Abstract
Purpose:
Dendritic cells (DCs) initiate adaptive immune responses through the uptake and presentation of antigenic material. In preclinical studies, intratumorally injected activated DCs (aDCs; DCVax®-Direct) were superior to immature DCs in rejecting tumors from mice.
Experimental Design:
This single-arm, open-label phase I clinical trial evaluated the safety and efficacy of aDCs, administered intratumorally, in patients with solid tumors. Three dose levels (2 million, 6 million, and 15 million aDCs per injection) were tested using a standard 3 + 3 dose-escalation trial design. Feasibility, immunogenicity, changes to the tumor microenvironment after direct injection, and survival were evaluated. We also investigated cytokine production of aDCs prior to injection.
Results:
In total, 39 of the 40 enrolled patients were evaluable. The injections of aDCs were well tolerated with no dose-limiting toxicities. Increased lymphocyte infiltration was observed in 54% of assessed patients. Stable disease (SD; best response) at week 8 was associated with increased overall survival. Increased secretion of interleukin (IL)-8 and IL-12p40 by aDCs was significantly associated with survival (p=0.023 and 0.024, respectively). Increased TNFα levels correlated positively with SD at week 8 (p<0.01).
Conclusions:
Intratumoral aDC injections were feasible and safe. Increased production of specific cytokines was correlated with SD and prolonged survival, demonstrating a link between the functional profile of aDCs prior to injection and patient outcomes.
Keywords: Activated dendritic cells, cancer immunotherapy, solid tumors, cytokines, intratumoral therapy
Introduction
Patients with unresectable, locally advanced, or metastatic solid tumors have a poor prognosis and few therapeutic options, especially after having failed standard therapies (1–3). Recently, there have been several promising advances in immune cancer therapies (4, 5). However, to mount an effective immune response against cancer, the immune system must first be primed to attack cancer cells (6). Specifically, tumor-specific antigens must be presented to naïve T cells by antigen-presenting cells, which induce T-cell differentiation into activated cytotoxic T cells (CTLs) (4, 7).
One of the most efficient antigen-presenting cells are dendritic cells (DCs) (8). DCs stimulate both B and T cells and generate costimulatory molecules, such as cytokines, to drive CTL clonal expansion (8). Given DCs’ ability to induce a broad immune response, DC-based immunotherapy research has grown rapidly in recent years. DC-based cancer vaccine clinical trials have shown various degrees of promise, and several products are currently in late-stage clinical trials (9). The various DC subsets found in blood are known for their efficient antigen cross-presentation and their ability to effectively migrate to draining lymph nodes. However, they comprise less than 1% of peripheral blood mononuclear cells, which means that there is insufficient cellular material to generate a vaccine (7, 10). As a result, researchers typically use ex vivo-generated DCs derived from monocytes collected from the patient via leukapheresis; however, strategies using other DC types are currently being investigated. (7, 10, 11). After generating DCs, the cells are pulsed with an antigen and injected back into the patient. The choice and source of the antigen (e.g., purified protein or cell lysate), as well as the loading method, have a large effect on outcome and vary widely between studies (10).
Another consideration in DC-based immunotherapy is DC maturation. Immature DCs take up antigens more readily; however, they also induce T-cell tolerance by triggering apoptosis, promoting anergy, or priming Treg differentiation in T cells (12). To ensure that the exogenously loaded DCs will elicit an active immune response, maturation agents must be used during vaccine preparation, either before or after antigen loading (10, 13). Maturation agents include Toll-like receptor (TLR) agonists or mixtures of cytokines, such as tumor necrosis factor α (TNFα), interleukin (IL)-1b, IL-6, and prostaglandin E2 (PGE2) (10). Differentiation protocols often include IL-4 to inhibit differentiation into macrophages instead of DCs (14), but this is not required if monocyte activation is avoided during purification (35). Some strategies supplement their maturation compounds with interferon γ (IFNγ), IFNα, and polyinosinic:polycytidylic acid to generate mature type-1 polarized DCs that secrete IL-12. The mature DCs induce a T-helper cell 1 (TH1)-type profile that elicits natural killer cell and CTL activation (15, 16). CTL activation triggers a pro-inflammatory state, stimulating these cells to kill tumor cells directly (17).
In addition to maturation, the administration method has a significant impact on outcomes. The administration route must allow the DCs to reach the lymph nodes, so they can induce T-cell differentiation. Several methods, including intravenous, intradermal, and intranodal injection, have been studied previously (10). Another promising route is intratumoral (i.t.) injection, which utilizes the necrotic or apoptotic milieu already present in tumors to load naïve DCs with endogenous antigens. I.t. injection of immature, unloaded DCs in animal models resulted in tumor regression and increased survival (18, 19). These therapeutic effects were enhanced by simultaneously treating the animals with other, more traditional treatment regimens, including chemotherapy (20–22) and radiation therapy (23–25). These approaches have also been tested in patients with various cancer types, and these studies produced varied results (26–29). As mentioned previously, immature DCs are proficient at antigen uptake and processing, whereas mature DCs are better at presenting antigens. Thus, we hypothesized that partially matured DC that could take up antigen in situ while being irrevocably committed to the maturation pathway could provide a more effective tumor vaccine. These partially matured DCs, called “activated DCs” (aDCs) express all the appropriate signaling molecules as well as unusually high levels of cytokines and can induce antigen-specific antitumor immune responses through MHC class I–mediated antigen presentation (30). aDCs can be generated using various agents, including Bacillus Calmette-Guerin (BCG) cell wall skeleton and a TLR-stimulating reagent (31). aDCs have been previously studied in mouse models (31) and humans (32).
We previously performed a preclinical study investigating intratumoral aDC injections combined with chemotherapy in mice xenografted with colon carcinoma cells. The immature DCs were activated using inactivated BCG and IFNγ. The aDCs expressed higher costimulatory molecule levels than immature DCs and secreted high levels of TNFα, IL-6, IL-8, IL-12, and other cytokines and chemokines. In this study, tumor clearance was higher for mice treated with combination therapy than for those with chemotherapy alone (33).
Based on the promising preclinical results, we conducted a phase I trial to test the safety and feasibility of aDCs administered using i.t. injection as a treatment for patients with unresectable, locally advanced, or metastatic solid tumors. Secondary outcomes included immune response measures, biopsy evaluations to determine local and systemic effects, and exploratory efficacy measures related to tumor size and patient survival. During this trial, we observed some variability in the autologous cell therapy products generated, possibly due to the inherent variability in monocytes obtained from different patients. Thus, we also investigated whether this variability translates to clinical efficacy.
Methods
Patients
Patients 18–75 years of age with locally advanced or metastatic disease and who had undergone at least one antitumor treatment regimen within 12 weeks of screening were eligible for the study. Other eligibility criteria included having an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, having at least one injectable tumor mass >1 cm in diameter and located away from major vascular structures or areas not amenable to swelling (e.g., upper airway tumors), producing a sufficient number of monocytes to manufacture the full dose course, having a life expectancy >6 months, and having adequate bone marrow and renal function. Patients with a history of autoimmune disease or organ transplants were excluded from the study. Other exclusion criteria included having positive status for HIV-1, 2, or HTLV-I,II; having heavily myelosuppressive or myelotoxic chemotherapy within 4 weeks prior to the first injection; receiving cancer immunotherapy within 2 years; having untreated brain metastases; needing ongoing steroid or anti-coagulant therapies; or having an acute or uncontrolled infection. Patient characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of treated patients
| Characteristics, n=39 | Total |
|---|---|
| Age, years, median (range) | 53 (30–73) |
| Sex, n (%) | |
| Male | 18 (46.2) |
| Female | 21 (53.8) |
| Disease type, n (%) | |
| Pancreatic adenocarcinoma | 5 (12.8) |
| Sarcoma | 9 (23.1) |
| Colorectal | 7 (17.9) |
| Neuroendocrine | 4 (10.3) |
| Melanoma | 6 (15.4) |
| Lung | 3 (7.7) |
| Breast | 2 (5.1) |
| Ovarian | 1 (2.6) |
| Bladder | 1 (2.6) |
| Cholangiocarcinoma | 1 (2.6) |
| No. of prior therapies, n (%) | |
| ≤2 | 20 (51.3) |
| 3–5 | 12 (30.8) |
| ≥6 | 7 (17.9) |
Study design
This was part 1 of a phase I/II open-label clinical trial evaluating the safety and efficacy of aDCs (ClinicalTrial.gov identifier NCT01882946). This dose-escalation portion of the trial used a “3 + 3” design. Three dose levels were included in this study: 2 million, 6 million, and 15 million aDCs. The study was conducted study in accordance with the International Conference on Harmonization principles of Good Clinical Practice and the Declaration of Helsinki (1989). The study and consent forms were approved by local Institutional Review Boards prior to commencing the study. All patients provided written informed consent. The study was conducted at two centers: University of Texas MD Anderson Cancer Center in Houston, TX, and Orlando Health in Orlando, FL.
Each patient underwent leukapheresis to collect monocytes, the DC precursor cells. The aDCs (trade name DCVax®-Direct) were prepared as described below. The first aDC injection took place approximately 3 weeks after the leukapheresis, and subsequent injections were administered at 1, 2, 8, 16, and 32 weeks after the first injection. All injections were administered to either a primary or metastatic tumor as follows. First, an 18G guide needle was placed using image guidance (computed tomography [CT], ultrasound, or magnetic resonance imaging [MRI]) and the commonly used coaxial technique, which provided access to the tumor. Then, a 20G trucut core biopsy device was inserted, followed by a thinner 22G needle to deliver the product directly into tumor tissue. For each immunization, cells were administered in 3–4 needle passes within tumor margins using a fanning technique. This technique enhanced aDCs’ exposure to dead and dying tumor cells while avoiding delivering a single bolus to the necrotic center of the tumor mass. After the injections, the subjects were observed for 2 hours with vital signs (heart rate, temperature, and blood pressure) taken every 30 minutes.
Dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD)
DLT was defined as any of the following: ≥grade 3 injection site reactions, development of clinical signs and symptoms of autoimmune disease, ≥grade 2 allergic reaction, ≥grade 2 immunological reaction that lasted for ≥3 days or required drug intervention, ≥grade 3 National Cancer Institute Common Toxicity Criteria (NCI CTC) v.4 toxicity, or grade 4 or life-threatening events that are not related to malignancy progression. The MTD was defined as the highest dose level at which no more than one third of subjects experience DLT.
Evaluation of efficacy
Treatment efficacy was evaluated by CT or MRI studies according to Response Evaluation Criteria in Solid Tumors v. 1.1 (34) or immune response-related criteria (35). Briefly, progressive disease (PD) was defined as a ≥20% increase in the sum of the target lesion’s diameters compared with the smallest sum observed during the study, and the absolute sum must increase ≥5 mm. SD was defined as having insufficient tumor shrinkage to qualify as a partial response (≥30% target lesion diameter reduction), while also having insufficient tumor growth to qualify as PD.
Preparation of aDCs
Monocytes were purified from the leukapheresis product using a proprietary tangential-flow filtration method. When comparing cytokine levels produced by aDCs differentiated with or without IL-4, we previously showed that those differentiated without IL-4 yielded higher levels of relevant cytokines (Supplemental Figure 1) (30). Therefore, aDCs used in this study were produced as follows. Cells were placed in Teflon tissue culture bags (Saint-Gobain, Malvern, PA) and differentiated into immature DC for 5 days in the presence of granulocyte macrophage colony-stimulating factor. Cells were cultured for 5 days, and then killed BCG mycobacteria and IFNγ were added to induce DC activation. The release criteria for patient administration was purity (i.e., CD86+, MHC-II+) >70% and cell viability >50%. Following activation, the cells were cryopreserved in single-dose aliquots. Flow cytometry was performed on cells looking for dendritic cell-activation markers (36).
Cytokine level determination
A custom multiplex magnetic (Luminex Corp., Austin, TX) bead set for TNFα, IL-4, IL-6, IL-8, IL-10, and IL-12p40 and a singleplex set for IL-12p70 (Invitrogen, Carlsbad, CA) were used to determine concentrations of cytokines produced during a 24-hour period in clarified supernatants from DCVax-Direct product cultures according to the manufacturer’s protocol. At the time the supernatant was harvested, a cell count was also performed. Cell viability was determined based on trypan blue exclusion. Data are reported as the average value of duplicate determinations normalized per million live DCs per 24-hour period.
Evaluation of tumor biopsies
Biopsied tumors were formalin fixed and paraffin embedded (FFPE) using standard methods. All immunohistochemistry was performed by QualTek Molecular Laboratories (Santa Barbara, CA).
In situ detection of IFNγ and TNFα transcripts in FFPE specimens was performed using the RNAscope assay with probes Hs-IFNγ and Hs-TNFα (cat#310501 and 310421, respectively, Advanced Cell Diagnostics [ACD], USA), as well as positive control probe PPIB (cat#313901), and the RNAscope 2.0 HD Reagent kit (Brown, cat#310035, ACD, USA) following procedures recommended by the manufacturer. To verify IFNγ and TNFα RNAscope specificity, PBMCs from three healthy donors were tested before and after T-cell stimulation. To stimulate T cells, PBMCs were isolated using Ficoll-Paque (Sigma-Aldrich), resuspended in RPMI-1640 medium supplemented with 10% fetal bovine serum, and treated with 50 ng/mL phorbol myristate acetate and 1 μg/mL ionomycin (Sigma-Aldrich) for 5 hrs at 37°C and 5% CO2. Cells were fixed in 10% neutral buffered formalin in Histogel, processed, and embedded into FFPE blocks. Sections (5 μm) were then tested using RNAscope as indicated above. The stimulated T cells demonstrated a strong increase in both IFNγ and TNFα compared with untreated cells. Digital images of the stained slides were acquired with an Aperio ScanScope XT digital slide scanner.
Statistical analysis
We performed statistical analyses to determine if cytokine levels were associated with outcome. In addition, we assessed whether the baseline characteristics or treatment factors were predictive of the cytokine levels or outcome. Response was measured based on two variables: SD at week 8 (best response) as a binary measure and duration of survival. We did not perform adjustments for testing multiplicity. A p value of 0.05 was considered statistically significant.
First, we generated descriptive measures for the cytokine levels, including correlations between the potency measures. Next, we assessed the association between baseline characteristics or treatment factors with cytokine levels using non-parametric ANOVA (Wilcoxon) methods. Scatter plots of the measures were reviewed for all of the pairs of cytokine levels. A proportional hazards model was used to fit the survival as a function of the individual cytokine levels, and a backward regression was used to determine if special measures were more predictive in a joint model. A logistic model was used to fit the SD at 8 weeks as a function of the individual cytokine levels, and a backward regression was used to determine if special measures were more predictive in a joint model. Proportional hazards models, logistic models, trend tests, or likelihood-ratio χ2 tests were used to evaluate the association of baseline characteristics and treatment factors with survival and SD at 8 weeks as appropriate to the measure and endpoint. For Kaplan-Meier plots of survival based on cytokine levels, we used the median value for each cytokine as the cutoff between the two groups.
Based on the analyses and a review of the scatter plots, a group of observations appeared to be potential outliers or possibly a unique set of subjects (described further in Results). The analyses were repeated with these subject records removed. All analyses were completed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Patients
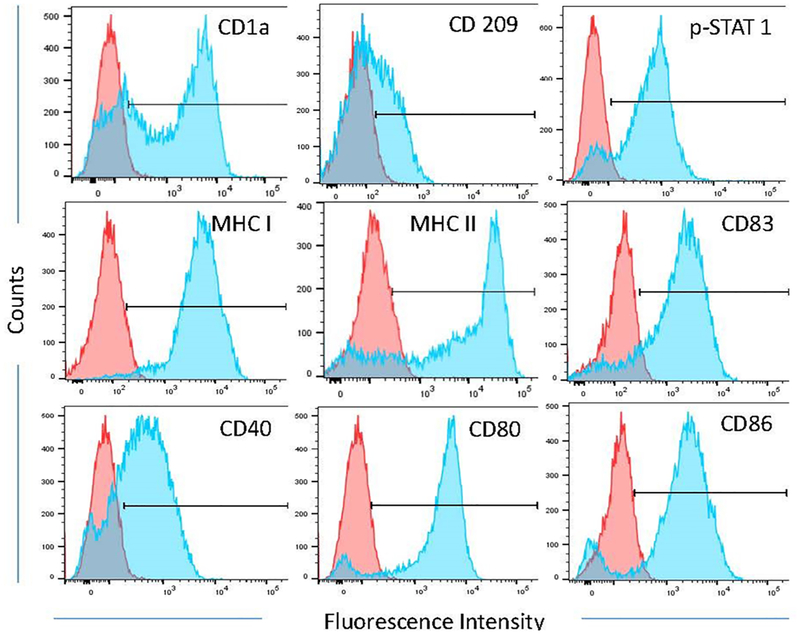
Overall, 40 patients were enrolled between July 2013 and June 2014 from two study centers, University of Texas MD Anderson Cancer Center in Houston, TX and Orlando Health in Orlando, FL. Prior to treatment, the aDCs generated from the leukapheresis product were evaluated for markers of dendritic cell-activation (Fig. 1). Following this analysis, one patient was deemed not evaluable due to an incorrect formulation of the aDCs. Patient demographics and clinical characteristics are presented in Table 1. The median patient age was 53 years (range 30–73 years). The study included 21 women (53.8%) and 19 men (48.7%). A large number of solid tumor types were eligible for enrollment in the study, with the most common being sarcoma (n=8), colorectal cancer (n=7), and melanoma (n=6). Patients had a median of three lesions (range, 1–5 lesions). The median number of prior treatments was two (average 3; range, 1–9). All aDC injections were performed on an outpatient basis using image guidance (CT, ultrasound, or MRI) facilitated by conscious sedation by an interventional radiologist. At the 2-million aDC dose, 16 patients were administered a median of four injections (range, 1–6 injections). At the 6-million aDC dose, 20 patients were administered a median of three injections (range, 2–6 injections). At the 15-million aDC dose, three patients were administered a median of four injections (range, 3–4 injections). Only one tumor was injected per patient.
Fig. 1.
The phenotype of activated dendritic cells (aDCs). Presented are representative flow cytometry histograms of various dendritic cell-activation markers. Red histograms are from the monocyte population harvested during leukapheresis. Blue histograms are from aDCs.
Safety and survival
Despite the high levels of cytokines produced by the aDC, there was no evidence of cytokine-mediated toxicity. No DLTs were observed during the dose-escalation trial design, and thus, an MTD was not determined. The maximum tested dose (15 million aDCs) was well tolerated. Adverse events related to the study treatment are reported in Table 2. Treatment-related adverse events were observed in 32 patients (82.1%), but the vast majority of these events were deemed to be grade 1 or 2 and most had resolved by the end of the study period. The most common adverse events were pyrexia (n=31; 79.5%), chills (n=16, 41.0%), fatigue (n=12, 30.8%), injection site pain or discomfort (n=11, 28.2%), night sweats (n=10, 25.6%), decreased appetite (n=9, 23.1%), and myalgia (n=7, 17.9%).
Table 2.
Treatment-related adverse events.
| Activated dendritic cells (aDCs/injection) | |||||||
|---|---|---|---|---|---|---|---|
| 2 million, n = 16 |
6 million, n = 20 |
15 million, n = 3 |
Total, n (%)b |
||||
| Adverse Eventa | G1–G2 | G3–G4 | G1–G2 | G3–G4 | G1–G2 | G3–G4 | |
| Pyrexia | 15 | 0 | 14 | 0 | 2 | 0 | 31 (79.5) |
| Chills | 10 | 0 | 5 | 0 | 1 | 0 | 16 (41.0) |
| Fatigue | 8 | 0 | 2 | 2 | 0 | 0 | 12 (30.8) |
| Injection site pain/discomfort | 8 | 0 | 3 | 0 | 0 | 0 | 11 (28.2) |
| Night sweats | 5 | 0 | 5 | 0 | 0 | 0 | 10 (25.6) |
| Decreased appetite | 6 | 0 | 2 | 0 | 1 | 0 | 9 (23.1) |
| Myalgia | 4 | 0 | 3 | 0 | 0 | 0 | 7 (17.9) |
| Headache | 3 | 0 | 1 | 0 | 0 | 0 | 4 (10.3) |
| Nausea | 3 | 0 | 0 | 0 | 1 | 0 | 4 (10.3) |
| Vomiting | 3 | 0 | 0 | 0 | 1 | 0 | 4 (10.3) |
| Anemia | 1 | 0 | 0 | 1 | 0 | 0 | 2 (5.1) |
| Influenza-like illness | 1 | 0 | 1 | 0 | 0 | 0 | 2 (5.1) |
| Pain | 0 | 0 | 2 | 0 | 0 | 0 | 2 (5.1) |
| Weight loss | 0 | 0 | 1 | 0 | 1 | 0 | 2 (5.1) |
| Abdominal pain | 0 | 0 | 1 | 0 | 0 | 0 | 1 (2.6) |
| Back pain | 0 | 0 | 1 | 0 | 0 | 0 | 1 (2.6) |
| Chest pain | 0 | 0 | 1 | 0 | 0 | 0 | 1 (2.6) |
| Dehydration | 1 | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Dry eye | 1 | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Dry mouth | 1 | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Dyspnea | 0 | 0 | 1 | 0 | 0 | 0 | 1 (2.6) |
| Face edema | 0 | 0 | 1 | 0 | 0 | 0 | 1 (2.6) |
| Hydronephrosis | 1 | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Hypokalemia | 0 | 0 | 0 | 1 | 0 | 0 | 1 (2.6) |
| Hypomagnesaemia | 1 | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Insomnia | 1 | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Musculoskeletal discomfort | 1 | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Peripheral edema | 1 | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Skin sensitization | 0 | 0 | 1 | 0 | 0 | 0 | 1 (2.6) |
| Systemic inflammatory response syndrome | 0 | 0 | 0 | 1 | 0 | 0 | 1 (2.6) |
| Tachycardia | 0 | 0 | 1 | 0 | 0 | 0 | 1 (2.6) |
Abbreviations: aDCs, activated dendritic cells; G, grade (according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4).
When adverse events were observed on multiple dates at different grades, the highest grade observed was listed.
Percent of total patients, N = 39.
There were four grade 3 (10.3%) and one grade 4 (2.6%) treatment-related serious adverse events, all at the 6 million aDCs per injection dose.
Histology
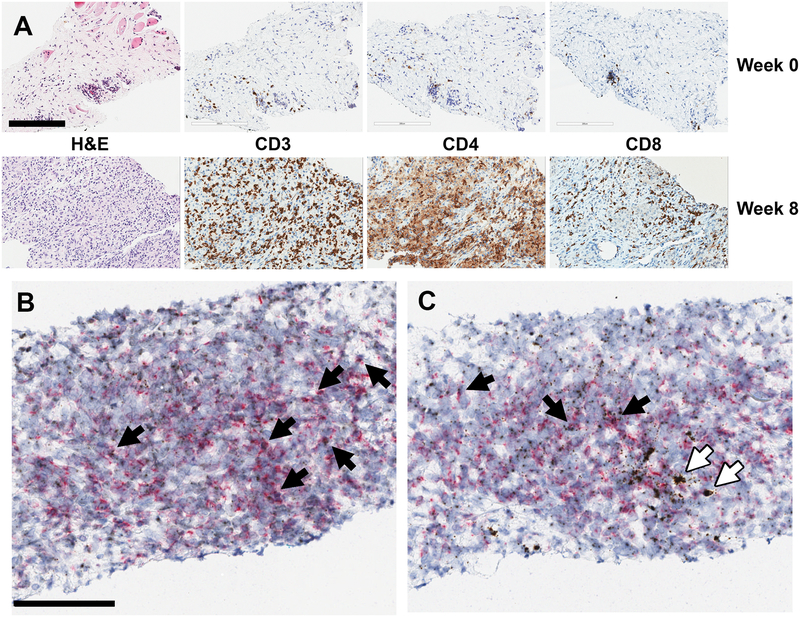
Following a protocol amendment, serial biopsy data were collected from 28 patients. In total, 104 biopsies were taken. New or increased necrosis was observed in 16 biopsied patients (57%). New or increased numbers of stromal lymphocytes were observed in 14 biopsied patients (50.0%); new or increased numbers of tumor-infiltrating lymphocytes were observed in 15 biopsied patients (54%); and both infiltrating and stromal lymphocytes were observed in 8 patients (29%). Biopsies were collected at week 3 and week 8, and peri- or intratumoral T cells were generally detected at 8-weeks post-treatment initiation (Fig. 2A). Therefore, the immune response was initiated somewhere within that timeframe. In some patients, the T-cell accumulation was detected at 2 or 3 weeks after the first injection. These T cells may represent a pre-existing antitumor immune response that localizes to the tumor following aDC injection.
Fig. 2.
Immunohistochemistry of biopsied tumor tissue. (A) T-cell infiltration following aDC treatment. Immunohistochemical staining shows that tumor-infiltrating lymphocytes, including CD3+ activated T cells, CD4+ helper cells, and CD8+ killer cells, increased from baseline in 15 of 27 biopsied patients. Representative images are from a clear cell sarcoma tumor treated with 6 million aDCs/injection. Two injections had been administered at the time of biopsy. Magnification is 20×, and the scale bar represents 200 μm. (B) and (C) Cytokine production by activated T cells. Tissue sections were probed for (B) IFNγ and (C) TNFα expression using RNAscope (brown dots) and co-stained for CD3 expression (red dots) using immunohistochemistry. Black arrows represent CD3+ activated T cells expressing either IFNγ or TNFα. White arrows represent CD3− cytokine-producing cells, likely macrophages. Representative images are from a clear cell sarcoma tumor treated with 6 million aDCs/injection. Two injections had been administered at the time of biopsy. Magnification is 20× and the scale bar represents 100 μm.
De novo or significantly enhanced PD-L1 expression was observed in 19 of 25 (76%) evaluated tumor biopsies. Among biopsies stained for both lymphocytes and PD-L1, new or increased PD-L1 expression was observed in 9 of 12 patients with new or increased infiltrating T cells and 11 of 12 patients with new or increased stromal lymphocytes. Among the 19 patients total with new or increased PD-L1 expression, 14 had either peritumoral or infiltrating lymphocytes.
When tumor-infiltrating T cells were observed, they were primarily a mixture of CD4+ and CD8+ cells; however, there were a few instances where either CD4+ or CD8+ T cells were detected exclusively. In some cases, the T cells constituted >30% of total cells in the biopsy section (see also Fig. 2A),
To assess tumor-associated and -infiltrating T-cell functionality, we performed RNAscope analysis for IFNγ and TNFα expression on selected tissues. The majority of T cells in the samples tested were positive for both cytokines (Fig. 2B and C), suggesting that fully functional T cells were recruited to the tumor. Tissue macrophages expressing TNFα were also detected.
Cytokine levels, survival, and SD
The cytokine levels of the aDCs were evaluated prior to injection in each patient. Because each batch of aDCs was derived from the patient’s own monocytes, there was a significant degree of inter-patient variability observed in the cytokine levels. To investigate this variability and its effects on treatment efficacy, we evaluated the internal correlations between cytokine levels and the associations between cytokine levels and baseline characteristics, treatment factors, survival, and SD at 8 weeks.
During statistical analyses, aDCs from three patients showed an aberrant pattern of cytokine production compared with those of aDCs from the majority of patients. The aDCs from these patients produced high levels of IL-8 and IL-6 but low levels of TNFα, and results from these patients consistently emerged as statistical outliers. The first outlier patient was a 51-year-old male melanoma patient from the 6-million aDC treatment group. He had five lesions and underwent one round of treatment previously. He received three injections, had SD at week 8, and died approximately 9 months after the first injection. The second was a 59-year-old female breast cancer patient in the 6-million aDC treatment group. She had three lesions and had undergone eight rounds of treatment previously. She received three injections and died approximately 1 month after the first injection. The third was a 52-year-old male lung cancer patient in the 15-million aDC treatment group. He had three lesions and underwent five rounds of treatment previously. He received three injections, had PD at week 8, and died approximately 3.5 months after the first injection. All three patients had a heavy burden of disease and an extremely poor prognosis. The three patients did not have other known prominent features that separated them from the rest of the trial subjects. An exploratory analysis of the aDCs from one of the patients using immunophenotyping suggested that the purified monocytes may have failed to completely transform into aDCs. Thus, we attributed the aberrant cytokine pattern to incomplete monocyte-to-DC differentiation that was not detectable initially based on the aDC release criteria used in the trial. In the subsequent analyses where DC quality was compared to patient outcomes, the outlier patient data were excluded.
Table 3 shows cytokine levels and expression of surface markers for the 36 patients that were included in the analyses.
Table 3.
Patient-level outcomes and biological markers.
| Patient1 | Sex | Organ/tumor type | Survival | Status2 | Disease3 | T cells4 | TNFα5 | IL-65 | IL-85 | IL-12 p405 | MHC-II MFI5,6 | CD86 MFI5,6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Pancreas | 48.5 | A | SD | N | ++ | + | + | + | n.d. | n.d. |
| 2 | M | Sarcoma/desmo | 8.2 | D | PD | n.d. | + | + | + | + | n.d. | n.d. |
| 3 | M | Soft tissue sarcoma | 24.0 | D | SD | Y | ++ | + | +++ | ++ | n.d. | n.d. |
| 4 | F | Soft tissue sarcoma | 11.2 | D | SD | N | + | + | ++ | ++ | ++ | +++ |
| 5 | M | Melanoma | 9.5 | D | PD | n.d. | ++ | + | ++ | + | ++ | +++ |
| 6 | M | Metastatic CRC | 3.7 | D | PD | n.d. | + | ++ | + | +++ | +++ | ++ |
| 7 | F | Metastatic CRC | 2.8 | L | PD | n.d. | + | ++ | + | ++ | ++ | ++ |
| 8 | M | Metastatic CRC | 4.7 | D | PD | n.d. | + | ++ | ++ | ++ | +++ | ++ |
| 9 | F | Pancreas | 8.8 | D | PD | n.d. | + | ++ | +++ | ++ | +++ | +++ |
| 10 | F | Soft tissue sarcoma | 13.8 | D | SD | Y | + | + | + | + | + | ++ |
| 11 | F | Pancreas | 2.4 | D | n.d. | n.d. | +++ | +++ | ++ | +++ | + | + |
| 12 | M | NET Pancreas | 45.5 | A | SD | Y | +++ | +++ | +++ | +++ | +++ | +++ |
| 13 | M | Melanoma | 45.0 | A | SD | Y | ++ | +++ | ++ | +++ | +++ | +++ |
| 14 | F | Melanoma | 7.5 | D | PD | n.d. | + | ++ | ++ | ++ | ++ | ++ |
| 15 | F | Cholangiocarcinoma | 21.8 | D | PD | Y | ++ | +++ | +++ | +++ | ++ | ++ |
| 16 | F | Melanoma | 5.3 | D | PD | Y | + | + | ++ | + | ++ | + |
| 17 | M | NSCLC | 1.5 | L | SD | n.d. | +++ | +++ | +++ | ++ | + | ++ |
| 18 | M | NET Pancreas | 5.6 | D | SD | Y | ++ | + | + | + | + | + |
| 19 | M | NET Lung | 42.6 | A | SD | N | +++ | +++ | ++ | n.d. | n.d. | n.d. |
| 21 | F | Metastatic CRC | 10.1 | D | SD | N | +++ | ++ | + | + | n.d. | n.d. |
| 22 | F | Ovary | 42.5 | A | SD | N | +++ | +++ | +++ | +++ | +++ | ++ |
| 23 | F | NSCLC | 34.7 | D | SD | Y | +++ | +++ | ++ | ++ | ++ | +++ |
| 24 | M | Pancreas | 4.1 | D | SD | Y | +++ | ++ | +++ | ++ | + | + |
| 25 | M | Soft tissue sarcoma | 13.4 | D | SD | N | ++ | + | + | + | + | + |
| 26 | M | Bladder | 22.0 | D | SD | N | +++ | +++ | +++ | +++ | ++ | + |
| 27 | F | NET | 28.6 | D | SD | N | +++ | +++ | +++ | +++ | n.d. | n.d. |
| 28 | F | Metastatic CRC | 7.1 | D | PD | Y | ++ | +++ | + | + | + | + |
| 29 | F | Pancreas | 17.4 | D | SD | Y | + | + | + | + | n.d. | n.d. |
| 30 | F | Soft tissue sarcoma | 9.3 | D | PD | Y | ++ | ++ | ++ | ++ | n.d. | n.d. |
| 31 | F | Soft tissue sarcoma | 15.4 | D | SD | n.d. | +++ | ++ | +++ | +++ | n.d. | n.d. |
| 32 | F | Soft tissue sarcoma | 36.2 | A | PD | Y | ++ | + | + | + | + | + |
| 33 | M | Metastatic CRC | 41.0 | D | SD | n.d. | ++ | + | +++ | +++ | n.d. | n.d. |
| 34 | M | Metastatic CRC | 16.1 | D | PD | N | ++ | ++ | + | +++ | +++ | +++ |
| 35 | F | Breast | 16.3 | L | n.d. | N | + | ++ | ++ | ++ | n.d. | n.d. |
| 36 | F | Melanoma | 45.1 | A | PD | Y | + | ++ | +++ | ++ | +++ | +++ |
| 39 | F | Soft tissue sarcoma | 15.1 | D | SD | N | +++ | +++ | ++ | +++ | n.d. | n.d. |
Abbreviations: CRC: colorectal cancer, Desmo: desmoplastic small-round-cell tumor, IL: interleukin, MHC: major histocompatibility complex, NET: neuroendocrine tumor, NSCLC: Non-small cell lung cancer, TNF: tumor necrosis factor
Excludes three patients determined to be statistical outliers and excluded from correlation analysis.
Status: A: Alive; D: Deceased; L: Lost to follow-up
Disease: SD: Stable disease at week 8; PD: progressive disease at week 8.
T cells: Y: de novo or significant increase in infiltrating T cells detected on biopsy; N: no de novo or significant increase in infiltrating T cells detected on biopsy; n.d.: not determined
+, ++, +++ indicate relative levels of expression: patients were divided into tertiles for each marker. Patients in the tertile with the lowest relative expression level are indicated by a ‘+’, in the mid-tertile by ‘++’, and in the top tertile by ‘+++’. n.d.: not determined
MFI: mean fluorescence intensity
Internal correlations between cytokine levels
To assess the quality of activation and the effect of the cytokines produced by aDCs, we determined the levels of TNFα, IL-6, IL-8, IL-10, IL-12p40, and IL-12p70 from the tissue culture supernatants prior to harvesting. There was a high level of internal correlation between the various cytokines evaluated. Those values were correlated with outcome (SD or survival) in univariate analyses. Separately, a backward regression model was used to assess the relative predictive strength of the measures and identify variable combinations based on a joint model, starting with all of the factors. The correlated cytokines were sorted into three groups. The first group included IL-6, IL-12p40, and to a lesser extent TNFα. The Pearson r value for IL-6 and IL-12p40 was 0.64 (p=0.004). The r value for IL-6 and TNFα was 0.88 (p<0.001). The r value for IL-8 and IL-12p40 was 0.641 (p<0.001). The second group was IL-10 and IL-8. The r value for IL-8 and IL-10 was 0.63 (p<0.001). The third group was IL-12p40 and IL-12p70, which had an r value of 0.55 (p<0.001). However, due to the short aDC activation time, the full complement of IL-12p70 production could not be detected.
Associations between cytokine levels and baseline characteristics and treatment factors
Next, we evaluated associations between cytokine levels and baseline characteristics and treatment factors, including indications, number of lesions, prior treatment, dose, number of injections, age, sum of the longest tumor diameter (SLD), and absolute lymphocyte count at screening (ALC), using regression analysis. SLD was negatively associated with levels of IL-8 (R2=0.20; p=0.006), IL-12p40 (R2=0.14; p=0.026), and IL-12p70 (R2=0.11; p=0.051), and positively associated with IL-10 levels (R2=0.14; p=0.023). ALC was positively associated with IL-12p40 (R2=0.26; p=0.002). Neither SLD nor ALC were independently associated with survival. We also investigated correlations between cytokine levels and quantitative measures of infiltrating T cells, but none were found. This lack of correlation could be a legitimate result or a sampling issue, as tumor sampling can be misleading due to tumor heterogeneity.
Associations between cytokine levels and survival
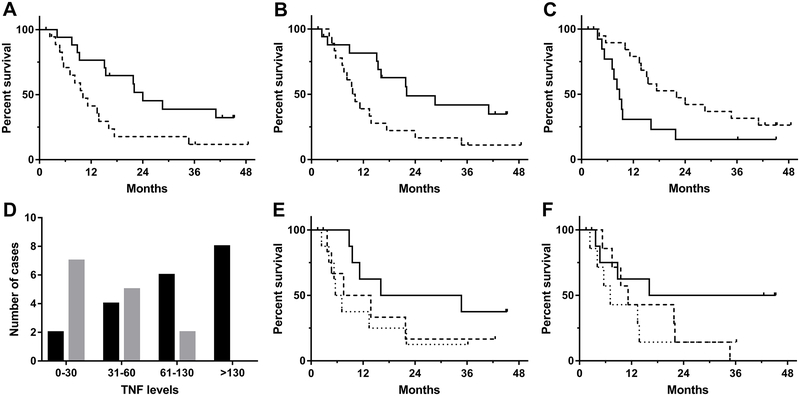
The aDC cytokine concentrations were individually fit in a proportional hazard model to determine whether they were predictive of survival. Univariate analysis indicated that IL-8 and IL-12p40 were associated with survival. Specifically, IL-8 levels greater than 985 ng/106 cells/day and IL-12p40 levels greater than 330 ng/106 cells/day were significantly correlated with longer overall survival (log-rank p=0.023 and p=0.024, respectively; Fig. 3A and B). We performed an exploratory analysis to evaluate a joint model of isolated cytokine pairs and assess potential factor interactions. The combination of IL-8 and IL-12p40 was associated with a potentially significant interaction term (p=0.020). The joint interaction model indicates that aDC preparations with high concentrations of both IL-8 and IL-12p40 were associated with longer overall survival in this patient population. This result suggests that there may be more complex relationships between DC potency measures and clinical outcomes.
Fig. 3.
Cytokine production and disease status subgroup analyses. (A) Correlation between IL-8 production (ng/106 DCs/day) and overall survival. Kaplan-Meyer curve of IL-8 production and survival. The dashed line indicates survival in patients injected with aDCs producing <985 ng IL-8/106 DCs/day (the median IL-8 production); the solid line represents those injected with cells producing ≥985 ng IL-8/106 DCs/day (p=0.03, log-rank). (B) Correlation between IL-12p40 production (ng/106 DCs/day) and overall survival. Kaplan-Meyer curve of IL-12p40 production and survival. The dashed line indicates survival in patients injected with aDCs producing <330 ng IL-12p40/106 DCs/day (the median IL-12p40 production); the solid line represents those injected with cells producing ≥330 ng IL-12p40/106 DCs/day (p=0.03, log-rank). (C) Stable disease (SD) at week 8 and survival. Kaplan-Meyer curve of patients with SD at week 8 compared with that of patients with progressive disease (PD) at week 8. The dashed line indicates survival in patients with PD at week 8; the solid line represents those patients with SD at week 8. The overall survival was significantly different between the two groups (p<0.05). (D) TNFα production by the aDCs and disease status at week 8. The number of patients with SD at week 8 is shown with black bars, and the number of patients with PD is shown with grey bars. There were no patients with PD at week 8 in patients with TNFα levels >130 ng/106 DCs/day (p<0.01, chi-squared). (E) Association between patient survival and expression levels of the cell surface marker CD86. The solid line indicates patients with cells having >3,400 MFI when stained for CD86; the dashed line indicates patients with cells having 2,000–3,400 MFI; and the dotted line represents patients with cells having <2,000 MFI (p=0.18, log-rank; p=0.07, log-rank for trend). (F) Association between patient survival and expression levels of the cell surface marker MHC-II. The solid line indicates patients with cells having >6,000 MFI when stained for MHC-II; the dashed line indicates patients with cells having 6,000–16,000 MFI; and the dotted line represents patients with cells having <16,000 MFI (p=0.21, log-rank; p=0.08, log-rank for trend). Three outliers were removed for (A), (B), and (D), see text for details. 24 of 39 patients were available for CD86 and MHC-II analyses.
Associations between cytokine levels and SD at week 8
Log-rank analysis showed that survival was significantly associated with SD at week 8 (p=0.047, Fig. 3C); thus, we determined whether there were cytokine markers associated with SD. The cytokine levels were individually fit into a logistic model to determine whether they were predictive of SD at week 8. Univariate analysis revealed a positive association between SD at week 8 and TNFα levels produced by aDCs (log-rank p<0.01, Fig. 3D), and this association was confirmed in a multivariate backward regression model (p=0.01).
Other measures of DC quality
The injected DC from 24 patients were analyzed for surface marker expression. Weak trend correlations were observed between survival and the levels of expression (mean fluorescence intensity divided into tertiles) of MHC class II (log-rank p=0.213, p for trend=0.085) and the CD86 costimulatory molecule (log-rank p=0.226, p for trend=0.088), lending further support for the hypothesis that patient outcome is at least partially a function of DC quality (Fig. 3E and F).
Discussion
In this phase I trial, we tested the safety and efficacy of activated autologous dendritic cells, injected intratumorally, as a treatment for patients with unresectable, locally advanced, or metastatic solid tumors. Patients were treated with 2-, 6-, or 15-million aDCs per injection at week 0, 1, 2, 8, 16, and 32 or until there were no more autologous aDCs to administer. We did not observe any DLTs, and thus, there was no MTD. This observation is consistent with other DC vaccine studies in which no DLTs or MTDs were identified (9, 37, 38). In our study, the maximum dose administered was 15 million aDCs per injection, and this dose was well tolerated. The literature is conflicted on whether larger doses improve outcomes (38) or do not add additional benefits (39–42). Given the results presented here, there is no clear indication of what dose is ideal for intratumorally injected aDCs. We observed relatively few low-grade, treatment-related adverse events in this study, and they were primarily associated with immune activation (e.g., pyrexia (43)). Collectively, these results indicate that aDCs are a safe treatment for solid tumors.
With respect to the efficacy of the aDCs, biopsies of injected tumors showed increased necrosis and infiltration of lymphocytes, including CD4+ helper cells and CD8+ killer cells. In individual cases, we observed immune reactivity with both rapid and delayed infiltration of T cells in patient biopsies and extensive necrosis. These observations preceded a demonstrable reduction in tumor size (Supplemental Figure 2). Studies have shown that increased infiltration and accumulation of certain types of T cells, such as stromal lymphocytes and CTLs, in tumors are strongly correlated with improved outcomes in several solid tumors (44–46). In addition, PD-L1 was upregulated in 19 of 25 tumors tested, and this upregulation likely reflects the tumor response to immune activation, particularly because tumor biopsies that tested positive for T cells were more likely to have increased PD-L1 expression. PD-L1 is a co-inhibitory molecule elicited during lymphocyte infiltration that downregulates T-cell activity to control excessive immune reactions, and tumors use it to evade immune responses (4, 10). Given that our PD-L1 data are from biopsied tumors, the emergence of PD-L1 expression may serve as a marker of successful antitumor immune response induction. Nevertheless, it is possible that the induced immune responses are suppressed by this immune checkpoint and that addition of a checkpoint blockade following DC therapy may further enhance efficacy. Overall, these results provide evidence that aDCs stimulate an effective T-cell response in solid tumors.
For the aDC treatment to be effective, it should also improve patient outcomes. We hypothesized that the survival mechanism was related to DC potency, as measured by the cytokines secreted by the aDCs. Therefore, we assessed cytokine levels of the aDCs prior to injecting them into the tumors. We observed that IL-12p40 was significantly associated with survival. IL-12p40 is one subunit of the heterodimeric IL-12 complex, also called IL-12p70. IL-12 is known to stimulate natural killer cells and mature T cells. It is also known to help convert TH2 cells to TH1 cells that have antitumor activity (47). Thus, IL-12–producing aDCs are ideal for an effective DC vaccine. We used IL-12p40 as a marker of IL-12 activity because the gene expression of IL-12p35, the other IL-12p70 subunit, is lower compared with that of IL-12p40 (48). The relatively short activation time of the aDCs was not sufficient for cells to make substantial quantities of IL-12p70 complex before harvesting cells, preventing us from exploring the associations between IL-12 p70 and survival.
IL-8 secretion also was associated with survival. Specifically, high IL-8 secretion showed significantly higher overall survival. IL-8 is largely considered to be negatively associated with cancer, and retention of intratumoral DCs through IL-8 has been demonstrated (49). IL-8 promotes angiogenesis, cell proliferation, and cell survival; however, it also promotes infiltration of immune cells into the tumor microenvironment (50), does not disrupt the ability of DCs to stimulate T cells, and may attract and retain neutrophils when secreted by DCs (4, 49). In the case of BCG immunotherapy, IL-8 was associated with the development of an antitumor immune response (51). It seems possible that the localized application of IL-8–producing aDCs stimulated infiltration of immune cells into the tumor.
The regression model indicated that the combination of IL-8 and IL-12p40 was positively associated with survival. To date, the literature is conflicted on whether cytokines, such as IL-8, are immunosuppressive or if they are beneficial by affecting the tumor microenvironment or playing a role in recruiting inflammatory cells. Our positive association with survival indicates that the combination of IL-8 and IL-12p40 (and possibly other cytokines), rather than individual cytokines, may be key for improved survival. It seems likely that these cytokines play multiple roles in the complex interactions between the tumor and immune system and that the overall effect is beneficial. It is also possible that these molecules solely serve as sensitive markers of overall aDC quality and potency. The observed associations between patient baseline parameters and cytokine production (i.e., aDC quality) suggest that factors, such as SLD and ALC, may predispose patients towards greater benefit from DC-based therapies, although the R2 values suggest that these baseline parameters only explain up to 25% of the variation in cytokine levels. This possibility deserves further attention in subsequent trials with more homogenous patient populations and will be the subject of future investigations.
IL-8 and IL-12 are known to play a role in the efficacy of i.t.-administered DCs. Several mouse and human studies evaluated the efficacy of i.t.-administered autologous DCs that were transfected to constitutively express IL-12, presuming they would elicit a stronger immune response. Mice with B16 melanoma treated intratumorally with IL-12-transfected DCs lived significantly longer than those treated with unmodified or GFP-modified DCs. Analysis of mouse spleen cells showed they were sensitized to generate CTLs (52). I.t. administration of IL-12-transfected DCs in mice with liver tumors significantly reduced tumor burden (based on liver weight). Further, mice who had previously been treated with IL-12-transfected DCs showed sustained immunity when challenged a second time with CMS4 tumor cells (53). In humans with metastatic gastrointestinal carcinomas, IL-12-transfected DCs injected intratumorally were safe, but the response was limited (54). A study investigating the limited response observed in humans found that IL-12-transfected DCs administered intratumorally stayed in tumors due to IL-8 expression by tumor cells (55). Further investigations showed that IL-8 affects DC migration in tumors, but does not affect T-cell stimulation (49). However, this barrier is not insurmountable (56). Nishioka et al. showed that IL-12-transfected DCs injected intratumorally were capable of migrating to the draining lymph in mice. Similarly, we observed decreased tumor size in distant lesions (Supplemental Figure 2), indicating that the i.t.-administered aDCs could be reaching the lymph. It seems likely that coupling aDCs with strategies to enhance migration to the lymph may be an effective means to improve aDC efficacy.
To evaluate clinical activity of antitumor vaccines, we must consider parameters other than tumor response criteria, as those may not be adequate or appropriate given the mode of action of these agents. In some cases, cancer immunotherapy may not show an apparent treatment effect, but still have an effect on survival (57). One example is the failed trial for tremelimumab. This trial was canceled due to futility, but later analysis revealed a survival benefit (58). Further, sipuleucel-T, the first FDA-approved cancer vaccine was approved based on improvements in overall survival (59). In our study, survival analysis of the aDC-treated patients showed that SD at week 8 was significantly correlated with survival. These data suggest that if the tumor can be stabilized by aDCs, then the odds of progression-free survival significantly increase, indicating clinical activity by aDCs. Based on this result, we investigated what cytokines were associated with SD at week 8. Analysis of the cytokine levels showed that TNFα was positively associated with SD at week 8. TNFα is a well-characterized cytokine extensively associated with upregulating the immune response, including DC maturation and T-cell priming, proliferation, and recruitment (60, 61). Human trials have shown that isolated limb perfusion of TNFα can be used to treat locally advanced soft-tissue sarcomas (62). In addition, TNFα has been shown to be critical for antitumor immune responses in mice (60). Our observed positive association between TNFα and efficacy outcomes is consistent with these results.
There were several limitations of this trial. First, this trial was primarily a safety study. The trial was conducted over a limited timeframe, and we collected limited biopsy material. Therefore, we could not investigate the mechanism action by aDCs. Some avenues for future research include looking at clinical activity with respect to PD-L1 and IDO expression; staining for cells that might be recruited by aDCs, such as tumor-infiltrating DCs and Treg cells (CD4+/Foxp3+cells); and performing more extensive chemokine profiling, including CCL5 and CXCL9–11. Second, while the quality of the aDCs was checked prior to patient administration, the aDC release criteria used for the trial may need to be expanded to identify incompletely differentiated aDCs. Third, while there are clear correlations between the quality of the cell product and outcomes, they cannot completely explain variations in clinical outcomes; thus, there are exceptions at the individual patient level. Cancer is a multifactorial disease, and other factors could have a substantial effect on clinical outcomes. However, given the explosive expansion of clinical cell products approved or in the pipeline for regulatory approval, it is clear that cell product quality, in the form of cytokine levels, should be considered as part of trial design and analysis and product distribution. Additional studies in the pipeline include more fully characterizing efficacy parameters, injecting multiple tumors, and biopsying distant tumors. Several of these topics are currently planned in the phase II portion of this trial.
In this study, we showed that aDCs are a safe, feasible treatment option for patients with solid tumors. We also identified specific cytokines that, when secreted by the aDCs, lead to stabilization of disease, resulting in prolonged survival. We showed that (1) T-cell infiltration of the tumor is either induced or enhanced following the therapy; (2) these T cells are functional CTLs based on in situ cytokine production; (3) PD-L1 is induced, indicating an immune-related mechanism of action; and (4) cytokine production (i.e., aDC quality) is correlated with clinical outcome, both in terms of arresting tumor growth (SD) and subsequent survival. Based on these data, it is clear that aDCs are a promising treatment to extend the survival of patients with unresectable, locally advanced, or metastatic solid tumors.
Supplementary Material
Statement of translational relevance.
Dendritic cells initiate adaptive immune responses through the uptake and presentation of antigenic compounds, such as proteins expressed on the surface of tumor cells. In preclinical studies, intratumorally injected activated dendritic cells (aDC; DCVax®-Direct) were superior to immature DCs in clearing tumors from mice. In this phase I clinical trial, we evaluated the safety and efficacy of intratumorally injected aDCs in patients with advanced solid tumors. aDCs were well tolerated. Lymphocyte infiltration increased in 54% of treated patients. aDCs that secreted higher levels of interleukin (IL)-8 and IL-12p40 were significantly associated with overall survival and those that expressed TNFα were associated with disease stabilization. These findings indicate that aDCs injected directly in tumors are safe and, when expressing high levels of certain cytokines, could prolong survival in patients with advanced tumors.
Acknowledgements
We thank the motivated patients and their families seeking to enroll in phase 1 clinical trials. We also thank all of the investigators and their staff, especially Dr. Omar Kayaleh at Orlando Health, Orlando, FL. This study was funded by Northwest Biotherapeutics. The University of Texas MD Anderson Cancer Center is supported in part by a Cancer Center Support Grant (CA016672) from the National Institutes of Health.
Financial Support: This study was funded by Northwest Biotherapeutics. The University of Texas MD Anderson Cancer Center is supported in part by a Cancer Center Support Grant (CA016672) from the National Institutes of Health.
Footnotes
Conflict of interest:
This study was funded by Northwest Biotherapeutics.
References
- 1.Amato RJ. Chemotherapy for renal cell carcinoma. Semin Oncol. 2000;27:177–86. [PubMed] [Google Scholar]
- 2.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003:Cd003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleger A, Perkhofer L, Seufferlein T. Smarter drugs emerging in pancreatic cancer therapy. Ann Oncol. 2014;25:1260–70. [DOI] [PubMed] [Google Scholar]
- 4.Ito A, Kondo S, Tada K, Kitano S. Clinical Development of Immune Checkpoint Inhibitors. Biomed Res Int. 2015;2015:605478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West H Immune checkpoint inhibitors. JAMA Oncology. 2015;1:115-. [DOI] [PubMed] [Google Scholar]
- 6.Melero I, Berman DM, Aznar MA, Korman AJ, Gracia JLP, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–72. [DOI] [PubMed] [Google Scholar]
- 7.Mac Keon S, Ruiz MS, Gazzaniga S, Wainstok R. Dendritic cell-based vaccination in cancer: therapeutic implications emerging from murine models. Front Immunol. 2015;6:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. [DOI] [PubMed] [Google Scholar]
- 9.Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. The Lancet Oncology. 2014;15:e257–e67. [DOI] [PubMed] [Google Scholar]
- 10.Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E, et al. Dendritic Cells as Pharmacological Tools for Cancer Immunotherapy. Pharmacol Rev. 2015;67:731–53. [DOI] [PubMed] [Google Scholar]
- 11.Chiang CL, Balint K, Coukos G, Kandalaft LE. Potential approaches for more successful dendritic cell-based immunotherapy. Expert Opin Biol Ther. 2015;15:569–82. [DOI] [PubMed] [Google Scholar]
- 12.Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–83. [DOI] [PubMed] [Google Scholar]
- 13.de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–100. [PubMed] [Google Scholar]
- 14.Hiasa M, Abe M, Nakano A, Oda A, Amou H, Kido S, et al. GM-CSF and IL-4 induce dendritic cell differentiation and disrupt osteoclastogenesis through M-CSF receptor shedding by up-regulation of TNF-alpha converting enzyme (TACE). Blood. 2009;114:4517–26. [DOI] [PubMed] [Google Scholar]
- 15.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–7. [DOI] [PubMed] [Google Scholar]
- 16.Trinchieri G Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 17.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–46. [DOI] [PubMed] [Google Scholar]
- 18.Candido KA, Shimizu K, McLaughlin JC, Kunkel R, Fuller JA, Redman BG, et al. Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents. Cancer Res. 2001;61:228–36. [PubMed] [Google Scholar]
- 19.Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Res. 2005;65:5958–64. [DOI] [PubMed] [Google Scholar]
- 20.Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Res. 2001;61:7530–5. [PubMed] [Google Scholar]
- 21.Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–94. [PubMed] [Google Scholar]
- 22.Tanaka F, Yamaguchi H, Ohta M, Mashino K, Sonoda H, Sadanaga N, et al. Intratumoral injection of dendritic cells after treatment of anticancer drugs induces tumor-specific antitumor effect in vivo. Int J Cancer. 2002;101:265–9. [DOI] [PubMed] [Google Scholar]
- 23.Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94:825–33. [DOI] [PubMed] [Google Scholar]
- 24.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–75. [PubMed] [Google Scholar]
- 25.Akutsu Y, Matsubara H, Urashima T, Komatsu A, Sakata H, Nishimori T, et al. Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. Int J Oncol. 2007;31:509–15. [PubMed] [Google Scholar]
- 26.Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, et al. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys. 2012;82:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiwara S, Wada H, Miyata H, Kawada J, Kawabata R, Nishikawa H, et al. Clinical trial of the intratumoral administration of labeled DC combined with systemic chemotherapy for esophageal cancer. J Immunother. 2012;35:513–21. [DOI] [PubMed] [Google Scholar]
- 28.Hasumi K, Aoki Y, Wantanabe R, Mann DL. Clinical response of advanced cancer patients to cellular immunotherapy and intensity-modulated radiation therapy. Oncoimmunology. 2013;2:e26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann DL, Celluzzi CM, Hankey KG, Harris KM, Watanabe R, Hasumi K. Combining conventional therapies with intratumoral injection of autologous dendritic cells and activated T cells to treat patients with advanced cancers. Ann N Y Acad Sci. 2009;1174:41–50. [DOI] [PubMed] [Google Scholar]
- 30.Bosch M, Trimble L, Tjoa B. IL-4 is not required for the generation and function of dendritic cells from non-activated monocytes. FASEB J; 2003: FEDERATION AMER SOC EXP BIOL 9650 ROCKVILLE PIKE, BETHESDA, MD 20814–3998 USA. p. C125-C. [Google Scholar]
- 31.Udagawa M, Kudo-Saito C, Hasegawa G, Yano K, Yamamoto A, Yaguchi M, et al. Enhancement of immunologic tumor regression by intratumoral administration of dendritic cells in combination with cryoablative tumor pretreatment and Bacillus Calmette-Guerin cell wall skeleton stimulation. Clin Cancer Res. 2006;12:7465–75. [DOI] [PubMed] [Google Scholar]
- 32.Rozera C, Cappellini GA, D’Agostino G, Santodonato L, Castiello L, Urbani F, et al. Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: results from a phase I trial in advanced melanoma. J Transl Med. 2015;13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEarchern J, Boynton A, Bosch M. Activation of Dendritic Cells Prior to Intratumoral Injection Enhances Tumor Clearance: Abstracts for the 18th Annual Scientific Meeting of the International Society for Biological Therapy of Cancer, Bethesda, Maryland, October 31–November 2, 2003. J Immunother. 2003;26:S1–S4. [Google Scholar]
- 34.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 35.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Autenrieth SE, Grimm S, Rittig SM, Grunebach F, Gouttefangeas C, Buhring H-J. Profiling of primary peripheral blood- and monocyte-derived dendritic cells using monoclonal antibodies from the HLDA10 Workshop in Wollongong, Australia. Clin Trans Immunol. 2015;4:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butterfield LH. Dendritic cells in cancer immunotherapy clinical trials: are we making progress? Front Immunol. 2013;4:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Draube A, Klein-Gonzalez N, Mattheus S, Brillant C, Hellmich M, Engert A, et al. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS One. 2011;6:e18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–75. [DOI] [PubMed] [Google Scholar]
- 40.Aarntzen EH, Srinivas M, Bonetto F, Cruz LJ, Verdijk P, Schreibelt G, et al. Targeting of 111In-labeled dendritic cell human vaccines improved by reducing number of cells. Clin Cancer Res. 2013;19:1525–33. [DOI] [PubMed] [Google Scholar]
- 41.Verdijk P, Aarntzen EH, Punt CJ, de Vries IJ, Figdor CG. Maximizing dendritic cell migration in cancer immunotherapy. Expert Opin Biol Ther. 2008;8:865–74. [DOI] [PubMed] [Google Scholar]
- 42.Celli S, Day M, Muller AJ, Molina-Paris C, Lythe G, Bousso P. How many dendritic cells are required to initiate a T-cell response? Blood. 2012;120:3945–8. [DOI] [PubMed] [Google Scholar]
- 43.Io Medicine. Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 44.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–71. [DOI] [PubMed] [Google Scholar]
- 45.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. [DOI] [PubMed] [Google Scholar]
- 46.Clemente CG, Mihm MC, Jr., Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–10. [DOI] [PubMed] [Google Scholar]
- 47.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–85. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri G Interleukin-12: A Cytokine at the Interface of Inflammation and Immunity In: Dixon FJ, editor. Adv Immunol: Academic Press; 1998. p. 83–243. [DOI] [PubMed] [Google Scholar]
- 49.Alfaro C, Suárez N, Martínez-Forero I, Palazón A, Rouzaut A, Solano S, et al. Carcinoma-Derived Interleukin-8 Disorients Dendritic Cell Migration Without Impairing T-Cell Stimulation. PLoS One. 2011;6:e17922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. [DOI] [PubMed] [Google Scholar]
- 51.de Boer EC, Somogyi L, de Ruiter GJ, de Reijke TM, Kurth KH, Schamhart DH. Role of interleukin-8 in onset of the immune response in intravesical BCG therapy for superficial bladder cancer. Urol Res. 1997;25:31–4. [DOI] [PubMed] [Google Scholar]
- 52.Akiyama Y, Watanabe M, Maruyama K, Ruscetti FW, Wiltrout RH, Yamaguchi K. Enhancement of antitumor immunity against B16 melanoma tumor using genetically modified dendritic cells to produce cytokines. Gene Ther. 2001;7:2113. [DOI] [PubMed] [Google Scholar]
- 53.Tatsumi T, Takehara T, Yamaguchi S, Sasakawa A, Miyagi T, Jinushi M, et al. Injection of IL-12 gene-transduced dendritic cells into mouse liver tumor lesions activates both innate and acquired immunity. Gene Ther. 2007;14:863–71. [DOI] [PubMed] [Google Scholar]
- 54.Mazzolini G, Alfaro C, Sangro B, Feijoó E, Ruiz J, Benito A, et al. Intratumoral Injection of Dendritic Cells Engineered to Secrete Interleukin-12 by Recombinant Adenovirus in Patients With Metastatic Gastrointestinal Carcinomas. J Clin Oncol. 2005;23:999–1010. [DOI] [PubMed] [Google Scholar]
- 55.Feijoó E, Alfaro C, Mazzolini G, Serra P, Peñuelas I, Arina A, et al. Dendritic cells delivered inside human carcinomas are sequestered by interleukin-8. Int J Cancer. 2005;116:275–81. [DOI] [PubMed] [Google Scholar]
- 56.Melero I, Arina A, Murillo O, Dubrot J, Alfaro C, Pérez-Gracia JL, et al. Immunogenic Cell Death and Cross-Priming Are Reaching the Clinical Immunotherapy Arena. Clin Cancer Res. 2006;12:2385–9. [DOI] [PubMed] [Google Scholar]
- 57.Hoos A Evolution of end points for cancer immunotherapy trials. Ann Oncol. 2012;23:viii47–viii52. [DOI] [PubMed] [Google Scholar]
- 58.Hoos A, Britten CM, Huber C, O’Donnell-Tormey J. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat Biotechnol. 2011;29:867. [DOI] [PubMed] [Google Scholar]
- 59.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med. 2010;363:411–22. [DOI] [PubMed] [Google Scholar]
- 60.Calzascia T, Pellegrini M, Hall H, Sabbagh L, Ono N, Elford AR, et al. TNF-α is critical for antitumor but not antiviral T cell immunity in mice. The Journal of Clinical Investigation. 2007;117:3833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. [DOI] [PubMed] [Google Scholar]
- 62.Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003;4:429–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.