Abstract
Background
Palbociclib administered with endocrine therapy was tolerable when the overall incidence of toxicities was assessed separately for three PALOMA studies. This study analyzed pooled, longer-term PALOMA safety data longitudinally.
Methods
Data were pooled from three randomized phase II and III studies (ClinicalTrials.gov: NCT00721409, NCT01740427, NCT01942135) of hormone receptor–positive/human epidermal growth factor receptor 2‒negative advanced breast cancer patients. Front-line patients were randomly assigned to receive letrozole with/without palbociclib (PALOMA-1) or letrozole plus palbociclib/placebo (PALOMA-2). In PALOMA-3, patients with prior endocrine resistance received fulvestrant plus palbociclib/placebo. The cumulative event rates of adverse events (AEs), reporting up to 50 months of treatment, were assessed over time.
Results
Patients who received endocrine therapy (n = 1343) were included in this pooled analysis (872 were also treated with palbociclib, and 471 were not). The most common AEs with palbociclib plus endocrine therapy were neutropenia and infections (any grade, 80.6% and 54.7%, respectively), which were higher than in the endocrine monotherapy arm (any grade, 5.3% and 36.9%). The most common hematologic AEs (≥15.0% in the palbociclib arm) were more likely to be reported in the initial months of the study, after which time the cumulative event rate did not substantially increase. With palbociclib plus endocrine therapy, any grade AEs leading to permanent discontinuation over three years occurred in only 8.3% of patients.
Conclusions
Based on these long-term safety analyses, there is no evidence of specific cumulative or delayed toxicities with palbociclib plus endocrine therapy, supporting the ongoing investigation of palbociclib plus endocrine therapy in early breast cancer (NCT02513394).
The novel and selective cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor palbociclib, used in combination with endocrine therapy, has demonstrated efficacy and tolerable safety in the management of metastatic or locally advanced breast cancer (1–4). Palbociclib targets dysregulated cell cycle machinery within the cyclin D-CDK4/6-retinoblastoma pathway to inhibit the uncontrolled cellular proliferation critical to pathophysiologic processes (5–7). In prospective, randomized phase II and III trials, palbociclib combined with endocrine therapy resulted in statistically significant (P < .001) improvement in progression-free survival (PFS) compared with endocrine therapy alone, in both firstline metastatic and previously treated hormone receptor−positive/human epidermal growth factor receptor 2−negative (HR+/HER2-) patients with advanced breast cancer (1–4).
The therapeutic window of any new treatment regimen needs to be evaluated for efficacy vs the clinically tolerable safety profile, particularly in the advanced disease setting, where maintaining quality of life and minimizing toxicity are a priority. Decisions on treatment with CDK4/6 inhibitors, including palbociclib in combination regimens, need to be based on an assessment of their immediate and long-term toxicity. The safety profile of palbociclib plus endocrine therapy has been reported for each of the three PALOMA studies at both interim and final analysis of the primary end point (investigator-assessed PFS) (1–3). Further analysis of the safety profile to characterize the incidence of adverse events (AEs) over time will enhance the ability of clinicians to manage toxicities, optimize treatment decisions, and appropriately counsel their patients (8). In this analysis, we evaluate the safety of palbociclib combined with endocrine therapy for up to 50 months—which extends beyond the cutoff dates of the safety data reported previously for PALOMA-1, -2, and -3—to characterize toxicities and identify any unusual trends and/or potential cumulative AEs based on a longitudinal analysis of a large, pooled clinical trial data set comprising all three randomized studies.
Methods
Study Design and Patients
This analysis includes safety data from the open-label, phase II PALOMA-1 study and the double-blind, phase III PALOMA-2 and -3 studies (ClinicalTrials.gov: NCT00721409 [9], NCT01740427 [10], NCT01942135 [11], respectively). All three PALOMA study designs, briefly shown in Supplementary Figure 1 (available online), have been described previously (1–4). Across all PALOMA studies, written informed consent was provided before study procedures were initiated; institutional review boards at participating centers approved all study-related procedures, which complied with the International Conference on Harmonisation, guidelines for Good Clinical Practice, and the Declaration of Helsinki. Overall survival has been reported for PALOMA-1 (12), and follow-up is in progress for the PALOMA-2 and PALOMA-3 studies.
Eligible patients were postmenopausal women (PALOMA-1 and -2) and women of any menopausal status (PALOMA-3) age 18 years or older with HR+/HER2- advanced breast cancer who had not received treatment for their advanced breast cancer (PALOMA-1 and -2), or whose advanced breast cancer had progressed on prior endocrine therapy while on or within 12 months of (neo)adjuvant therapy (including some endocrine therapies), or while on or within one month of endocrine therapy for advanced breast cancer (PALOMA-3). Other key inclusion criteria appear in Supplementary Table 1 (available online). Study treatments have been previously described (1–3) and are summarized in the Supplementary Methods (available online).
Safety Assessments
AEs in all three PALOMA studies were reported from the time patients provided informed consent until at least 28 days after their last study drug treatment and before the initiation of any new anticancer therapy, or until all toxicities resolved or were characterized as chronic/stable in PALOMA-1, whichever occurred later. AEs were assessed at least monthly; laboratory tests were performed biweekly during the first two cycles and on day 1 of subsequent cycles; blood counts were performed on day 15 ± 1 day for the first two cycles, on day 1 of subsequent cycles, and at the end of treatment or withdrawal; physical examinations were performed at screening and on day 1 of every cycle until the end of treatment or withdrawal.
The severity of all-causality AEs was recorded by investigators and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v3.0 (PALOMA-1) and v4.0 (PALOMA-2 and -3). Serious AEs were also assessed.
Data and Statistical Analyses
AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) v19.0. The frequencies of maximum grade events for each patient during study treatments were summarized by treatment arms. Related preferred terms were aggregated into cluster terms (Supplementary Methods, available online). To evaluate the long-term safety of palbociclib combined with endocrine therapy, the cumulative event rates of hematologic and selected nonhematologic AEs—recognized as being of clinical relevance—were assessed using Kaplan-Meier estimates. Frequently reported AEs, in 15% or more of palbociclib-treated patients, were assessed at six-month intervals (0–<6, 6–<12, 12–<18, 18–<24, 24–<30, 30–<36 months).
To assess the risk of having an AE in palbociclib-treated patients (compared with patients receiving endocrine monotherapy), hazard ratios for each AE of interest were estimated using a Cox model stratified by study. A log-rank test was used to derive P values (two-sided) without adjustment for multiplicity. P values of less than .05 were considered statistically significant. In addition, person-years analysis was conducted for selected nonhematologic AEs to adjust the duration of drug exposure to the risk of having the AE. For venous thromboembolic events, treatment duration–adjusted incidence rates per person per year were based on the Standardised MedDRA Query v20.0 for venous embolic and thrombotic events.
Mean CTCAE grades for selected hematologic (laboratory-assessed) and nonhematologic adverse events were calculated by cycle for the first 18 cycles of treatment. The frequency of AEs leading to temporary dose discontinuations (dose interruptions within a cycle or a delay of the next treatment cycle) over six-month intervals and cumulatively was also summarized.
Results
Patients
The experimental arm of this pooled data set includes 875 patients randomly assigned to the palbociclib arms in the three PALOMA studies (intent-to-treat population: PALOMA-1, n = 84, data cutoff date, January 2, 2015; PALOMA-2, n = 444, February 26, 2016; PALOMA-3, n = 347, July 31, 2015). Pooled analyses excluded one patient in PALOMA-1 and two patients in PALOMA-3 who were randomly assigned to palbociclib but not treated (safety population: n = 872). The comparator arm includes 477 patients randomly assigned to endocrine monotherapy or endocrine therapy ± placebo. Four patients in PALOMA-1 and 2 patients in PALOMA-3 were randomized but did not receive therapy (safety population: n = 471). The mean age of the pooled intent-to-treat population was similar in the palbociclib plus endocrine arm and endocrine monotherapy arm, and most patients were postmenopausal (Table 1).
Table 1.
Patient demographic and baseline clinical characteristics (intent-to-treat population)
| Characteristic | Palbociclib + endocrine therapy (n = 875) | Endocrine therapy* (n = 477) |
|---|---|---|
| Age, y | ||
| Mean (range) | 60 (30–89) | 60 (28–88) |
| <65, No. (%) | 571 (65.3) | 314 (65.8) |
| ≥65, No. (%) | 304 (34.7) | 163 (34.2) |
| ≥75, No. (%) | 83 (9.5) | 32 (6.7) |
| Race, No. (%) | ||
| White | 672 (76.8) | 377 (79.0) |
| Asian | 145 (16.6) | 65 (13.6) |
| Black | 21 (2.4) | 12 (2.5) |
| Other† | 37 (4.2) | 23 (4.8) |
| Menopausal status, No. (%) | ||
| Pre-/perimenopausal | 72 (8.2) | 36 (7.5) |
| Postmenopausal | 803 (91.8) | 441 (92.5) |
| ECOG PS, No. (%) | ||
| 0 | 509 (58.2) | 263 (55.1) |
| 1 | 357 (40.8) | 211 (44.2) |
| 2 | 9 (1.0) | 3 (<1.0) |
| Disease sites, No. (%) | ||
| Visceral | 452 (51.7) | 257 (53.9) |
| Nonvisceral | 423 (48.3) | 220 (46.1) |
| No. of disease sites†, No. (%) | ||
| 1 | 272 (31.1) | 144 (30.2) |
| 2 | 231 (26.4) | 118 (24.7) |
| 3 | 203 (23.2) | 114 (23.9) |
| 4 | 106 (12.1) | 63 (13.2) |
| ≥5 | 63 (7.2) | 38 (8.0) |
| Measurable disease, No. (%) | ||
| Yes | 671 (76.7) | 375 (78.6) |
| No | 204 (23.3) | 102 (21.4) |
| Disease stage at initial diagnosis, No. (%) | ||
| I | 77 (8.8) | 43 (9.0) |
| II | 257 (29.4) | 124 (26.0) |
| III | 141 (16.1) | 86 (18.0) |
| IV | 224 (25.6) | 108 (22.6) |
| Other | 21 (2.4) | 9 (1.9) |
| Unknown/missing | 71 (8.1) | 26 (5.5) |
| Not collected | 84 (9.6) | 81 (17.0) |
| Prior systemic therapy, No. (%) | ||
| Hormonal | 623 (71.2) | 328 (68.8) |
| Chemotherapy | 495 (56.6) | 283 (59.3) |
| None | 211 (24.1) | 118 (24.7) |
Comparator arm received endocrine therapy ± placebo. ABC = advanced breast cancer (includes metastatic breast cancer); ECOG = Eastern Cooperative Oncology Group; PS = performance status.
Includes patients with missing/unreported information.
Exposure
Median duration of treatment was longer (all three studies), and median relative dose intensities slightly lower (for PALOMA-2 and PALOMA-3 studies), in the palbociclib-treated arms than the corresponding endocrine monotherapy arms (Table 2). In 119 of 527 (22.6%) patients in PALOMA-1 and -2, the combination of palbociclib and letrozole as firstline treatment was continued for 24 to less than 30 months, and 11 patients were treated for more than 36 months. For 140 of 345 (40.5%) patients in PALOMA-3, palbociclib plus fulvestrant was received for 12 or more months. Dose reductions were required in 36.9% of the pooled safety population that received palbociclib plus endocrine therapy (Table 2). Median durations of exposure to endocrine therapy in the comparator arms were consistently less than for the palbociclib arm, irrespective of the line of therapy. The total duration of treatment was 2.4-fold longer in the palbociclib arm than the comparator arm (Table 2).
Table 2.
Exposure to palbociclib or placebo (safety population)
| Pooled analysis |
PALOMA-1 |
PALOMA-2 |
PALOMA-3 |
|||||
|---|---|---|---|---|---|---|---|---|
| Exposure | Palbociclib + endocrine therapy (n = 872) | Endocrine therapy ± placebo (n = 471) | Palbociclib + endocrine therapy (n = 83) | Endocrine therapy (n = 77) | Palbociclib + endocrine therapy (n = 444) | Endocrine therapy + placebo (n = 222) | Palbociclib + endocrine therapy (n = 345) | Endocrine therapy + placebo (n = 172) |
| Treatment duration, mo | ||||||||
| Median | — | — | 13.83 | 7.59 | 19.81 | 13.57 | 10.84 | 4.50 |
| Range | — | — | 0.23−53.06 | 0.92−40.77 | 0.03−34.07 | 0.33−35.18 | 0.03−19.58 | 0.46−20.07 |
| Total person-years exposure | 1017.8 | 423.8 | 127.0 | 76.4 | 627.3 | 258.2 | 263.5 | 89.3 |
| Average daily dose, mg | ||||||||
| Median | — | — | 125 | — | 125 | 125 | 125 | 125 |
| Range | — | — | 79−267* | — | 77−125 | 105−126 | 78−131 | 104−129 |
| Relative dose intensity†, % | ||||||||
| Median | — | — | 100 | — | 93.0 | 99.6 | 89.8 | 100 |
| Range | — | — | 95−100 | — | 40−110 | 56−105 | 22−107 | 80-100 |
| Mean | — | — | 99.5 | — | 86.8 | 98.2 | 85.6 | 97.1 |
| Standard deviation | — | — | 1.04 | — | 14.5 | 5.52 | 15.4 | 6.0 |
| Dose modifications (any cause) | ||||||||
| Dose reductions‡, No. (%) | ||||||||
| ≥1 dose reductions | 322 (36.9) | 6 (1.5)§ | 34 (41.0) | — | 160 (36.0) | 3 (1.4) | 128 (37.1) | 3 (1.7) |
| 1 dose reduction | 214 (24.5) | 6 (1.5)§ | 20 (24.1) | — | 97 (21.8) | 3 (1.4) | 97 (28.1) | 3 (1.7) |
| 2 dose-level reductions | 116 (13.3) | 0§ | 12 (14.5) | — | 63 (14.2) | 0 | 41 (11.9) | 0 |
| Dose interruptions‖, No. (%) | 646 (74.1) | 220 (46.7) | 50 (60.2) | 22 (28.6) | 310 (69.8) | 94 (42.3) | 286 (82.9) | 104 (60.5) |
| Cycle delays¶, No. (%) | 560 (64.2) | 82 (20.8)§ | 70 (84.3) | — | 303 (68.2) | 60 (27.0) | 187 (54.2) | 22 (12.8) |
Maximum value pertains to a single patient who was misdosed. — = no data.
Relative dose intensity = [(actual dose)/(intended dose)]*100%.
Dose reduction is any dose reduction from the initial prescribed dose regardless of its duration; dose interruptions are not counted as reduction.
Denominator is n = 394, includes patients in the endocrine therapy plus placebo arms of PALOMA-2 and -3 only.
Includes any missing dose, gaps within a cycle, or not completing 21 doses per cycle (except last cycle).
Defined as any cycle start delay (PALOMA-1), delays beyond 31 days (PALOMA-2), or delays of two or more days (cycles 1 and 2) or seven or more days (cycle 3 or later; PALOMA-3).
AE Incidence
A summary table of any grade treatment-emergent adverse events reported in 15.0% or more of patients in either treatment arm of this pooled study is shown in Table 3. Grade 3−4 hematologic toxicities were reported for 98.6% of patients in the palbociclib-treated arm and for 3.6% of patients in the endocrine monotherapy arm. Any grade neutropenia, the most common hematologic AE in the palbociclib-treated arm, was reported for 80.6% and 5.3% patients in the palbociclib-treated and endocrine monotherapy arms, respectively.
Table 3.
Summary of any grade treatment-emergent adverse events reported for ≥15.0% of patients in either treatment arm of the PALOMA-1, -2, and -3 pooled safety study—all-causality and all cycles in the as-treated population
| Palbociclib + endocrine therapy (n = 872) |
Endocrine therapy* (n = 471) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Preferred term | All grades, No. (%) | Grades 1/2, No. (%) | Grade 3, No. (%) | Grade 4, No. (%) | All grades, No. (%) | Grades 1/2, No. (%) | Grade 3, No. (%) | Grade 4, No. (%) |
| Any AEs†,‡ | 863 (99.0)§ | 186 (21.3) | 542 (62.2) | 120 (13.8) | 434 (92.1)‖ | 317 (67.3) | 101 (21.4) | 9 (1.2) |
| Hematologic AEs¶ | ||||||||
| Neutropenia | 703 (80.6) | 133 (15.3) | 482 (55.3) | 88 (10.1) | 25 (5.3) | 20 (4.2) | 4 (0.8) | 1 (0.2) |
| Leukopenia | 394 (45.2) | 161 (18.5) | 228 (26.1) | 5 (0.6) | 17 (3.6) | 15 (3.2) | 1 (0.2) | 1 (0.2) |
| Anemia | 241 (27.6) | 201 (23.1) | 38 (4.4) | 2 (0.2) | 50 (10.6) | 41 (8.7) | 9 (1.9) | 0 |
| Thrombocytopenia | 166 (19.0) | 149 (17.1) | 14 (1.6) | 3 (0.3) | 5 (1.1) | 4 (0.8) | 1 (0.2) | 0 |
| Nonhematologic AEs | ||||||||
| Infections¶ | 477 (54.7)# | 431 (49.4) | 39 (4.5) | 6 (0.7) | 174 (36.9) | 159 (33.8) | 12 (2.5) | 0 |
| Fatigue | 342 (39.2) | 320 (36.7) | 20 (2.3) | 2 (0.2) | 129 (27.4) | 125 (26.5) | 4 (0.8) | 0 |
| Nausea | 298 (34.2) | 295 (33.8) | 3 (0.3) | 0 | 117 (24.8) | 111 (23.6) | 6 (1.3) | 0 |
| Stomatitis¶ | 252 (28.9) | 246 (28.2) | 6 (0.7) | 0 | 57 (12.1) | 56 (11.9) | 1 (0.2) | 0 |
| Alopecia | 226 (25.9) | 226 (25.9) | —** | —** | 48 (10.2) | 48 (10.2) | —** | —** |
| Arthralgia | 223 (25.6) | 216 (24.8) | 7 (0.8) | 0 | 120 (25.5) | 117 (24.8) | 3 (0.6) | 0 |
| Diarrhea | 214 (24.5) | 205 (23.5) | 9 (1.0) | 0 | 85 (18.0) | 80 (17.0) | 5 (1.1) | 0 |
| Headache | 196 (22.5) | 193 (22.1) | 3 (0.3) | 0 | 100 (21.2) | 96 (20.4) | 4 (0.8) | 0 |
| Cough | 188 (21.6) | 188 (21.6) | 0 | — | 73 (15.5) | 73 (15.5) | 0 | —** |
| Back pain | 167 (19.2) | 155 (17.8) | 11 (1.3) | 1 (0.1) | 90 (19.1) | 87 (18.5) | 3 (0.6) | 0 |
| Constipation | 167 (19.2) | 165 (18.9) | 2 (0.2) | 0 | 68 (14.4) | 67 (14.2) | 1 (0.2) | 0 |
| Hot flush | 166 (19.0) | 166 (19.0) | 0 | —** | 107 (22.7) | 106 (22.5) | 1 (0.2) | —** |
| Vomiting | 149 (17.1) | 145 (16.6) | 4 (0.5) | 0 | 66 (14.0) | 61 (13.0) | 5 (1.1) | 0 |
| Rash¶ | 144 (16.5) | 138 (15.8) | 6 (0.7) | 0 | 43 (9.1) | 42 (8.9) | 1 (0.2) | 0 |
| Decreased appetite | 138 (15.8) | 131 (15.0) | 7 (0.8) | 0 | 39 (8.3) | 38 (8.1) | 1 (0.2) | 0 |
| Pain in extremity | 122 (14.0) | 121 (13.9) | 1 (0.1) | 0 | 72 (15.3) | 66 (14.0) | 6 (1.3) | 0 |
Endocrine therapy with placebo (PALOMA-2 and -3) or without placebo (PALOMA-1). AE = adverse event.
AEs were graded in accordance with the maximum Common Terminology Criteria for Adverse Events (CTCAE; version 4.0) and the Medical Dictionary for Regulatory Activities (version 19.0) coding dictionary applied.
Includes data up to 28 days after last dose of study drug.
In the palbociclib plus endocrine therapy arm, grade 5 events were reported for 15 patients (1.7%) as follows: disease progression (six events), pulmonary embolism¶, general physical health deterioration, hepatic failure, acute myocardial infarct, cardiogenic shock, cardiopulmonary failure, cardiovascular insufficiency, death, disseminated intravascular coagulation, and respiratory failure (one event each).
In the endocrine therapy (±placebo) arm, grade 5 events were reported for seven patients (1.5%) as follows: infections¶ (three events), pulmonary embolism¶, acute respiratory distress syndrome, breast cancer, cardiac arrest, cerebral hemorrhage (one event each).
Cluster terms applied as defined in the Supplementary Appendix (available online).
A single missing or unknown infection occurred that is not included in the total.
Maximum CTCAE grade does not apply.
Any grade infections, the most common nonhematologic AEs in the palbociclib-treated and endocrine monotherapy arms, were reported for 54.7% and 36.9% patients, respectively, and in 431 of 477 (90.4%) and 159 of 174 (91.4%) of those patients, events were of grade 1−2 severity. There were no grade 5 infections reported for palbociclib-treated patients, whereas three events of grade 5 severity (pneumonia, lower respiratory tract infection, and peritonitis bacterial) were reported for patients receiving endocrine monotherapy (Table 3). The five most common any grade infections with palbociclib plus endocrine therapy were nasopharyngitis (13.6% and 9.1% with endocrine monotherapy), upper respiratory tract infection (11.7% and 8.3%, respectively), urinary tract infection (10.0% and 6.8%), and oral herpes (4.2% vs 0.8%) (data not shown).
Fatigue and nausea were among the other most common nonhematologic toxicities in both treatment arms; these events were predominantly of grade 1−2 severity.
Timing and Cumulative Event Rates of AEs
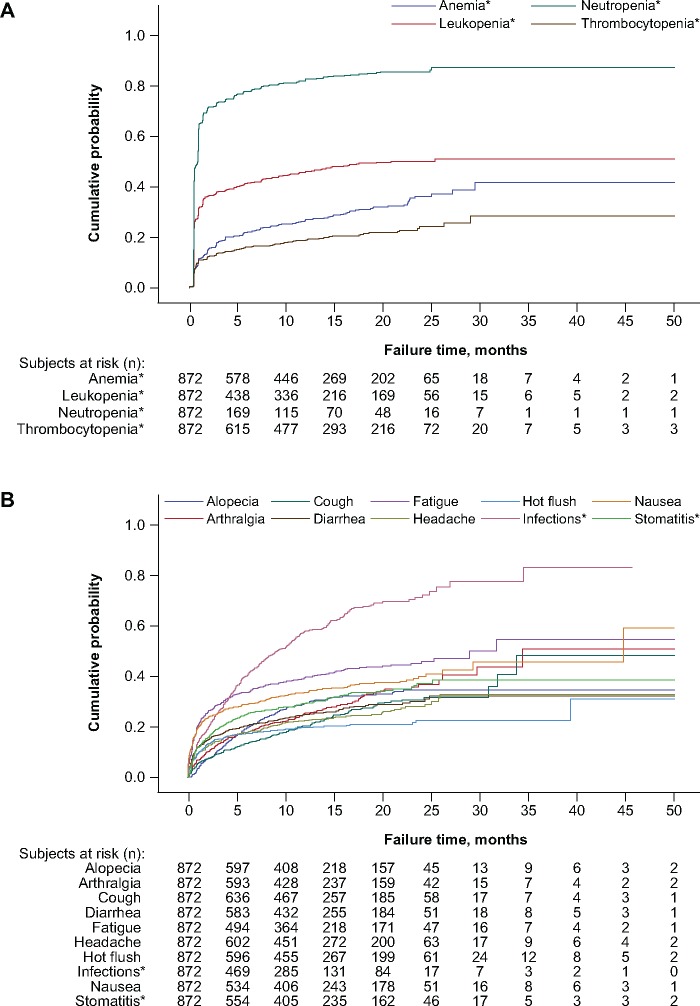
The cumulative event rates of the four most common all-causality hematologic AEs over all cycles for up to 50 months are depicted for the 872 patients treated with palbociclib plus endocrine therapy in Figure 1A. Kaplan-Meier plots show that the probability of any grade neutropenia being reported was greater than 0.6 within the first two months of treatment, after which time the increase of the cumulative event rate was minimal and relatively gradual (>0.8 by month 10 and <0.9 over the next 40 months). The cumulative event rate of any grade leukopenia being reported was greater than 0.3 within the initial months of the study, it reached greater than 0.4 by month 10, and thereafter it increased to approximately 0.5 and plateaued. The probability of any grade anemia and thrombocytopenia being reported was greater than 0.1 within the initial three months of treatment, after which time it reached greater than 0.3 for reports of anemia and greater than 0.2 for thrombocytopenia by month 20; the cumulative event rate for reporting anemia and thrombocytopenia reached a plateau of approximately 0.4 and less than 0.3, respectively, by approximately month 30.
Figure 1.
Kaplan-Meier curves showing cumulative event rates over time among palbociclib-treated patients for the (A) hematologic adverse events (AEs) of neutropenia, leukopenia, anemia, and thrombocytopenia and for (B) selected nonhematologic AEs. *Cluster terms were used and are defined in the Supplementary Methods (available online).
Among the selected nonhematologic AEs of any grade (Figure 1B), the cumulative event rates for fatigue, nausea, infections, and stomatitis were greater than 0.2 within the initial few months of palbociclib plus endocrine therapy. The cumulative event rate of reporting infections surpassed those for all other AEs before five months (Figure 1B), increasing to approximately 0.6 by 15 months, and increasing more slowly to greater than 0.8 by 35 months. By comparison, the cumulative event rates for reports of all other nonhematologic AEs were less than 0.5 by 30 months of palbociclib treatment, most of which had plateaued by that time.
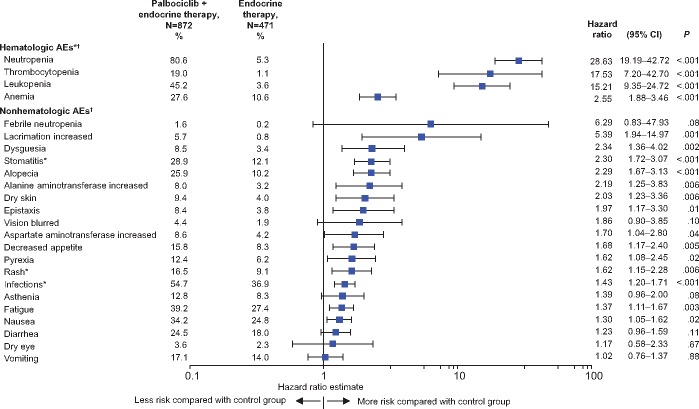
To evaluate the risk of an AE in palbociclib-treated patients compared with patients receiving endocrine monotherapy, a forest plot of hazard ratios for a group of selected clinically relevant AEs is presented in Figure 2. Although all selected AEs in the forest plot show that there is an increased risk in the palbociclib-treated arm compared with the control arm, the hematologic AEs are statistically significantly higher in the palbociclib-treated arm (all P < .001).
Figure 2.
Forest plot depicting the estimated hazard ratios and 95% confidence intervals (CIs) in descending order for hematologic and selected nonhematologic adverse events (AEs; all causalities, all cycles) in the as-treated population. A log-rank test was used to derive P values (two-sided) without adjustment for multiplicity. *Cluster terms were used and are defined in the Supplementary Methods (available online). †Includes data up to 28 days after last dose of study drug, and the time to an event in days was calculated as the date of AE onset minus the date of the first dose of palbociclib/placebo + 1. Endocrine therapy included placebo in PALOMA-2 and -3, but not in the PALOMA-1 study.
Adverse Events Adjusted for Exposure
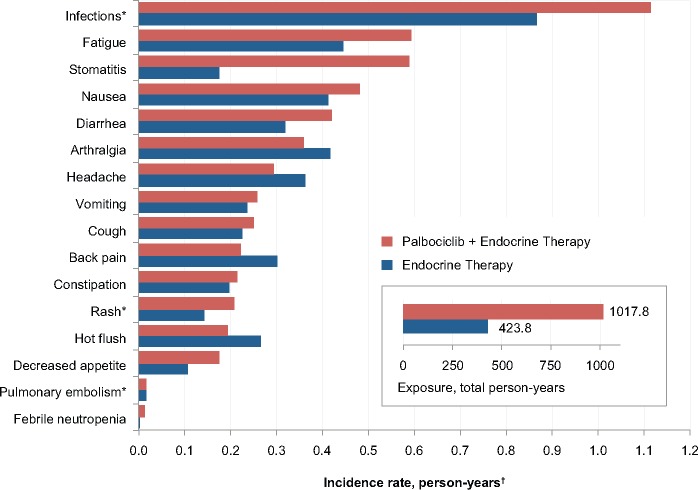
The incidence rate of selected AEs was assessed in each of the treatment arms after adjusting for the duration of exposure to treatment (Figure 3). Between treatment arms, the incidence rate of stomatitis was higher in palbociclib-treated patients after controlling for the duration of exposure to study drugs; however, differences between treatment arms for febrile neutropenia, infections, fatigue, and nausea, among other AEs, were mitigated to some extent after controlling for duration of treatment. The rate of pulmonary embolism was the same in both arms after adjusting for duration of exposure to treatment (0.017 person-years in both arms), and the rate for all venous thromboembolic events was 0.025 person-years in the palbociclib-treated arm vs 0.027 in the endocrine monotherapy arm.
Figure 3.
Adverse event (AE) incidence rate of selected nonhematologic AEs in the safety population after adjusting by person-years of exposure to study drugs. *Cluster terms were used and are defined in the Supplementary Methods (available online). †Incidence rate for selected AEs (preferred term/cluster of preferred terms) = (sum of total number of events)/(sum of total person-years).
Adverse Events and Dose Modifications Over Time
Treatment-emergent AEs were assessed at six-month intervals for less than 36 months. The incidence of any grade AEs reported for 15.0% or more of patients had a similar trend over time; all toxicities peaked during the first six months of treatment (Supplementary Figure 2, available online) and generally decreased over time, with the greatest decrease occurring after the first six months of treatment. With the exception of neutropenia, leukopenia, and infections, the incidence of individual AEs remained below 15.0% at each time interval after the first six months.
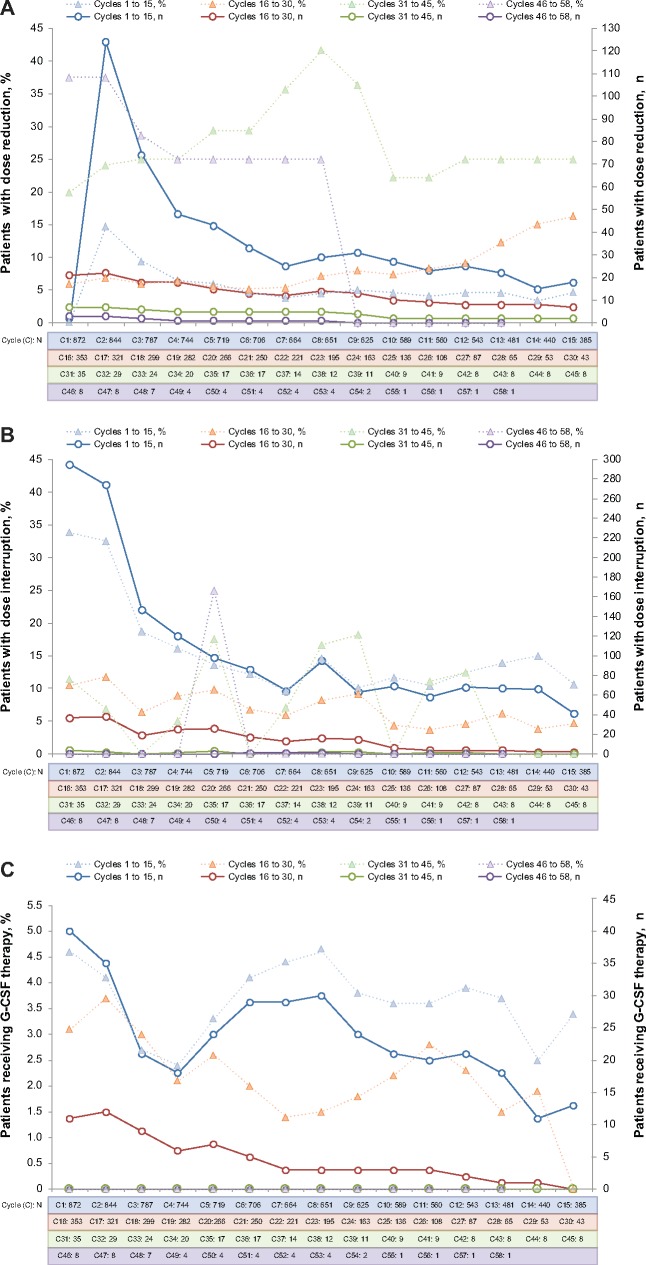
The rate of dose modifications associated with AEs showed a similar temporal pattern, with dose reductions and temporary discontinuations (dose interruptions and cycle delays) most commonly implemented during the first six months of treatment with palbociclib plus endocrine therapy (Table 4). The frequency of dose reductions and temporary discontinuations decreased over time. Any grade AEs leading to a dose reduction and/or a temporary discontinuation over three years were 36.0% and 71.4%, respectively. The maximum number of dose reductions and dose interruptions were initiated in cycles 2 and 1, respectively, and the frequency of each generally declined over the course of subsequent treatment cycles, reaching 0% in later cycles (Figure 4). Overall, neutropenia and leukopenia were the most common AEs associated with dose reductions (29.8% and 3.1%, respectively) and temporary discontinuations (62.5% and 12.6%); however, few patients (1.5% and 0%) permanently discontinued treatment because of neutropenia and leukopenia.
Table 4.
Dose reductions and discontinuations associated with AEs over time
| Time interval, mo |
||||||
|---|---|---|---|---|---|---|
| Dose reductions and discontinuations | 0 to <6 (n = 872), No. (%) | 6 to <12 (n = 676), No. (%) | 12 to <18 (n = 491), No. (%) | 18 to <24 (n = 289), No. (%) | 24 to <30 (n = 119), No. (%) | 30 to <36 (n = 27), No. (%) |
| Dose reductions* | ||||||
| Any AE | 243 (27.9) | 69 (10.2) | 33 (6.7) | 18 (6.2) | 3 (2.5) | 0 |
| Neutropenia† | 210 (24.1) | 49 (7.2) | 24 (4.9) | 16 (5.5) | 3 (2.5) | 0 |
| Leukopenia† | 25 (2.9) | 3 (0.4) | 1 (0.2) | 0 | 0 | 0 |
| Thrombocytopenia† | 8 (0.9) | 1 (0.1) | 0 | 1 (0.3) | 0 | 0 |
| Infections† | 4 (0.5) | 4 (0.6) | 2 (0.4) | 1 (0.3) | 0 | 0 |
| Temporary discontinuations‡ | ||||||
| Any AE | 539 (61.8) | 326 (48.2) | 198 (40.3) | 118 (40.8) | 41 (34.5) | 8 (29.6) |
| Neutropenia† | 480 (55.0) | 271 (40.1) | 174 (35.4) | 107 (37.0) | 35 (29.4) | 6 (22.2) |
| Leukopenia† | 89 (10.2) | 23 (3.4) | 12 (2.4) | 8 (2.8) | 2 (1.7) | 0 |
| Infections† | 24 (2.8) | 31 (4.6) | 22 (4.5) | 6 (2.1) | 3 (2.5) | 0 |
| Fatigue | 12 (1.4) | 6 (0.9) | 8 (1.6) | 2 (0.7) | 1 (0.8) | 0 |
| Thrombocytopenia† | 13 (1.5) | 4 (0.6) | 3 (0.6) | 2 (0.7) | 0 | 1 (3.7) |
| Nausea | 13 (1.5) | 4 (0.6) | 2 (0.4) | 1 (0.3) | 0 | 0 |
| ALT increased | 8 (0.9) | 6 (0.9) | 1 (0.2) | 0 | 0 | 0 |
| AST increased | 9 (1.0) | 3 (0.4) | 0 | 1 (0.3) | 1 (0.8) | 0 |
| Diarrhea | 9 (1.0) | 3 (0.4) | 0 | 3 (1.0) | 0 | 0 |
| Rash† | 9 (1.0) | 3 (0.4) | 1 (0.2) | 0 | 0 | 0 |
| Stomatitis† | 8 (0.9) | 4 (0.6) | 3 (0.6) | 1 (0.3) | 0 | 0 |
| Anemia† | 9 (1.0) | 3 (0.4) | 2 (0.4) | 4 (1.4) | 2 (1.7) | 0 |
| Vomiting | 8 (0.9) | 3 (0.4) | 2 (0.4) | 1 (0.3) | 0 | 0 |
| Pyrexia | 8 (0.9) | 2 (0.3) | 1 (0.2) | 1 (0.3) | 0 | 0 |
| Permanent discontinuations§ | ||||||
| Any AE | 37 (4.2) | 20 (3.0) | 8 (1.6) | 3 (1.0) | 3 (2.5) | 1 (3.7) |
| Neutropenia† | 6 (0.7) | 4 (0.6) | 1 (0.2) | 1 (0.3) | 1 (0.8) | 0 |
| ALT increased | 3 (0.3) | 1 (0.1) | 0 | 0 | 0 | 0 |
| Infections† | 2 (0.2) | 2 (0.3) | 0 | 0 | 0 | 0 |
| Fatigue | 2 (0.2) | 1 (0.1) | 1 (0.2) | 0 | 1 (0.8) | 1 (3.7) |
| Disease progression | 2 (0.2) | 0 | 1 (0.2) | 0 | 0 | 0 |
AEs associated with dose reductions in 1% or more of patients during the first three years of palbociclib treatment are shown. AE = adverse event; ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Cluster terms were used and are defined in the Supplementary Appendix (available online).
AEs associated with temporary treatment discontinuations in 1% or more of patients during the first three years of palbociclib treatment are shown. A temporary dose discontinuation included any interruption and resumption of treatment within the same cycle or at the start of a new cycle (ie, cycle delays).
AEs associated with permanent discontinuation in more than two palbociclib-treated patients during the first three years of treatment are shown.
Figure 4.
Dose reductions (A), dose interruptions (B), and granulocyte colony-stimulating factor (G-CSF) therapy (C) by cycle. Patients could have had a dose reduction and/or dose interruption in more than one cycle. Patients with one or more dose reductions or one or more dose interruptions in a cycle are only reported once in the respective cycle. Patients with one or more G-CSF medications (including filgrastim, pegfilgrastim, lenograstim, and/or G-CSF) concomitant in the respective cycle are reported. Percentages were computed as n/N*100. n = number of patients with at least one dose reduction or dose interruption per dosing records in the respective cycle; N = number of patients taking palbociclib during the cycle.
Serious AEs, Permanent Discontinuations, and Grade 5 Events
All causalities, all cycles, any grade treatment-emergent serious AEs (SAEs) were reported in a total of 160 of 872 (18.3%) patients treated with palbociclib and in 65 of 471 (13.8%) patients receiving endocrine monotherapy (data not shown). The most common SAEs in palbociclib-treated patients were pulmonary embolism, reported in 11 (1.3%) patients, and febrile neutropenia, reported in nine (1.0%); no other SAEs occurred in more than 1.0% of patients, including patients receiving endocrine monotherapy (comparator arm: pulmonary embolism, three [0.6%] patients; febrile neutropenia, one [0.2%] patient). The overall permanent discontinuation rate due to any grade AEs in those receiving palbociclib was 8.3% (72/872 patients) over three years, and 91.4% remained on palbociclib regardless of treatment combination. No treatment-related deaths occurred during the trial or the 28 days after the last dose of study drug among patients receiving palbociclib in the three pooled PALOMA studies. During the safety period evaluated, grade 5 events occurred in 15 of 872 (1.7%) patients receiving palbociclib plus endocrine therapy and in seven of 471 (1.5%) in the endocrine therapy comparator arm.
Adverse Events of Interest
The average burden of AEs for patients receiving palbociclib plus endocrine therapy over the initial 18 treatment cycles was consistently low for infections, nausea, and fatigue, with the average CTCAE grade of these events remaining below 2 (Supplementary Figure 3, available online). Among patients who experienced grade 3−4 neutropenia (laboratory-based findings), the mean CTCAE grade of these events decreased slightly after the second cycle, from 3.1 in cycle 1 to 3.0 by cycle 3, and then remained within this narrow range through cycle 18. The mean CTCAE grade for neutropenia remained stable over time with the implementation of dose modifications during the same period.
The incidence of any grade febrile neutropenia in palbociclib-treated patients during the first 18 months of treatment was low (0−<6 months, 11/872 [1.3%]; 6−<12 months, 2/676 [0.3%], and 12−<18 months, 1/491 [0.2%]); there were no subsequent events, and the reported incidence over the entire study period was 14 (1.6%) for febrile neutropenia of any grade (data not shown).
Hepatic Function
Pooled incidences of any grade (grades 1−4) laboratory elevations in the palbociclib-treated and comparator arms were as follows: alanine aminotransferase (ALT) 40.8% and 31.1%, respectively; aspartate aminotransferase (AST) 48.4% and 40.8%; alkaline phosphatase 36.1% and 43.7%; and total bilirubin 7.9% and 6.0% (data not shown). Overall, the rate of grade 3−4 hepatic toxicities was low, although grade 3–4 laboratory values were more frequently observed in the palbociclib-treated patients than in the comparator arm for ALT, 2.3% and 0.2%, and AST, 3.3% and 1.9%, respectively. No cases meeting the criteria for Hy’s Law (13) were observed in palbociclib-treated patients.
Growth Factors
Granulocyte colony-stimulating factors (G-CSFs) usage peaked during cycles 1 and 8 (40 of 872 [4.6%] and 35 of 658 [4.6%] patients, respectively); thereafter, the percentage of patients receiving G-CSFs gradually declined over subsequent cycles and reached no G-CSF use at all by cycle 30 until cycle 58 (Figure 4C).
Discussion
Based on pooled data from the 3 PALOMA studies, the current report represents the largest and most comprehensive analysis of the long-term safety of palbociclib combined with endocrine therapy in patients with HR+ disease, the most common breast cancer subtype. The safety profile of palbociclib plus endocrine therapy in this pooled analysis remains consistent with previously reported results for palbociclib plus letrozole in patients who were front-line metastatic (PALOMA-1 and -2) and for those who received palbociclib plus fulvestrant to treat disease that progressed on prior endocrine therapy in the advanced breast cancer setting (PALOMA-3) (1–3). With regard to QTc prolongation, which has been shown to be a concern with certain CDK4/6 inhibitors, even in patients not considered to be at high risk for such an event (14), the recent results for corrected QT (QTc) evaluations in PALOMA-2 patients indicated that palbociclib plus letrozole does not prolong QTc to a clinically relevant extent at the recommended 125 mg/d dosing regimen (15).
Hematologic AEs, particularly cytopenias, are among the most commonly observed toxicities with palbociclib treatment. Although neutropenia is the most frequently reported AE of any grade, relatively few patients experienced febrile neutropenia, and the rate of permanent treatment discontinuation associated with neutropenia was low. Among patients who experienced grade 3−4 neutropenia, the average severity grade of neutropenia remained relatively stable and at the low end of the grade 3−4 range over time, indicating a lack of cumulative myelotoxicity with prolonged palbociclib treatment. Additionally, the average severity of neutropenia in this study decreased slightly after the second cycle, possibly because of dose modifications triggered by grade 4 neutropenia events that tend to occur early during treatment.
Previous reports showed that one or more dose modifications (dose reduction, dose interruption, or cycle delay) for the management of neutropenia or any AE did not significantly alter the treatment effect of palbociclib plus endocrine therapy on PFS (16–19). These observations are consistent with the proposed noncytotoxic mechanism of palbociclib-induced bone marrow suppression and are unlike the effects of chemotherapy (20).
The incidence of hematologic and nonhematologic AEs peaked within the first six months of palbociclib plus endocrine therapy, temporally coinciding with the period of most dose modifications (cycles 1 and 2). Early recognition of AEs is crucial for their successful management because it allows continued treatment and optimization of treatment outcomes. AEs associated with palbociclib plus endocrine therapy, including the most common toxicities (ie, neutropenia, infections, leukopenia, fatigue, nausea), generally tapered off over time. The majority of patients (63%) did not require a dose reduction and remained on the full 125 mg/d dose during treatment. The cumulative incidence of AEs during up to 50 months of treatment was very similar over time, and the rate of permanent discontinuation associated with AEs remained low, indicating that these toxicities were well managed. The most frequently used toxicity management techniques were dose reductions and temporary discontinuations. Although cytopenias (particularly neutropenia and leukopenia) were the most common reason for dose modifications, clinical management of other AEs was achieved effectively using these methods, and only four nonhematologic AEs (fatigue, increased alanine aminotransferase, infections, and disease progression) were associated with permanent treatment discontinuation in more than two patients. Relevant resources that provide recommendations on the management of dose modifications in response to toxicities are available to treating physicians (21,22).
At present there are no definitive predictive markers for short- or long-term toxicity to indicate which patients may better tolerate and benefit more from the addition of a CDK4/6 inhibitor to endocrine treatment. However, it has been reported on the basis of a univariate analysis of PALOMA-2 study data that Asian ethnicity and a baseline absolute neutrophil count lower than 3.68×103/mm3 represent potential risk factors for the development of grade 3–4 neutropenia (17). Further research on this topic is warranted given that it is information that can facilitate the conservation of health care resources in cases when endocrine monotherapy may suffice. Such insights could also reduce the likelihood of toxicity in potentially at-risk patients in both the adjuvant and advanced disease settings to minimize the impact on day-to-day quality of life (QoL). It is worth noting, however, that QoL can be maintained in patients treated with palbociclib plus endocrine therapy (23,24) through the use of appropriate supportive care and dose modifications as needed to manage AEs such as neutropenia, the most common AE associated with palbociclib (3).
Another therapeutic strategy to enhance the efficacy of endocrine therapies in patients with HR+/HER2- advanced breast cancer involves combining the mechanistic target of rapamycin (mTOR) pathway inhibitor everolimus with exemestane (25). BOLERO-2 clinical trial reports, which showed the effects of everolimus plus exemestane in patients with HR+ breast cancer who had relapsed on nonsteroidal aromatase inhibitors (26), indicate that toxicities tended to occur early after treatment initiation and necessitated dose reduction(s) and more discontinuations than the placebo plus exemestane arm, although the AE incidence with everolimus decreased over time (25), as was observed in this analysis with palbociclib plus endocrine therapy. In the BOLERO-2 study, the most common any grade AEs, reported for 15.0% or more of the 238 patients who received the less intensive regimen of endocrine therapy (exemestane) plus placebo after a median of 12 weeks’ exposure, were nausea (27%), fatigue (26%), diarrhea (16%), and arthralgia (16%) (26). In the endocrine monotherapy arm of this pooled safety study, those same AEs (any grade) were reported in 25%, 27%, 18%, and 25% of patients, respectively, demonstrating that AEs associated with endocrine therapy alone are consistently less toxic and that their safety profile is not altered over time for up to three years.
Currently, most of the safety data pertaining to CDK4/6 inhibitors received concomitantly during endocrine-based therapy for HR+/HER2- advanced breast cancer in women represent an evaluation after a relatively short follow-up period. The longest safety follow-up of any CDK4/6 inhibitor in the breast cancer setting is five years for palbociclib plus letrozole in the PALOMA-1 study (December 30, 2016, data cutoff), reported in conjunction with overall survival (12). Although these data were used to assess cumulative incidence annually over five consecutive years to reveal no new safety concerns, as a single study, it included only 83 patients, and, although informative, it must be viewed in that context. This pooled analysis assesses AEs reported for 872 patients treated with palbociclib plus endocrine therapy across three phase III clinical trials after a follow-up of up to 50 months and represents the most comprehensive safety report of CDK4/6 inhibitor use for advanced breast cancer at this time.
Some limitations may exist with respect to the generalizability of these safety findings. The representation of blacks in this pooled analysis was 2.5% or less of the study population in either arm; Asian patients constituted 16.6% and 13.6% of the women in the palbociclib-treated and endocrine therapy only arms, respectively. Additionally, patients with certain comorbidities or those taking certain concomitant medications were excluded from taking part in the clinical trials that formed the basis of our analyses; thus, the findings of our analyses may not be applicable to those patients who were excluded from these trials.
Based on these long-term safety analyses, there is no evidence of specific cumulative or delayed toxicities resulting from prolonged treatment with palbociclib combined with endocrine therapy for HR+/HER2- ABC, supporting the use of palbociclib plus endocrine therapy in the ongoing investigation of their utility in early breast cancer as adjuvant treatments (NCT02513394).
Funding
These analyses, and the studies included in these analyses, were sponsored by Pfizer Inc.
Notes
Affiliations of authors: Institut Curie, Paris, France (VD); University of California San Francisco Helen Diller Family Comprehensive Cancer Center, San Francisco, CA (HSR); Pfizer Inc, Collegeville, PA (PS, CHB); British Columbia Cancer Agency, Vancouver, BC, Canada (KG); Robert H. Lurie Comprehensive Cancer Center, Feinberg School of Medicine, Chicago, IL (MC); Peter MacCallum Cancer Centre, University of Melbourne, Melbourne, VIC, Australia (SL); Istituto Europeo di Oncologia, Milan, Italy (MC); Pfizer Inc, La Jolla, CA (DRL); Pfizer Inc, Milano, Italy (AM); Pfizer Inc, San Francisco, CA (EG); David Geffen School of Medicine, Los Angeles, CA (DJS, RSF); Institute of Cancer Research and Royal Marsden Hospital, London, UK (NCT).
Pfizer Inc funded the study designs, conduct, data collection, and data analysis. All authors were responsible for data interpretation, writing or revising the manuscript for intellectual content, and the decision to submit the manuscript for publication. The authors have no conflicts of interest.
We thank the patients, investigators, and study personnel who participated in the PALOMA trials. PALOMA studies 1, 2, and 3 (NCT00721409, NCT01740427, NCT01942135, respectively) were sponsored by Pfizer. Editorial support was provided by Susan Reinwald, PhD, and Johna Van Stelten, PhD, both of Complete Healthcare Communications, LLC (West Chester, PA), a CHC Group Company, and was funded by Pfizer.
Supplementary Material
References
- 1. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;161:25–35. [DOI] [PubMed] [Google Scholar]
- 2. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;174:425–439. [DOI] [PubMed] [Google Scholar]
- 3. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;37520:1925–1936. [DOI] [PubMed] [Google Scholar]
- 4. Turner NC, Huang Bartlett C, Cristofanilli M.. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;37317:1672–1673. [DOI] [PubMed] [Google Scholar]
- 5. O'Leary B, Finn RS, Turner NC.. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;137:417–430. [DOI] [PubMed] [Google Scholar]
- 6. Boér K. Impact of palbociclib combinations on treatment of advanced estrogen receptor-positive/human epidermal growth factor 2-negative breast cancer. OncoTargets Ther. 2016;9:6119–6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finn R, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;115:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thanarajasingam G, Atherton PJ, Novotny PJ, Loprinzi CL, Sloan JA, Grothey A.. Longitudinal adverse event assessment in oncology clinical trials: The Toxicity Over Time (ToxT) analysis of Alliance trials NCCTG N9741 and 979254. Lancet Oncol. 2016;175:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Study of letrozole with or without palbociclib (PD-0332991) for the first-line treatment of hormone-receptor positive advanced breast cancer (NCT00721409). https://clinicaltrials.gov/ct2/show/NCT00721409?term=NCT00721409&rank=1. Accessed July 12, 2017.
- 10.A study of palbociclib (PD-0332991) + letrozole vs. letrozole for 1st Line treatment of postmenopausal women with ER+/HER2- advanced breast cancer (PALOMA-2) (NCT01740427). https://clinicaltrials.gov/ct2/show/NCT01740427?term=NCT01740427&rank=1. Accessed July 12, 2017.
- 11.Palbociclib (PD-0332991) combined with fulvestrant in hormone receptor+ HER2-negative metastatic breast cancer after endocrine failure (PALOMA-3) (NCT01942135). https://clinicaltrials.gov/ct2/show/NCT01942135?term=NCT01942135&rank=1. Accessed July 12, 2017.
- 12. Finn RS, Crown J, Lang I, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole vs letrozole alone for first-line treatment of ER+/HER2‒ advanced breast cancer (PALOMA-1; TRIO-18). J Clin Oncol .2017;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Temple R. Hy's law: Predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;154:241–243. [DOI] [PubMed] [Google Scholar]
- 14. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;37518:1738–1748. [DOI] [PubMed] [Google Scholar]
- 15. Durairaj C, Ruiz-Garcia A, Gauthier ER, et al. Palbociclib has no clinically relevant effect on the QTc interval in patients with advanced breast cancer. Anticancer Drugs. 2018;293:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verma S, Huang Bartlett C, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;2110:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diéras V, Harbeck N, Joy AA, et al. PALOMA-2: Neutropenia patterns in patients with estrogen receptor−positive/human epidermal growth factor receptor 2−negative first-line advanced breast cancer receiving palbociclib plus letrozole. Paper presented at: 42nd Congress of the European Society for Medical Oncology; September 8–12, 2017; Madrid, Spain.
- 18. Zheng J, Yu Y, Durairaj C, et al. Palbociclib exposure-response analyses in the treatment of hormone-receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2–) advanced breast cancer (ABC). Paper presented at: 40th Annual San Antonio Breast Cancer Symposium; December 5–9, 2017; San Antonio, TX.
- 19. Sun W, Yu Y, Hoffman J, Turner NC, Cristofanilli M, Wang D. Palbociclib exposure-response analyses in second-line treatment of hormone receptor−positive advanced breast cancer. Paper presented at: American Society of Clinical Oncology Annual Meeting; June 2–6, 2017; Chicago, IL.
- 20. Hu W, Sung T, Jessen B, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res. 2016;228:2000–2008. [DOI] [PubMed] [Google Scholar]
- 21. IBRANCE (Palbociclib). Summary of Product Characteristics. Kent, UK: Pfizer Limited; 2018. [Google Scholar]
- 22. Ibrance (Palbociclib). Full Prescribing Information. New York: Pfizer; 2018. [Google Scholar]
- 23. Rugo H, Diéras V, Gelmon K, et al. Impact of palbociclib plus letrozole on health-related quality of life compared with letrozole alone in treatment-naive postmenopausal patients with ER+ HER2− advanced/metastatic breast cancer: Results from PALOMA-2. Paper presented at: 41st Congress of the European Society for Medical Oncology; October 7–11, 2016; Copenhagen, Denmark.
- 24. Harbeck N, Iyer S, Turner N, et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: Patient-reported outcomes from the PALOMA-3 trial. Ann Oncol. 2016;276:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rugo HS, Pritchard KI, Gnant M, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: Insights from BOLERO-2. Ann Oncol. 2014;254:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;3666:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.