 20152220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.B. Groombridge, R. Luxmore, The Green Turtle and Hawksbill (Reptilia: Cheloniidae): World Status, Exploitation and Trade (IUCN Conservation Monitoring Centre, 1989), 601 pp. [Google Scholar]
- 31.Jenkins C. N., Van Houtan K. S., Global and regional priorities for marine biodiversity protection. Biol. Conserv. 204, 333–339 (2016). [Google Scholar]
- 32.C. Johnson, MITI and the Japanese Miracle: The Growth of Industrial Policy: 1925–1975 (Stanford Univ. Press, 1982). [Google Scholar]
- 33.Fearnside P. M., Transmigration in Indonesia: Lessons from its environmental and social impacts. Environ. Manag. 21, 553–570 (1997). [Google Scholar]
- 34.Rivalan P., Delmas V., Angulo E., Bull L. S., Hall R. J., Courchamp F., Rosser A. M., Leader-Williams N., Can bans stimulate wildlife trade? Nature 447, 529–530 (2007). [DOI] [PubMed] [Google Scholar]
- 35.N. J. Pilcher, E. H. Chan, R. Trono, “Mass turtle poaching: A case study from Southeast Asia,” in SWOT: The State of the World’s Sea Turtles (2008); https://static1.squarespace.com/static/5b80290bee1759a50e3a86b3/t/5bb3e68f104c7b401473214a/1538516626051/r3_case-study.pdf.
- 36.Fabinyi M., Pido M., Harani B., Caceres J., Uyami-Bitara A., de las Alas A., Buenconsejo J., Ponce de Leon E. M., Luxury seafood consumption in China and the intensification of coastal livelihoods in Southeast Asia: The live reef fish for food trade in Balabac, Philippines. Asia Pac. Viewp. 53, 118–132 (2012). [Google Scholar]
- 37.Clarke S. C., Understanding pressures on fishery resources through trade statistics: A pilot study of four products in the Chinese dried seafood market. Fish Fish. 5, 53–74 (2004). [Google Scholar]
- 38.Van Houtan K. S., Kittinger J. N., Historical commercial exploitation and the current status of Hawaiian green turtles. Biol. Conserv. 170, 20–27 (2014). [Google Scholar]
- 39.Jacquet J., Alava J. J., Pramod G., Henderson S., Zeller D., In hot soup: Sharks captured in Ecuador’s waters. Environ. Sci. 5, 269–283 (2008). [Google Scholar]
- 40.Österblom H., Sumaila U. R., Bodin Ö., Sundberg J. H., Press A. J., Adapting to regional enforcement: Fishing down the governance index. PLOS ONE 5, e12832 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrossian G. A., Preventing illegal, unreported and unregulated (IUU) fishing: A situational approach. Biol. Conserv. 189, 39–48 (2015). [Google Scholar]
- 42.Lin J., Tackling southeast Asia’s illegal wildlife trade. S.Y.B.I.L. 9, 191–208 (2005). [Google Scholar]
- 43.Detsis E., Brodsky Y., Knudtson P., Cuba M., Fuqua H., Szalai B., Project Catch: A space based solution to combat illegal, unreported and unregulated fishing: Part I: vessel monitoring system. Acta Astronaut. 80, 114–123 (2012). [Google Scholar]
- 44.Österblom H., Bodin Ö., Global cooperation among diverse organizations to reduce illegal fishing in the Southern ocean. Conserv. Biol. 26, 638–648 (2012). [DOI] [PubMed] [Google Scholar]
- 45.E. Myers, S. Yozell, “Civil-military cooperation to combat illegal, unreported, and unregulated (IUU) fishing: A summary of the September 2017 National Interagency Advisory Group Meeting” (The Stimson Center, Environmental Security Program, 2018). [Google Scholar]
- 46.Pinsky M. L., Reygondeau G., Caddell R., Palacios-Abrantes J., Spijkers J., Cheung W. W. L., Preparing ocean governance for species on the move. Science 360, 1189–1191 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Fabinyi M., Historical, cultural and social perspectives on luxury seafood consumption in China. Environ. Conserv. 39, 83–92 (2012). [Google Scholar]
- 48.J. A. Bennett, Wealth of the Solomons: A History of a Pacific Archipelago, 1800-1978 (University of Hawaii Press, 1987). [Google Scholar]
- 49.C. J. Limpus, A Biological Review of Australian Marine Turtle Species. 3. Hawksbill turtle, Eretmochelys imbricata (Linnaeus) (Queensland Environmental Protection Agency, 2009). [Google Scholar]
- 50.C. J. Limpus, J. D. Miller, Australian Hawksbill Turtle Population Dynamics Project (Queensland Environmental Protection Agency, 2008). [Google Scholar]
- 51.B. Halkyard, Exploiting green and hawksbill turtles in Western Autralia: The commercial marine turtle fishery, in Historical Perspectives of Fisheries Exploitation in the Indo-Pacific, J. Christensen, M. Tull, Eds. (Springer, 2014), vol. 12, pp. 211–230. [Google Scholar]
- 52.K. L. Eckert, “Biology and population status of marine turtles in the North Pacific Ocean,” in NOAA Technical Memorandum (NOAA-TM-NMFS-SWFSC-186, 1993), pp. 1–166.
- 53.J. A. Mortimer, Sea Turtle Biology & Conservation in the Arnavon Marine Conservation Area (AMCA) of the Solomon Islands (The Nature Conservancy, 2002). [Google Scholar]
- 54.M. Donnelly, “Trade routes for tortoiseshell,” in SWOT : The State of the World’s Sea Turtles Report III (2008), pp. 24–25.
- 55.N.-s. Nomukyoku, Suisan hakurankai dai 4 ku shuppinn sinsahokoku tokei-bu [Statictical reporst of exhibits of the group four in the first Fisheries Exposition] (1884), pp. 94.
- 56.Pauly D., Christensen V., Guénette S., Pitcher T. J., Sumaila U. R., Walters C. J., Watson R., Zeller D., Towards sustainability in world fisheries. Nature 418, 689–695 (2002). [DOI] [PubMed] [Google Scholar]
- 57.G. J. Abel, migest: Methods for the indirect estimation of bilateral migration, version 1.7.4 (2018).
- 58.Gu Z., Gu L., Eils R., Schlesner M., Brors B., circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014). [DOI] [PubMed] [Google Scholar]
- 59.M. R. Guevara, D. Hartmann, M. Mendoza, diverse: An R package to analyze diversity in complex systems (R Statistical Software, 2017).
- 60.Watson R. A., A database of global marine commercial, small-scale, illegal and unreported fisheries catch 1950–2014. Sci. Data 4, 170039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.M. D. Spalding, C. Ravilious, E. P. Green, World Atlas of Coral Reefs (UNEP World Conservation Monitoring Centre, 2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav5948/DC1
Fig. S1. Japan was the major importer of tortoiseshell from 1844 to 1992.
Fig. S2. Turtle exports per country varied in relation to CITES trade ban.
Fig. S3. Illegally trafficked hawksbill sea turtle parts and products are seized entering the United States from more than 70 countries.
Fig. S4. Seized hawksbill sea turtles are trafficked to the United States largely by air transportation and in raw forms more frequently than processed tortoiseshell.
Fig. S5. Power law models show the relationship between calculated precise and crude areas for individual scutes and scute groupings from hawksbill sea turtles.
Fig. S6. Hawksbill sea turtle specimens used in this study were a range of sizes.
Fig. S7. Demographically explicit scenarios produce different estimates of the number of hawksbill sea turtles harvested.
Table S1. Hawksbill sea turtle export data curation methods.
Table S2. Model parameters for tortoiseshell morphometric relationships in Fig. 1.
Table S3. Complete list of United Nations country abbreviations (alpha 2) used in Fig. 4 and figs. S1 and S3.
Dataset S1. Hawksbill sea turtle historical trade records.
Dataset S2. Hawksbill sea turtle scute morphometrics.
Dataset S3. U.S. seizure records of hawksbill sea turtles.