Abstract
Skeletal muscle powers all movement of the vertebrate body and is distributed in multiple regions that have evolved distinct functions. Axial muscles are ancestral muscles essential for support and locomotion of the whole body. The evolution of the head was accompanied by development of cranial muscles essential for eye movement, feeding, vocalization, and facial expression. With the evolution of paired fins and limbs and their associated muscles, vertebrates gained increased locomotor agility, populated the land, and acquired fine motor skills. Finally, unique muscles with specialized functions have evolved in some groups, and the diaphragm which solely evolved in mammals to increase respiratory capacity is one such example. The function of all these muscles requires their integration with the other components of the musculoskeletal system: muscle connective tissue (MCT), tendons, bones as well as nerves and vasculature. MCT is muscle’s closest anatomical and functional partner. Not only is MCT critical in the adult for muscle structure and function, but recently MCT in the embryo has been found to be crucial for muscle development. In this review, we examine the important role of the MCT in axial, head, limb, and diaphragm muscles for regulating normal muscle development, discuss how defects in MCT-muscle interactions during development underlie the etiology of a range of birth defects, and explore how changes in MCT development or communication with muscle may have led to the modification and acquisition of new muscles during vertebrate evolution.
Keywords: Muscle, muscle connective tissue, myogenesis, FAPs, development, birth defects, evolution, axial muscle, limb, head, diaphragm
INTRODUCTION
Skeletal muscle powers many critical vertebrate functions: locomotion, postural support, feeding, respiration, and communication. Accounting for 30–50% of vertebrate body mass (Romer and Parsons, 1986), skeletal muscle is distributed in multiple regions that have evolved distinct functions. A shared feature of chordates are the axial muscles, metameric muscles that stabilize and flex the notochord or vertebrae to enable support and locomotion of the body (Figure 1; reviewed by Wotton et al., 2015). The evolution of the head in vertebrates has been accompanied by the development of muscles that allow for eye movement, feeding, vocalization, and facial expressions (Figure 1). With the evolution of paired fins and limbs and their associated musculature, vertebrates have gained increased locomotor agility, populated the land, and been able to acquire fine motor skills, such as writing. In addition to axial, head, and limb muscles, different groups of vertebrates have evolved novel muscles that allow for specialized functions. Such novel muscles include the syringeal muscles which control song production in birds, facial muscles that allow for suckling in mammals, and the mammalian diaphragm that is essential for respiration and separating the thoracic and abdominal cavities. Development of all these different muscle groups has been crucial for the evolutionary success of vertebrates.
Figure 1. During the course of chordate and vertebrate evolution, axial and limb muscles become increasingly complex.
A-B. Simple axial muscles in Amphioxus (A) and tail of ascidian free-swimming larva (B) (based on Walker and Homberger, 1992). C. Lamprey myomeric axial muscle subdivided into epaxial muscle (red), innervated by dorsal roots, and hypaxial muscle (pink), innervated by ventral roots (based on Fetcho, 1987). D. Myomeric axial muscle of Chondrichythian, Actinopterygian, and Sarcopterygian fishes is subdivided into epaxial and hypaxial muscles innervated by dorsal and ventral roots, respectively, and separated by a horizontal septum (based on Romer and Parsons, 1986). E - F. Epaxial and hypaxial muscles of tetrapods, e.g. amphibians (E) and reptiles (F) are separated by vertebral transverse processes and subdivided into multiple muscles (based on Romer and Parsons, 1986). G. Simple shark ventral pectoral fin muscles extend from pectoral girdle to dermal rays (based on Walker and Homberger, 1992). H. Multiple ventral fin muscles of ray-finned perch extend pectoral girdle to bony rays (based on Winterbottom, 1974). I. Dorsal muscles of lungfish Neoceratodus pelvic fin are subdivided into two proximal-distal groups (after Diogo, Johnston, Molnar, Esteve-Altava 2016). J. Dorsal muscles of salamander Ambystoma subdivided into three proximal-distal groups (after Diogo et al., 2016). K. Dorsal and ventral muscles of grouse Dendragapus subdivided into three proximal-distal groups (after Kardon, 1998).
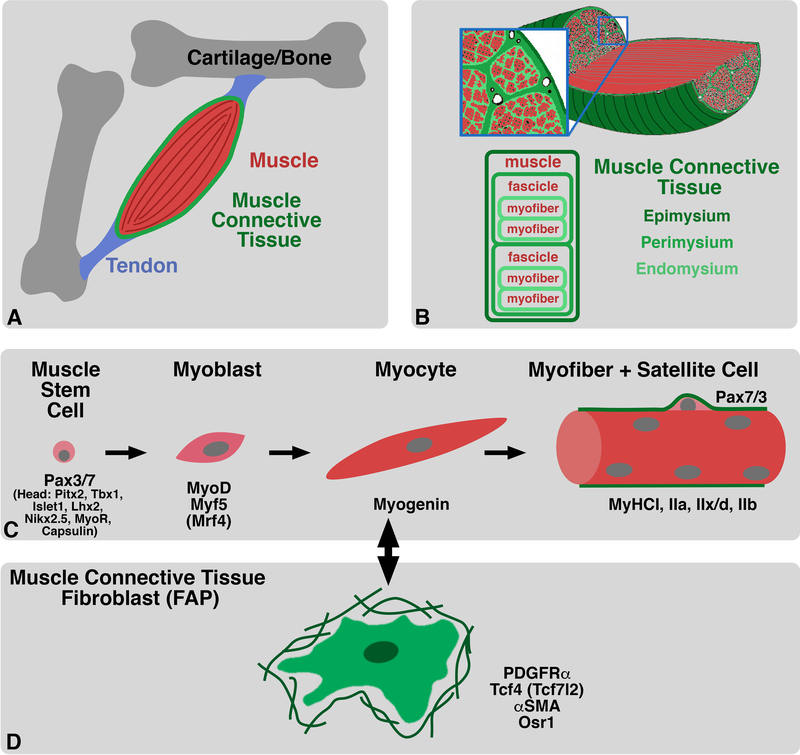
Muscle function requires its integration with the other components of the musculoskeletal system: bones, tendons, and muscle connective tissue as well as nerves and vasculature (Figure 2A). The bones are the rigid part of the musculoskeletal system that support, and in response to muscle contraction, move the body. The tendons are the tensional links between the bones and muscles. The muscle connective tissue (MCT, sometimes called dense irregular connective tissue; Nassari et al., 2017) is the muscle’s closest anatomical and functional partner (Figure 2B). MCT is composed of three layers: the endomysium ensheathes individual myofibers, the perimysium bundles myofibers into fascicles, and the epimysium surrounds anatomical muscles and links to the tendons at the myotendinous junctions (Sanes, 2004). All three MCT layers are composed primarily of extracellular matrix, largely collagens and proteoglycans, as well as the MCT fibroblasts that produce this matrix (Andrade and Brandan, 1991; Lipton, 1977; Sanes, 2004; Zou et al., 2008). The MCT is essential for maintaining muscle’s structural integrity as well as transmitting muscle’s contractile force to tendon and bone. In addition to MCT’s important structural and functional roles in the adult, recently MCT has been found to have critical roles in regulating muscle development.
Figure 2. Muscle and muscle connective tissue (MCT) structure, molecular markers, and development.
A – B. MCT surrounds myofibers, fascicles, and whole muscles to transmit contractile force of muscle to tendon and bone. C. During myogenesis muscle progenitors/stem cells (which express Pax3/7 in axial and limb muscles and variety of transcription factors in the head) become committed MyoD/Myf5+ myoblasts, differentiate into Myogenin+ myocytes, which fuse into post-mitotic, multinucleate myofibers. D. MCT fibroblasts (also known as FAPs in the adult) express Pdgfra, Tcf7l22, and Osr1 and secrete ECM.
Muscle development occurs via two interrelated processes, myogenesis and muscle morphogenesis. Myogenesis is the cellular process by which muscle progenitors differentiate into multinucleate myofibers (Figure 2C). The first step in myogenesis is the establishment of muscle progenitors. Axial and limb muscle progenitors originate from the somites, transient epithelial blocks of paraxial mesoderm located bilateral to the neural tube, while head muscle progenitors largely come from cranial pre-chordal and pharyngeal mesoderm, lateral to the developing brain (Bryson-Richardson and Currie, 2008; Buckingham, 2006; Michailovici et al., 2015; Murphy and Kardon, 2011; Wotton et al., 2015). Reflecting their different embryonic origins, the axial and limb muscle progenitors express the transcription factors Pax3 and Pax7, while the head progenitors express a heterogeneous array of transcription factors, including Pitx2, Tbx1, Islet1, Lhx2, Nkx2.5, MyoR and Capsulin (Michailovici et al., 2015; Murphy and Kardon, 2011; Relaix et al., 2005; Sambasivan et al., 2011; Tzahor, 2009). Subsequently, in all regions of the body muscle progenitors become committed myoblasts that express the myogenic regulatory transcription factors MyoD, Myf5 and/or Mrf4. Finally, myoblasts terminally differentiate into committed post-mitotic myocytes that express Myogenin, synthesize muscle-specific proteins (e.g. myosin heavy chain), fuse into multinucleate myofibers (Abmayr and Pavlath, 2012; Sampath et al., 2018), and mature and diversify into myofibers that differ in their contractile and metabolic properties (Schiaffino et al., 2007). Concurrent with myogenesis is muscle morphogenesis, the process by which individual myofibers are assembled into defined, unique muscles (e.g. biceps) and these muscles are arranged into a stereotyped spatial array. This process involves the migration of muscle progenitors to particular regions, regulation of the number of myoblasts, selective differentiation of muscle progenitors into myofibers, elongation and increase in size of myofibers via selective myoblast fusion, and the integration of myofibers with MCT, tendons, and bones.
Considerably less in known about the development of the MCT (Figure 2D). MCT fibroblasts are the cellular source of the MCT extracellular matrix, and they originate from multiple different embryonic sources. The MCT fibroblasts associated with the axial muscles likely derive from the somites (Nowicki et al., 2003). However, the MCT fibroblasts associated with limb muscles arise from the lateral plate mesoderm, the mesoderm that will form the limb bud and give rise not only to the MCT, but the tendons, ligaments, and bones of the limb (Kardon et al., 2003; Pearse et al., 2007). Finally, the MCT fibroblasts of head muscles originate from a third source, the neural crest cells (Le Lièvre and Le Douarin, 1975; Noden, 1983a; Olsson et al., 2001). Study of MCT fibroblasts had been hampered by the lack of molecular markers for these cells. Recently, several markers of embryonic and adult MCT fibroblasts have been identified, and these include Tcf4 (formally known as Tcf7l2), plate-derived growth factor receptor alpha (PDGRFRα), and alpha smooth muscle actin (αSMA) (Kardon et al., 2003; Mathew et al., 2011; Murphy et al., 2011; Olson and Soriano, 2009; Tomasek et al., 2002). These proteins are expressed in MCT fibroblasts associated with muscles throughout the body. In addition, the transcription factor odd skipped related 1 (Osr1) was recently found to identify a sub-population of embryonic limb MCT fibroblasts (Vallecillo-García et al., 2017). In the embryo Osr1 is expressed in limb MCT fibroblasts, with Osr1 and Tcf4 co-expressed in sub-populations of fibroblasts, and in the adult Osr1 is expressed in activated MCT fibroblasts following muscle injury (Stumm et al., 2018; Vallecillo-García et al., 2017). Interest in MCT fibroblasts in the adult has been accelerated by the finding of fluorescence-activated cell sorting strategies that allow MCT fibroblasts to be isolated from adult muscle and its associated MCT (Joe et al., 2010; Uezumi et al., 2010). As these adult MCT fibroblasts can give rise to both fibroblasts and adipogenic cells, they have been called fibroadipogenic progenitors (FAPs; Joe et al., 2010; Uezumi et al., 2010). Since embryonic Osr1+ cells have been explicitly shown to give rise to FAPs (Vallecillo-García et al., 2017) and most adult MCT fibroblasts express Tcf4 and PDGFRα and activated FAPs express Osr1 (Murphy et al., 2011; Stumm et al., 2018; Uezumi et al., 2010), MCT fibroblasts and FAPs are likely the same cell population. However, molecular heterogeneity and sub-populations within these fibroblasts undoubtedly exist (also see discussions in Nassari et al., 2017; Wosczyna and Rando, 2018).
Development of skeletal muscle and its MCT are tightly linked and recent studies show that MCT fibroblasts are crucial regulators of muscle development throughout the vertebrate body. In this review we discuss and synthesize our current knowledge of the cellular and molecular mechanisms by which the MCT regulates myogenesis and muscle morphogenesis in four major types of vertebrate muscle: axial muscles, head muscles, limb muscles, and the mammalian-specific diaphragm. Defects in MCT development or its communication with neighboring muscle have also emerged as a source of congenital birth defects in head and limb muscles and the diaphragm. We review these studies and explore how studies of normal muscle-MCT development interactions and genetic mouse models of limb and diaphragm defects together enhance and deepen our understanding of normal development and the etiology of birth defects. Finally, we review new studies that suggest that changes in MCT development may have been important developmental innovations that led to the modification or acquisition of new muscles during vertebrate evolution.
AXIAL MUSCLE
Locomotion of chordates and their vertebrate descendants requires the axial muscles. Axial muscles lie in close proximity to the dorsal notochord, vertebrae, and the ventral trunk regions and served as the main propulsive force prior to the evolution of limbs (Romer and Parsons, 1986). The ancestral axial muscle was likely similar to that of the Cephalochordate, Amphioxus, and the swimming larvae of Urochordates, both close relatives of vertebrates (Figure 1A-B). In Amphioxus axial muscle extends from the head to the tip of the tail and consists of a series of V-shaped myomeres, separated by connective tissue myosepta (Holland, 1996). These myomeres function as a series of separate contractile units that laterally flex the notochord and enable undulatory swimming and stabilize the back. With the evolution of jawless vertebrates (lampreys and hagfish), the axial muscle became subdivided into epaxial and hypaxial muscles (Figure 1C; Fetcho, 1987). The epaxial muscles are the intrinsic muscles of the back that are closely associated with the vertebral column and innervated by the dorsal rami of the spinal cord ventral roots, while the hypaxial muscles lie ventral to the vertebrae and are innervated by the ventral rami (Fetcho, 1987). This division of axial muscle into two separately innervated muscles increased the range of motion, allowing for both lateral undulations and dorsal-ventral flexion in the earliest vertebrates (Ahmed et al., 2017). Subsequently in jawed vertebrates, epaxial and hypaxial muscles became further functionally separated, as a horizontal septum of connective tissue developed between these muscles (Figure 1D; reviewed in Wotton et al., 2015). While in sharks, some fishes, and salamanders the epaxial muscle is primarily composed of one muscle (the dorsalis trunci; Figure 1E), in many reptiles and mammals the epaxial muscle is subdivided into three muscles: the transversospinalis, longissimus dorsi, and iliocostalis (Figure 1F; Romer and Parsons, 1986). Mammals further elaborate epaxial muscles into a complex and regionally varied series of muscles that are important for dynamic and static stabilization, spinal extension, and lateral bending (Figure 1F; Goodrich, 1958; Romer and Parsons, 1986; Schilling and Carrier, 2010; Webster et al., 2014). Similarly, the hypaxial muscles (e.g. intercostal and abdominal muscles) became progressively more elaborate and regionally varied during the evolution of tetrapods (Figure 1E-F; Romer and Parsons, 1986). Interestingly, there are both epaxial and hypaxial muscles that lose their segmental arrangement and fuse to form sheets of muscle that are critical for locomotion (Carrier, 1990; Romer and Parsons, 1986).
Epaxial and hypaxial trunk muscles develop from the somites (Figure 3; Bryson-Richardson and Currie, 2008; Buckingham, 2006; Wotton et al., 2015). The somites mature to form a dorsal epithelial layer, the dermomyotome, which gives rise to the overlying dermis and, via multiple waves of migrating muscle progenitors, the underlying primary myotome (Figure 3A; Gros et al., 2004; Hollway and Currie, 2005; Kalcheim et al., 1999; Venters and Ordahl, 2002). The myotome is a metameric series of muscles transiently present in all amniote embryos. Ultimately, the dorsomedial lip of the dermomyotome and the medial myotome give rise to the epaxial muscles, while the ventrolateral lip of the dermomyotome and the lateral myotome give rise to the hypaxial muscles (Figure 3A-C; Cinnamon et al., 1999; Denetclaw et al., 1997; Gros et al., 2004; Pu et al., 2013). On a molecular level, the transcription factor, Engrailed, appears to be essential for establishing the boundary between the epaxial and hypaxial muscle domains (Ahmed et al., 2017; Wotton et al., 2015).
Figure 3. Development of axial muscles in tetrapods.
A. Epaxial muscle derives from the dorsomedial region of the dermomyotome and myotome, while the hypaxial muscle derives from the ventrolateral region of the dermomyotome and myotome. DML, dorsomedial lip and VLL, ventrolateral lip. B-C. Dorsomedial dermomyotome and myotome give rise to epaxial back muscles with MCT presumably derived from the somite (shown as red muscles outlined in grey). Hypaxial dermomyotome and myotome give rise to primaxial hypaxial muscles (e.g. intercostal muscles, pink muscles outlined in grey) with MCT presumably derived from the somite and also abaxial hypaxial muscles (e.g. abdominal muscles, pink muscles outlined in green) with MCT derived from lateral plate mesoderm (green).
The somites are also the embryonic source for the cartilages, bones, and tendons of the axial musculoskeletal system. As the somites mature, they not only form the dorsal epithelial dermomyotome and myotome but a ventromedial sclerotome, a region of mesenchymal cells (Figure 3A; reviewed in Scaal, 2016). The ventral sclerotome gives rise to the vertebral column and also the ribs (Scaal, 2016). In addition, in response to signals from the overlying myotome, the dorsal cranial and caudal edges of the sclerotome form the syndetome, the region that gives rise to the tendons of the axial muscles (Figure 3A; Brent et al., 2005; Brent et al., 2003; Brent and Tabin, 2004). Thus developmental interactions between the muscle cells in the myotome and the cartilage progenitors in the sclerotome lead to the induction of the tendon progenitors that are ultimately needed to connect muscle to bone. Furthermore, the close spatial relationship between the myotome, syndetome, and sclerotome prefigures and ensures the future linkage of muscle, tendon, and bone.
The developmental origin of the MCT of the axial muscles is complex and less well known. Lineage studies using quail-chick chimeras or using the Prx1Cre transgene in mice to follow the fate of lateral plate mesoderm have determined that the MCT of a subset of hypaxial trunk muscles (distal intercostals, pectoralis, and transverse, internal/external oblique, and rectus abdominal muscles) are derived from the lateral plate mesoderm (Figure 3; Burke and Nowicki, 2003; Christ et al., 1983; Durland et al., 2008). This subset of hypaxial muscles with MCT derived from the lateral plate have been termed abaxial muscles (Burke and Nowicki, 2003). The MCT associated with the other hypaxial muscles (e.g. intercostals and longissimus dorsi) as well as the epaxial muscles are assumed to come from the somites and these muscles have been termed primaxial muscles (Burke and Nowicki, 2003). Thus there appears to be two different developmental sources for the MCT of the axial muscles: MCT of lateral plate origin that surrounds the abaxial muscles and MCT of somitic origin that surrounds the primaxial muscles (Nowicki et al., 2003). The boundary between the primaxial and abaxial muscles, with MCT of different developmental origins, has been termed the lateral somitic frontier. However, it should be noted that there is a dearth of formal evidence that the MCT of the primaxial muscles derives from the somites. Transplantation of quail somites into chick has shown that somitic cells can contribute to the MCT of some axial muscles (Saberi et al., 2017). In addition, analysis of a ScxGFP transgene that labels the syndetome and tendon cells also labels MCT fibroblasts in some epaxial muscles (Deries et al., 2010). Together these data suggest that MCT fibroblasts of primaxial muscles may derive from the syndetome. However, a definitive test of the extent and particular location of somitic contribution to the MCT of primaxial muscles will require more extensive and detailed analyses of quail-chick chimeras or Cre-lineage experiments in mice in which particular regions of the somites are targeted.
The morphogenetic processes that transform the myotome and dermomyotome into the diverse array of epaxial and hypaxial muscles are just beginning to be elucidated. Via a detailed analysis of a developmental series of embryos labeled via whole mount immunofluorescence, Deries and colleagues (2010) have determined that the epaxial muscles develop gradually by the translocation, re-orientation, and elongation of the myotomal myocytes followed by cleavage of the myotomal masses. How the hypaxial muscles form has not been as fully detailed. Flank hypaxial muscles (both primaxial and abaxial) arise from epithelial extensions of the dermomyotome and the myotome (Christ et al., 1983; Evans, 2003). The primaxial hypaxial muscles undergo morphogenesis into individual muscles in the context of presumably somite-derived MCT and those muscles in the thoracic region develop in close association with the sclerotome forming the ribs (Figure 3; Burke and Nowicki, 2003; Chevallier, 1975; Durland et al., 2008; Evans, 2003). The abaxial hypaxial muscles derive from the lateral-most regions of the dermomyotome that extend into the lateral plate mesoderm, and their morphogenesis occurs in the context of lateral plate-derived MCT (Figure 3; Burke and Nowicki, 2003; Durland et al., 2008).
Not only does the MCT surround epaxial and hypaxial muscles as they develop, but it is a source of signals regulating muscle development. The morphogenesis of the dermomyotome and myotome into epaxial muscles appears to be tightly correlated with changes in the extracellular matrix (Deries et al., 2012). The dermomyotome and myotome are surrounded by a laminin matrix that is remodeled at distinct stages of myotome development. When the myotome forms the epaxial muscle masses, the laminin matrix disassembles and the fibronectin matrix increases in density around the myocytes. This correlation suggests that the fibronectin matrix may regulate the morphogenesis of the epaxial muscles (Deries et al., 2012). As MCT fibroblasts are intercalated amongst the myogenic cells of the myotome (Deries et al., 2010), this suggests that these fibroblasts may be the source of fibronectin and thus regulate epaxial muscle morphogenesis.
Several studies have provided evidence that the MCT fibroblasts regulate the morphogenesis of abaxial hypaxial muscles. The first evidence that MCT fibroblasts play a role in hypaxial muscle morphogenesis comes from quail-chick chimera studies (Nowicki and Burke, 2000). As with most axial tissues, axial muscle expresses Hox genes appropriate to their anterior-posterior location. Interestingly, the Hox gene expression of transplanted somites and their abaxial muscle derivatives gets re-specified to match the Hox genes of the surrounding lateral plate mesoderm, which will give rise to MCT (Nowicki and Burke, 2000). This suggests that the MCT in the lateral plate mesoderm may be an important regulator, via Hox expression, of abaxial muscle morphogenesis. Subsequently, two other studies have specifically examined the role of the lateral plate mesoderm in regulating morphogenesis of abaxial muscles. Genetic deletion of the transcription factor Pitx2 leads to the almost complete absence of abdominal abaxial muscles (external and internal obliques and transverse and rectus abdominus) (Eng et al., 2012). As Pitx2 is most strongly expressed in the lateral plate mesoderm when the muscle defects appear, the authors suggest that Pitx2 in the lateral plate mesoderm, via its regulation of Hox genes, regulates abdominal muscle morphogenesis. A more direct test of the role of the lateral plate in muscle morphogenesis comes from an analysis of the development of the cutaneous maximus muscle, an abaxial muscle present in many mammals (but not humans) that functions to twitch the skin over the back (Pan et al., 2012). Fat1 is an atypical Fat-like cadherin planar cell polarity molecule that is critical for development of the cutaneous maximus. While Fat1 does have a cell-autonomous role controlling the polarity of muscle progenitor migration (Caruso et al., 2013), it also is essential in the surrounding lateral plate mesoderm for regulating spreading and differentiation of muscle progenitors (Helmbacher, 2018). Deletion of Fat1 in the lateral plate mesoderm, via Prx1Cre, leads to hypoplasia of the ventral cutaneous maximus with disorganized myofibers and a reduction in the number of muscle progenitors (Helmbacher, 2018). Thus this study explicitly demonstrates that the lateral plate mesoderm (presumably the MCT fibroblasts) controls the morphogenesis of an abaxial hypaxial muscle.
In summary, during chordate and vertebrate evolution axial muscle becomes progressively more complex and allows for a greater range of motion. The muscle, tendon, and bone of the axial musculoskeletal system all derive from different regions of the somite and their close spatial relationship allows for their coordinated development. In contrast, the MCT of axial muscle arises from two different developmental sources: the somites and the lateral plate mesoderm. Similar to other regions of the embryo (see sections below), the MCT is likely a key source of patterning information that regulates axial muscle morphogenesis.
CRANIAL MUSCLE
The evolution of the head has been a major innovation in vertebrates that concentrated the sense organs, neural tissues, and structures for feeding, breathing, and communication in the rostral region of the animal. Cranial muscles are essential for many of these functions, including feeding, breathing, talking and facial expressions (Edgeworth, 1935; Haas, 2001; Holliday and Witmer, 2007). Craniofacial muscles can be broadly divided into three groups: 1) extraocular muscles, which move and rotate the eye; 2) muscles developmentally associated with the pharyngeal arches (bilateral swellings on either side of the pharynx) that control the jaw, facial expressions, pharynx, and 3) tongue muscles (Figure 4). Development of these muscles involves the complex interplay of different embryonic tissues, and defects in these interactions are a source of cranial birth defects. Changes in development have also led to major evolutionary innovations in cranial muscles; for example, duplication of jaw muscles to increase the predation capabilities of Tetraodontoid fishes (Friel and Wainwright, 1997), evolution and diversification of facial expression muscles to allow increased modes of feeding and communication (Burrows, 2008); and the expansion of laryngeal muscles in humans to increase vocalization abilities (Diogo et al., 2008). Interactions between the MCT and muscle are critical for morphogenesis of cranial muscle and developmental changes in these interactions may be important for the etiology of birth defects and the evolutionary origin and modification of cranial muscles.
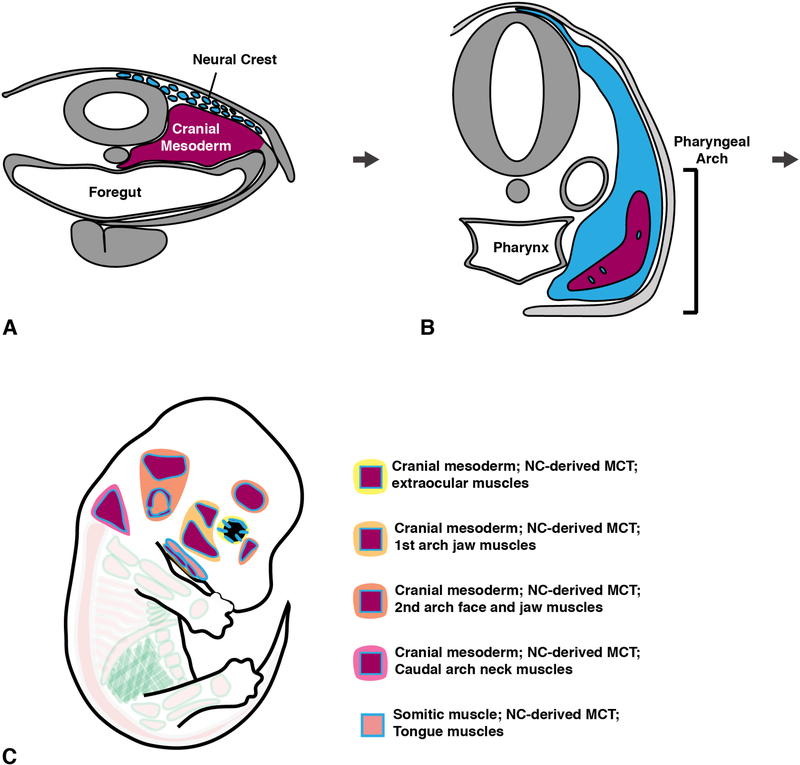
Figure 4. Development of cranial muscle in tetrapods.
(A) Transverse section through early head showing cranial neural crest migration and cranial mesoderm. (B) Transverse section through later developing head showing pharyngeal arch with a cranial mesodermal core surrounded by neural crest that has begun to infiltrate the mesoderm. (C) Groups of cranial muscles with their neural-crest derived MCT.
The head muscles and their MCT derive from several embryonic sources (Figure 4). Most of the head muscles derive from the cranial mesoderm, mesoderm lying on either side of the neural tube and extending rostrally from the prosencephalon and caudally toward the somites. Unlike the paraxial mesoderm that segments into somites in the trunk, the cranial mesoderm is unsegmented, but does contain different regions that give rise to different cranial muscles (reviewed in Michailovici et al., 2015; Ziermann et al., 2018). The most rostral region (also termed the prechordal mesoderm) gives rise to the extraocular muscles. Pharyngeal mesoderm gives rise to the masticatory muscles (associated with pharyngeal arch 1), facial muscles (associated with pharyngeal arch 2), and the pharyngeal and laryngeal muscles (associated with more caudal arches). Interestingly, the pharyngeal cranial mesoderm region does not give rise exclusively to head muscles, but has also been found to give rise to major components of the heart (Lescroart et al., 2015; Lescroart et al., 2010). The tongue muscles derive from anterior somites, from which muscle progenitors migrate to the tongue region (reviewed in Diogo et al., 2015; Ziermann et al., 2018). The MCT comes from a third embryonic tissue, the neural crest. The neural crest is a migratory population derived from the dorsal neural tube and not only give rises to the head’s MCT, but also the tendons, facial bones, many bones of the skull (Couly et al., 1993; Noden, 1983b; Olsson et al., 2001; Piekarski et al., 2014). Unlike the true head muscles, the neck muscles and MCT represent a transitional region in which muscle derives from somites and cranial mesoderm and MCT derives from neural crest and the lateral plate mesoderm (Durland et al., 2008; Heude et al., 2018; Lescroart et al., 2015; Matsuoka et al., 2005; Piekarski and Olsson, 2007; Sefton et al., 2016; Theis et al., 2010).
The development of the head muscles begins with the concurrent movement of muscle progenitors and neural crest cells and with the specification of muscle cells (Figure 4). Muscle progenitors move into locations surrounding the eye, pharyngeal arches, and regions associated with the pharynx, larynx, and tongue. Concurrently, cranial neural crest cells collectively migrate in stereotyped streams to these same regions (Horigome et al., 1999; Landacre, 1921; Noden, 1975). In the pharyngeal region, muscle progenitors form the core of each arch, and neural crest cells circumscribe and infiltrate the muscle progenitors, leading to extensive mixing of neural crest cells and muscle progenitors (Grenier et al., 2009; Hacker and Guthrie, 1998; Noden, 1983a; Trainor et al., 1994). During this time, muscle progenitors begin the process of myogenesis and are specified into myoblasts. Unlike axial or limb muscles, Pax3 and Pax7 are not the initial key regulators of myogenesis, but instead distinct regulatory networks of multiple transcription factors including Pitx2, Tbx1, Islet1, Lhx2, Nkx2.5, MyoR and Capsulin are required, but then converge on the requirement of MyoD and Myf5 for myoblast specification and Myogenin for myocyte differentiation (reviewed by Diogo et al., 2015; Harel et al., 2012; Michailovici et al., 2015; Tzahor, 2009; Ziermann et al., 2018). The initial steps of myogenesis leading up to the specification of MyoD+ myoblasts are independent of signals from the neural crest (Tzahor et al., 2003).
The neural crest plays a key role in regulating the morphogenesis and pattern of cranial muscles (reviewed by Noden and Francis-West, 2006; Noden and Trainor, 2005; Ziermann et al., 2018). Initial evidence for the important role of the neural crest came from embryological transplantation experiments. When grafted in birds or amphibians to different anteroposterior levels, neural crest cells are able to dictate the pattern of muscles that develop (Horstadius, 1946; Noden, 1983b). In contrast, prior to the emigration of cranial neural crest, all cranial mesoderm is equivalent in their potential to form different head muscles (Trainor et al., 1994; von Scheven et al., 2006). Extirpation of neural crest in axolotls reveals that the formation of head muscle with properly organized myofibers and origins and insertions depends on the neural crest (Ericsson et al., 2004; Olsson et al., 2001). Experimental manipulations of chick embryos and analysis of mouse mutants further reveals that while neural crest regulates muscle morphogenesis, it is not essential for the initial steps of myogenesis. In the absence of neural crest, MyoD+ and Myf5+ myoblasts are specified (Rinon et al., 2007; Tzahor et al., 2003). However, neural crest via inhibition of WNT and BMP signaling regulates the proliferation, differentiation, and position of myoblasts and this, in turn, regulates the pattern of muscles formed (Rinon et al., 2007; Tzahor et al., 2003). Thus, initiation of myogenesis is independent of cranial neural crest, but subsequent patterning and proliferation rely on neural crest derived signals.
Analyses of mouse mutants in which genes are deleted in neural crest have explicitly demonstrated that the neural crest regulates muscle morphogenesis in the head and also suggests that the MCT, which derives from the neural crest, may play a key role. First, analysis of the transcription factor Pitx2, which is required for development of extraocular muscles, has provided insights into neural crest function. Germline null mutations in Pitx2 lead to the complete loss of extraocular muscles (Gage et al., 1999; Kitamura et al., 1999; Lu et al., 1999), but the expression of Pitx2 in both the cranial mesoderm and the neural crest (Gage et al., 2005) made it unclear in which tissue Pitx2 was required. Subsequently, tissue-specific deletion of Pitx2 determined that while Pitx2 is required in cranial mesoderm cell-autonomously for survival of muscle primordia and activation of Myf5 and MyoD in extraocular muscles (Zacharias et al., 2011), Pitx2 is also important in the neural crest (Evans and Gage, 2005). Neural crest specific deletion of Pitx2 via Wnt1Cre demonstrated that Pitx2 is required in neural crest to regulate the orientation of extraocular muscles relative to the optic cup. In a second series of experiments, the transcription factors Dlx5/6 were shown to be critical in neural crest cells for development of the masseter muscle. By analyzing mice null for Dlx5/6 or its regulator Endra, Heude and colleagues (2010) determined that Dlx5/6 in non-skeletal neural crest cells is critical for formation of this jaw muscle. Furthermore, the expression of Dx5/6 in neural crest-derived cells closely associated with the masseter suggested that Dlx5/6+ MCT in particular may be critical for masseter morphogenesis. Finally, multiple studies have shown that signals from the neural crest are critical for several aspects of tongue muscle development (reviewed by Cobourne et al., 2018). The first step in tongue development is the migration of muscle progenitors following the migration of neural crest cells to the tongue bud (Han et al., 2012). Conditional deletion experiments have determined that cilia-dependent GLI processing in the neural crest is required for survival and migration of muscle progenitors into the bud (Millington et al., 2017). Once in the tongue bud, conditional mutagenesis experiments have established that reception of TGFβ signaling either through Tgfβr2 or Alk5 in the neural crest is essential for regulating proliferation and differentiation of tongue muscle cells (Han et al., 2014; Hosokawa et al., 2010; Iwata et al., 2013). The disorganized collagen and elastin fibers associated with the aberrant tongue muscle in Tgfβr2 mutants further suggests that Tgfβr2 is acting within the MCT to affect muscle morphogenesis (Iwata et al., 2013). Altogether these experiments demonstrate that the neural crest regulates the proliferation and differentiation of myoblasts and ultimately the pattern of head muscles that develop and suggests that signaling from the MCT may be key for this regulation.
Of babies born with birth defects, approximately one third exhibit craniofacial defects (Gorlin et al., 1990) and usually these defects are attributed to problems in neural crest (Trainor, 2010). Several of these birth defects include aberrant cranial muscles and comparison of these human birth defects with mouse genetic experiments suggest that defects in signaling between neural crest and muscle progenitors may underlie the etiology of these defects. Human patients with hemifacial macrosomia have unilateral underdevelopment of facial structures that develop from pharyngeal arches 1 and 2 (Heude et al., 2011). In addition to underdevelopment of bones, they also exhibit poorly developed masticatory muscles. The finding of patients with masseter muscle defects but normal jaw bones (Heude et al., 2011), coupled with the mouse genetic data on the development of the masseter (Heude et al., 2010), suggests that defects in the MCT associated with the masseter may be the cause of the defects in these patients. Another interesting example of cranial muscle defects is Axenfeld-Rieger syndrome, an autosomal dominant disorder characterized by defects in the eye (Lines et al., 2004; Meyer-Marcotty et al., 2008; Semina et al., 1996), and although extraocular muscles are present, they may be smaller or have anomalous insertions (Bhate and Martin, 2012; Park et al., 2009). PITX2 mutations are frequently associated with this syndrome and based on the mouse genetic analysis of the importance of Pitx2 in the neural crest in patterning extraocular muscle insertions (Evans and Gage, 2005), it is possible that defects in the neural crest are responsible for this phenotype.
The head muscles have undergone substantial changes during vertebrate evolution and developmental modifications in neural crest are likely to be important drivers of these morphological changes. Comparative studies and interspecific transplantation experiments have demonstrated that species-specific muscle patterning information resides within the neural crest. Studies in amphibians provided the first evidence that the neural crest were the source of species-specific information in the head musculoskeleton; neural crest transplants from a toad into a newt resulted in a donor-specific cartilage as well as the induction of a host-derived, but toad-specific muscle (Wagner, 1959). Subsequent data largely comes from the study of bird development. Quails and ducks have distinctly different jaw skeletal and muscle morphologies, associated with their different feeding habits (Tokita and Schneider, 2009). Transplantation of quail cranial neural crest into a duck host resulted in the production of quail-derived skeleton and MCT that transformed duck-derived muscle to acquire a quail-like shape and attachment sites (Tokita and Schneider, 2009). This establishes that, similar to amphibians, species-specific patterning information resides within the neural crest. Careful analysis of this transformation process, revealed that the expression levels and location of the MCT marker, Tcf4, and tendon marker, Scx, reflected the donor neural-crest program. Furthermore, analysis of cranial myogenesis and muscle morphogenesis showed that the neither muscle specification nor differentiation were changed by the presence of quail-derived neural crest, but the shape and attachment sites of muscle were transformed. This suggests that the neural-crest derived MCT and tendons determine the species-specific shape and attachment of head muscles. Subsequently, developmental studies of parrots, which have acquired two anatomically unique jaw muscles to allow for feeding on hard-shelled nuts and seeds, suggest that changes in the neural-crest derived MCT and tendon may also lead to the evolution of entirely new muscles (Tokita et al., 2013). Altogether these studies strongly implicate an important role for the neural crest in determining the species-specific pattern of cranial muscles and suggest that changes in neural crest development may be the underlying driver of the evolutionary modification and generation of new cranial muscles.
LIMB MUSCLE
The origin and diversification of paired appendages have played critical roles in vertebrate evolution (Figure 1G-K). Muscle is a crucial component of fins and limbs, as the number, arrangement, and anatomy of muscles has been modified to enable activities such as swimming, running, flying, and manipulating tools. Tetrapod limbs contain over 40 distinct muscles and each muscle extends over one or two joints, attaching to bone via tendons that originate proximally and insert distally (Figure 1J-K). Muscles are organized into three proximaldistal groups associated with the upper arm/thigh, forearm/shank, and hand/foot, and each of these groups has a dorsal subdivision that extends or ventral subdivision that flexes the limb (Figure 5). Compared with other regions of the body, the morphogenesis of limb muscles has been the most intensively studied because of the accessibility of the limb to surgical manipulation in chick embryos, identification of genetic reagents in mice to manipulate components of the limb musculoskeleton, and the nonessential requirement of the limb for viability. These studies have revealed that an assemblage of transcription factors expressed in the MCT is critical for limb muscle morphogenesis. In addition, recent evidence suggests that mutations in these transcription factors are a cause of congenital limb abnormalities that include muscle defects and changes in the expression of these factors can lead to the evolution of novel modifications of limb muscles.
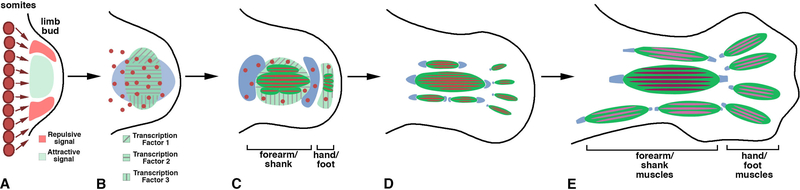
Figure 5. Development of limb muscles in tetrapods.
A. Muscle progenitors (red) migrate from limb-level somites into the core of the limb, which contains attractive HGF and SDF signals, and avoids peripheral regions, which contains repulsive EphrinA5 signals. B. Muscle progenitors populate MCT (green) regions that express an array of transcription factors (Tbx3–5, Tcf4, Osr1, Hoxa11/d11) and tendon (blue) regions. C. Muscle progenitors that migrate into tendon regions do not differentiate, while those that migrate into MCT regions do differentiate. D-E. In MCT regions muscle progenitors differentiate, and so the MCT pattern pre-figures the future pattern of anatomical muscles. Tendons develop at the origin and insertion ends of forming muscles and their associated MCT.
The limb’s muscle and MCT originate from two embryonic sources that are each accessible to surgical manipulation in chick and Cre-mediated manipulation in mouse (Figure 5). Limb muscle derives from the ventrolateral dermomyotome of somites lying adjacent to the developing limb buds (Chevallier et al., 1977; Christ et al., 1977; Ordahl and Le Douarin, 1992). Quail-chick transplantations and chick viral lineage studies have provided a detailed map of which somites contribute to particular muscles in the fore and hind limbs (Beresford, 1983; Kardon et al., 2002; Lance-Jones, 1988). Quail-chick studies have also determined that the lateral plate mesoderm proliferates to form the limb bud and the MCT, tendons, ligaments, and bones of the limb (Chevallier et al., 1977; Christ et al., 1977). In addition, more recent viral lineage studies have determined that the limb mesoderm gets progressively specified into the various cells types, with cartilage and perichondral cells coming from a common lineage and MCT and tendon deriving from another lineage (Pearse et al., 2007). The different embryonic sources of muscle and MCT have allowed each of these tissues to be individually genetically manipulated. Pax3Cre and Pax7Cre (and tamoxifen-inducible Pax7CreERT2) have been used to lineage trace and manipulate muscle progenitors and their derivatives (Engleka et al., 2005; Keller et al., 2004; Lepper et al., 2011; Murphy et al., 2011); Pax3Cre recombines in the earliest muscle progenitors, but also recombines in somitically-derived endothelial cells that populate the limb, while Pax7Cre and Pax7CreERT2 recombine slightly later, but exclusively in muscle progenitors (Hutcheson et al., 2009). The Prx1Cre transgene (and tamoxifen-inducible Prx1CreERT2) recombines in the lateral plate-derived mesoderm of the limb and so has been a useful reagent to manipulate the limb’s MCT, tendons, and bones (Hasson et al., 2010; Logan et al., 2002).
The first step in the development of limb muscles is the emigration of muscle progenitors from the somites into the limb, and attractive and repulsive signals from the limb bud mesoderm are critical for this process (Figure 5A-B). Two signaling molecules have been found to act as chemoattractants for muscle progenitors. The ligand Hepatocyte Growth Factor (HGF) is strongly expressed in the limb mesoderm, while the migrating muscle progenitors express the receptor tyrosine kinase MET (Adachi et al., 2018). Genetic loss-of-function experiments in mice and HGF bead experiments in chick demonstrate that HGF/MET signaling is necessary and sufficient to guide muscle progenitors into the limb (Bladt et al., 1995; Brand-Saberi et al., 1996; Dietrich et al., 1999; Heymann et al., 1996). A second chemokine ligand, Stromal Cell-Derived Factor 1 (SDF1 or Cxcl12) expressed in the limb bud mesoderm, and its receptor Cxcr4 expressed in muscle progenitors, also regulate muscle migration (Vasyutina et al., 2005). SDF1 bead experiments in chick and Cxcr4 null mice show that SDF1/Cxcr4 signaling positively regulates the migration and survival of muscle progenitors into the limb. In addition to these positive regulators of migration, Ephrina5/Epha4 signaling negatively regulates limb muscle migration, as the ligand Ephrina5 expressed in the peripheral limb bud mesoderm repulses receptor Epha4-expressing muscle progenitors from populating these regions of the limb (Swartz et al., 2001). Thus a combination of attractive HGF and SDF1 ligands expressed in the central limb and repulsive Ephrina5 ligand in the limb periphery determine where receptor Met+, Cxcr4+, Epha4+ muscle progenitors migrate to and ultimately reside in the limb (Figure 5B). Although not yet explicitly tested, HGF and SDF1 are likely expressed in MCT fibroblasts or their precursors.
After entering the limb, muscle progenitors undergo a complex process of morphogenesis eventually resulting in the complex pattern of limb muscles. A detailed descriptive analysis of this process in developing chick hind limbs reveals that muscle progenitors initially form dorsal and ventral masses that gradually become subdivided into three dorsal and ventral muscle masses associated with the three proximal-distal limb regions and then these six masses subdivide into individual anatomical muscles (Figure 5; Kardon, 1998). Early experiments in the chick suggested that the MCT may be an important regulator of this morphogenetic process. In particular, MCT forms in a muscle pre-pattern even in the absence of muscle, and MCT can organize even non-muscle cells to form muscle-like structures (Grim and Wachtler, 1991; Jacob and Christ, 1980; Lanser and Fallon, 1987). Subsequently, Tcf4 was identified as the first gene expressed in MCT of the limb in a muscle pre-pattern, independent of muscle (Kardon et al., 2003). Furthermore, virally expressed ectopic Tcf4 in chick limb non-muscle mesoderm led to induction of ectopic muscle and disruption of Tcf4 led to muscle mis-patterning (Kardon et al., 2003). These data suggested a model whereby the lateral-plated derived MCT establishes a pre-pattern in the limb that determines where muscle progenitors differentiate and ultimately the number and location of limb muscles (Figure 5C-E).
Elucidation of the important role of MCT in regulating limb muscle morphogenesis has been facilitated by the identification in mouse of transcription factors that are expressed in the MCT and functionally important in establishing the pattern of limb muscles. Six transcription factors are expressed in different limb MCT regions. The T-box transcription factors Tbx5 and Tbx4 are expressed throughout the mesoderm of fore and hind limbs, respectively, including the MCT (Hasson et al., 2007; Naiche and Papaioannou, 2007), while Tbx3 is expressed in the anterior and posterior MCT, posterior bones and a subset of bone eminences (eminences are the protusions on bones to which tendons attach; Colasanto et al., 2016). Hoxa11 is expressed in the MCT and also the tendons and perichondrium of the forearm and shank regions of the fore and hind limbs (Swinehart et al., 2013). The Odd skipped-related 1, Osr1, transcription factor is expressed in multiple MCT regions of the fore and hind limb and also in the synovial joints (Stricker et al., 2006; Vallecillo-García et al., 2017). Finally, analysis in mouse shows that Tcf4 is expressed in MCT throughout the limb (as well as some bones and bone eminences), but Tcf4 lineage studies show that it is most strongly expressed in MCT near the elbow and knee (Colasanto et al., 2016; Mathew et al., 2011 ). Genetic deletion of these genes leads to defects in the muscles in regions where these genes are expressed. Thus genetic deletion of Tbx5 and Tbx4 leads to mis-patterned fore and hind limb muscles, respectively (Hasson et al., 2010); compound mutants of Hoxa11 and Hoxd11 paralogue causes defects in forearm and shank muscles (Swinehart et al., 2013); mutations in Tcf4 lead to muscle truncations near the knee (Mathew et al., 2011); and Osr1 null mice have various muscle defects (Vallecillo-García et al., 2017). The phenotype of Tbx3 mutants is most striking because two specific anterior muscles, the lateral triceps and brachialis, are absent in these mice (Colasanto et al., 2016). In addition to affecting the size and shape of muscles, Tcf4 also affects myofiber maturation and fiber type (Mathew et al., 2011). It is thought that all of these genes primarily function in the MCT, and at least in the case of Tbx3 and Tcf4 it has been demonstrated that these genes do not function cell-autonomously within muscle cells (via Pax3Cre deletion; Colasanto et al., 2016; Mathew et al., 2011). However, it should be noted that none of these transcription factors are exclusively expressed in nor have been specifically deleted just in the MCT and so may function in other tissues (e.g. tendons, bones, or bone eminences) to regulate muscle patterning.
The array of transcription factors expressed in the MCT regulate limb muscle morphogenesis through several mechanisms. Studies of Tbx3, Tbx5, and Osr1 examined when loss of these genes affected muscle development and all found that defects appeared by E12.5, two days before the pattern of limb muscles is established at E14.5 (Colasanto et al., 2016; Hasson et al., 2010; Vallecillo-García et al., 2017). Interestingly, while Osr1 and Tbx5 cell non-autonomously regulate the number and pattern of MyoD+ myoblasts at E12.5, Tbx3 does not regulate the number of myoblasts, but rather their differentiation into myofibers. There are at least three mechanisms by which these transcription factors may act within MCT fibroblasts to ultimately affect muscle morphogenesis. First, as found with Osr1, the transcription factors may regulate MCT fibroblast cell fate, and in the absence of Osr1 lateral plate-derived limb mesodermal cells adopts a more chondrogenic or tendon-like fate (Vallecillo-García et al., 2017). Second, the transcription factors may regulate the number and/or pattern of MCT fibroblasts in particular limb regions, and in Hoxa11/d11 mutants alterations in the pattern of Tcf4+ MCT fibroblasts are present (Swinehart et al., 2013). Finally, the transcription factors may regulate the MCT fibroblasts’ secretion of signaling molecules or extracellular matrix that regulate the development of neighboring muscle cells; in Tbx5 mutants MCT fibroblasts express less and more disorganized N-Cadherin and β-Catenin (Hasson et al., 2010) and OSR1 regulates a large number of secreted signals (e.g. SDF and Bmp4) and extracellular matrix proteins (e.g. Col6; Vallecillo-García et al., 2017).
Analysis of mutations of the transcription factors expressed in the MCT also provides insights into the relationships between the developing MCT and muscle with the developing tendons and bones. Analysis of Hoxa11/d11, Tbx5, and Tbx3 mutants indicates that while muscle and MCT development are de-coupled from bone development, they are tightly linked with tendon and bone eminence development. In particular, in Hoxa11/d11 compound mutants with one wild-type allele of either gene muscle and associated tendons in the forearm and shank are highly aberrant, but the bones appear normal (Swinehart et al., 2013). Careful analysis of the temporal requirements for Tbx5 and Tbx4 indicate that while these genes are required early (prior to E10.5–11.5) to regulate hind and fore limb bones, at later developmental times these genes coordinately regulate muscle and tendon morphogenesis without affecting bones (Hasson et al., 2010). However, a similar study of the temporal requirements of Tbx3 indicates that while Tbx3 is required early (prior to E9.25) for ulna and posterior digit development, the degree of development of the lateral triceps and brachialis muscles is tightly linked with the degree of development of their bone eminence attachment sites (Colasanto et al., 2016). Together these data, as well as lineage studies in the limb (Pearse et al., 2007), suggest that the long bones of the limb are specified and patterned early, while the MCT, tendons, and bone eminences are specified and undergo coordinated morphogenesis later in limb development.
In summary, an array of transcription factors are expressed in the lateral-plate derived mesoderm in different regions that are critical for patterning the musculoskeletal system. Some of these factors (e.g. Tbx3, 4, and 5) are expressed and required early in the limb (prior to E11.5 in mouse) for proper development of the long bones. All of the factors are expressed in the MCT as well as other lateral plate derivatives, such as the tendons and bone eminences. The expression of these transcription factors in the MCT is critical for regulating the morphogenesis of neighboring muscles and the expression of single or overlapping sets of transcription factors may function as a molecular address specifying particular muscles. The specification of two specific muscles by Tbx3 supports such a hypothesis (Colasanto et al., 2016). Mechanistically, these transcription factors act either by regulating the specification of MCT fibroblasts, the number and pattern of these fibroblasts, and/or the signaling or extracellular matrix molecules secreted by these fibroblasts. The MCT fibroblasts, in turn, determine the pattern of muscles that forms via their regulation of the number, survival, and/or differentiation of muscle progenitors. Finally, the coordinate morphogenesis of MCT, with muscle, tendon, and bone eminences ensures that a functional musculoskeleton develops.
The MCT is not only important for regulating the normal development of limb muscles, but defects in MCT likely underlie the abnormal muscles found in congenital limb abnormalities. Mutations in TBX3 and TBX5 have been identified as the cause of two congenital limb syndromes, Ulnar-Mammary syndrome and Holt-Oram syndrome (Bamshad et al., 1997; Basson et al., 1997; Li et al., 1997). Individuals with Ulnar-Mammary syndrome exhibit a variety of limb phenotypes, ranging from the absence of the forearm and hand, to posterior bone defects, including loss of the ulna and/or posterior digits (Bamshad et al., 1999; Bamshad et al., 1997). Individuals with Holt-Oram syndrome are characterized by deformities in the forelimb, ranging from malformations of carpal bones, thumb abnormalities or phocomelia (Basson et al., 1997). In addition to the obvious bone defects, muscle defects are also associated with both syndromes. Individuals with Holt-Oram syndrome have been found to have hypoplastic pectoralis major, deltoid, and shoulder muscles (Newbury-Ecob et al., 1996), and recently at least one individual with Ulnar-Mammary syndrome has completely lost the lateral triceps in one limb (Colasanto et al., 2016). Based on the genetic data from mouse mutants (Colasanto et al., 2016; Hasson et al., 2010), it is likely that loss of either Tbx5 or Tbx3 expression in the MCT is the cause of these muscle defects.
During vertebrate evolution fins and limbs have been subject to many evolutionary modifications, including changes in their musculature. One example of limb evolution is the striking variation in the feathering of feet in domestic pigeons, with some birds exhibiting many large feathers (Domyan et al., 2016). Intriguingly, the presence of feathered feet is also accompanied by changes in the leg musculature (Domyan et al., 2016). Underlying these morphological changes are genetic changes such that the presence of feathered feet and changed musculature results from a partial transformation of the hindlimb to a forelimb mediated by the decreased expression of the normally hindlimb-specific transcription factor Pitx1 and the increased expression of the normally forelimb-specific Tbx5. Based on the data in mouse supporting a role of Tbx5 in MCT regulating forelimb muscle morphogenesis, it is likely that the increased expression of Tbx5 in the MCT during development of feathered footed pigeons is the cause the morphological changes in their limb muscles. Potentially other changes in the genetic regulation of developing MCT underlie evolutionary changes in limb musculature.
DIAPHRAGM
The diaphragm is a unique mammalian muscle that has been crucial for the evolutionary success of mammals. It is a domed muscle lying at the base of the thoracic cavity that is essential for the inspiratory phase of respiration and for separating the abdominal contents from the overlying thoracic cavity containing the heart and lungs (Merrell and Kardon, 2013). In humans, the common birth defect, Congenital Diaphragmatic Hernia (CDH), highlights the diaphragm’s critical function. In CDH, the diaphragm fails to form properly and weaknesses in the diaphragm allow abdominal contents to herniate into the thoracic cavity and impede lung development. The resulting lung hypoplasia leads to 50% mortality and high morbidity and thus demonstrates the importance of proper diaphragm development (Kardon et al., 2017; Merrell and Kardon, 2013). The diaphragm is not only critical for humans, but also for all mammals. The evolution of mammals involved the appearance of tidally ventilated, alveolar lungs and increased aerobic requirements (Farmer, 2015; Perry et al., 2010). It is thought that the evolution of the diaphragm in mammals was essential for obtaining maximal lung function, as the diaphragm both opens the thoracic cavity during inspiration (allowing for influx of air) and keeps the abdominal contents caudal. Thus understanding the development of the diaphragm has important implications for human health and the overall evolutionary success of mammals. Strikingly, recent studies show that the MCT is critical for the development of the diaphragm, an important cellular source of CDH, and may play a key role in the evolution of the diaphragm.
During development the diaphragm’s muscle and MCT originate from two different embryonic sources. Similar to axial and limb muscles, the diaphragm’s muscle arises from the somites and recently was shown to specifically come from cervical-level somites (Sefton et al., 2018). The diaphragm’s MCT comes from the pleuroperitoneal folds (PPFs), a lateral plate-derived pyramidal tissue located between the thoracic (pleural) and abdominal (peritoneal) cavities (Figure 6A). Histological, immunofluorescent, and genetic lineage studies in mice reveal that the PPFs expand dorsally and ventrally to give rise to the diaphragm’s MCT and central tendon (Figure 6; Babiuk et al., 2003; Merrell et al., 2015; Sefton et al., 2018). The muscle progenitors migrate from the cervical somites to the PPFs, and as the PPFs spread the muscle cells are carried throughout the nascent diaphragm and differentiate into the radially oriented myofibers of the costal diaphragm in close association with the MCT (Figure 6; Merrell et al., 2015; Sefton et al., 2018).
Figure 6. Development of the mammalian diaphragm.
A. Muscle progenitors (red) migrate from cervical somites to the pleuroperitoneal folds (PPFs, green). The PPFs are also the target for the axons of the phrenic nerve (orange). B-C. As PPFs spread dorsally and ventrally they carry the muscle progenitors, which differentiate into radially oriented myofibers. Outgrowth of phrenic nerves is also regulated by PPF expansion. D. PPFs give rise to the MCT guiding expansion and differentiation of muscle and ultimately surrounding myofibers of the costal diaphragm.
Multiple studies demonstrate that the PPFs, and likely the PPF-derived MCT, control development of the diaphragm and are the cellular source of CDH. The normal morphogenetic expansion of the PPFs ahead of muscle (and also nerve and vasculature; Merrell et al., 2015; Sefton et al., 2018), as well as their expansion in the absence of muscle (Merrell et al., 2015) suggested that the PPFs regulate the morphogenesis of the diaphragm’s muscle. Further studies conditionally deleting the CDH-associated genes, Gata4, WT1, and β-catenin, in specific embryonic tissues have now definitively shown that mutations in the PPFs (or their associated mesothelium) lead to defective diaphragm development and hernias (Carmona et al., 2016; Merrell et al., 2015; Paris et al., 2015) and so establish that PPFs regulate normal diaphragm development and are a cellular source of CDH. Detailed analysis of Gata4 additionally revealed that the PPFs regulate the proliferation and survival of adjacent muscle progenitors, and because the PPF cells closest to the muscle are PPF-derived MCT fibroblasts, this suggests that these fibroblasts are the critical regulators of the diaphragm’s muscle (Merrell et al., 2015). Thus PPF-derived MCT fibroblasts guide normal diaphragm muscle development and defects in MCT development or MCT-muscle communication lead to CDH. What precisely is the molecular communication between the MCT fibroblasts and the muscle progenitors remains an open question.
How the diaphragm evolved at the origin of mammals during the Permian era is a major unanswered question (Farmer, 2015; Perry et al., 2010), but studies of diaphragm development in mouse suggest that the PPFs may be critical for diaphragm evolution. One way to elucidate the evolutionary origin of the diaphragm is to compare the developmental innovations present in mammals, but absent from birds and reptiles, which do not have a muscularized diaphragm. While cervical-level somites give rise to neck and limb muscles in birds, reptiles, and mammals, it is only in mammals that muscle progenitors migrate to the nascent diaphragm (Hirasawa et al., 2015; Hirasawa and Kuratani, 2013; Sefton et al., 2018). Thus recruitment of muscle progenitors from cervical-level somites to this region is a crucial event uniquely present in mammals. In mammals, the PPFs not only are the target of the migrating muscle progenitors, but they subsequently guide muscle morphogenesis (Babiuk et al., 2003; Hitachi et al., 2009; Merrell et al., 2015; Sefton et al., 2018). This suggests that the PPFs, which give rise to the diaphragm’s MCT, may be the developmental innovation that allowed for the formation of a muscularized diaphragm. Critical to determine will be whether mammals are unique in their possession of PPFs or whether PPFs are present more broadly in amniotes, but only in mammals are the PPFs able to recruit muscle progenitors and guide their morphogenesis.
CONCLUSION
Muscles in different regions of the vertebrate body have evolved distinct anatomical structures and functions, but in all regions muscle connective tissue plays a central role in regulating muscle morphogenesis and determining the pattern of muscles that develop. The muscles in the trunk, head, limb, and diaphragm are composed of similar multinucleate post-mitotic myofibers, but they differ in the contractile and metabolic properties of their myofibers, the size and organization of myofibers, and the overall shape and attachment to tendons and bones. The generation of myofibers by the process of myogenesis is largely similar in axial, cranial, limb, and diaphragm muscles; following a heterogeneous network of genes regulating the development of muscle progenitors, the molecular and cellular processes of myoblast specification, differentiation, and fusion into myofibers are similar in all muscles (Figure 2C). However, it is during the process of muscle morphogenesis that the distinctive pattern and anatomy of different muscles emerges, and MCT plays a crucial role in this developmental process. The MCT of the trunk, head, limbs, and diaphragm arise from different embryonic tissues – somites, neural crest, lateral plate mesoderm, and pleuroperitoneal folds – but nevertheless they function equivalently. In all regions, MCT does not regulate the initial development of muscle progenitors or their specification into myoblasts. Instead, MCT regulates several aspects of muscle morphogenesis. First, in the head, limbs, and diaphragm, MCT guides migration of muscle progenitors to target regions likely, as indicated by limb experiments (Bladt et al., 1995; Brand-Saberi et al., 1996; Dietrich et al., 1999; Heymann et al., 1996; Swartz et al., 2012; Vasyutina et al., 2005), by a combination of attractive (e.g. HGF, SDF), repulsive (e.g. Ephrin/Eph), and potentially permissive signals. Once muscle progenitors populate target regions, the MCT then locally promotes the proliferation, survival, and differentiation of neighboring myoblasts into myofibers. These localized regions of differentiated myofibers form the nucleus of anatomical muscles. Thus an attractive model is that the MCT serves as a mesodermal pre-pattern that determines the sites of myofiber differentiation and ultimately determines the number, location, size, and shape of muscles. Consistent with such a model, development of the MCT in the limb and diaphragm is independent of muscle development (Kardon et al., 2003; Merrell et al., 2015; Vallecillo-García et al., 2017). The data in the limb suggests that spatially complex and overlapping sets of transcription factors expressed in MCT fibroblasts in different regions may function as molecular addresses that specify particular muscles. The specification of two specific muscles by the transcription factor Tbx3 in the forelimb supports such a notion (Colasanto et al., 2016). How such genes expressed in MCT fibroblasts ultimately regulate muscle morphogenesis is unclear. These genes may regulate MCT cell fate (e.g. Osr1; Vallecillo-García et al., 2017), regulate the number and pattern of MCT fibroblasts, and/or the secretion by MCT fibroblasts of signaling molecules or extracellular matrix proteins (e.g. Hasson et al., 2010; Iwata et al., 2013) that regulate myoblast proliferation, survival, and/or differentiation. The experiments on MCT regulation of muscle morphogenesis also shed light on how the different components of the musculoskeletal system are assembled. Experiments in the limb and head (Colasanto et al., 2016; Hasson et al., 2010; Heude et al., 2010) suggest that specification of bones precedes and their development can be de-coupled from the rest of the musculoskeleton, while the development of the muscles, MCT, tendons, and bone eminences is tightly linked. The tight developmental linkage between muscles, MCT, tendons, and their bone attachment sites ensures that a functional musculoskeletal system develops.
While much progress has been made in our understanding of muscle morphogenesis, particularly with reference to limb muscles, many outstanding questions remain. First, the array of transcription factors in the limb suggests that overlapping sets of genes expressed in the MCT in different domains of the vertebrate body may be critical for specifying the pattern of muscles that develop. However, our current understanding of gene expression in MCT fibroblasts and their regulation of muscle patterning does not explain the complex array of axial, head, and limb muscles present. Second, our understanding of how MCT fibroblasts regulate the proliferation, survival, and differentiation of myoblasts is limited. For instance, the very first myofibers to differentiate develop in an orientation that prefigures the position of the future muscle (Kardon, 1998), and so this initial myofiber orientation determines the origin and insertion and therefore the function of the muscle that develops. Yet, we have little understanding of the molecular or cellular processes that determine where myofibers differentiate and their orientation. Finally, the coupled development of muscle, MCT, tendons, and bones is critical for development of a functional musculoskeleton. While signals from the MCT are critical for coupling muscle and MCT development, it is unclear how MCT, tendons, and bones are specified from a common embryonic source (e.g. somites in the trunk, neural crest in the head, or lateral plate in the limb) and then their development subsequently coordinated. In fact, one of the major impediments to studying MCT has been the lack of molecular markers and genetic reagents that are specific for MCT. To date, all MCT markers and reagents label MCT as well as at least one other component of the musculoskeleton, e.g. tendon or bone eminence, and this likely reflects their close lineage relationship. Finding unique molecular markers and creating more specific MCT Cre lines is a challenge for future research. Birth defects in the development of the head, trunk, limbs, and diaphragm commonly arise in humans. Defects in development of the diaphragm muscle have long been recognized since CDH is both common and has major health consequences (Lally, 2016). In contrast, while birth defects in bone development have been readily recognized in the head and limbs, it is only more recently that abnormalities in cranial and limb muscle development have been identified (Colasanto et al., 2016; Heude et al., 2011; Newbury-Ecob et al., 1996). Strikingly, mouse models of CDH and cranial and limb birth defects suggest that MCT fibroblasts are an important cellular source for these muscle defects (Colasanto et al., 2016; Hasson et al., 2010; Heude et al., 2010; Merrell et al., 2015). Thus increasing our understanding of the normal role of MCT in regulating muscle morphogenesis will be essential for elucidating the molecular and cellular processes underlying the etiology of these birth defects.
Finally, the progressive modification of the form and function of different muscles and the acquisition of new muscles have been instrumental to the evolutionary success of vertebrates. How muscles have been modified or new muscles originate during evolution has been an outstanding question. Recent comparative studies in birds suggest that developmental modifications of the MCT have been key to evolutionary changes in their jaw musculature and have allowed birds to exploit new food sources (Tokita et al., 2013; Tokita and Schneider, 2009). Developmental innovations in the MCT may also underlie the evolution of novel muscles, such as the syringeal muscles in songbirds and the diaphragm in mammals. Future studies of the development of their MCT and muscle should provide insights into the mechanisms underlying the evolutionary origin of these important muscles.
In summary, the MCT is essential for regulating morphogenesis of muscles throughout the body; defects in MCT development have emerged as a source of congenital birth defects in head, limb, and diaphragm muscles; and developmental changes in MCT may have been key developmental innovations that enabled the evolution of the vertebrate musculature. Therefore the MCT serves as a critical link for understanding muscle development, birth defects, and evolution.
REFERENCES
- Abmayr SM, Pavlath GK, 2012. Myoblast fusion: lessons from flies and mice. Development 139, 641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Pascual-Anaya J, Hirai T, Higuchi S, Kuratani S, 2018. Development of hypobranchial muscles with special reference to the evolution of the vertebrate neck. Zoological Lett 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MU, Maurya AK, Cheng L, Jorge EC, Schubert FR, Maire P, Basson MA, Ingham PW, Dietrich S, 2017. Engrailed controls epaxial-hypaxial muscle innervation and the establishment of vertebrate three-dimensional mobility. Dev Biol 430, 90–104. [DOI] [PubMed] [Google Scholar]
- Andrade W, Brandan E, 1991. Isolation and characterization of rat skeletal muscle proteoglycan decorin and comparison with the human fibroblast decorin. Comp Biochem Physiol B 100, 565–570. [DOI] [PubMed] [Google Scholar]
- Babiuk RP, Zhang W, Clugston R, Allan DW, Greer JJ, 2003. Embryological origins and development of the rat diaphragm. J Comp Neurol 455, 477–487. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Le T, Watkins WS, Dixon ME, Kramer BE, Roeder AD, Carey JC, Root S, Schinzel A, Van Maldergem L, Gardner RJ, Lin RC, Seidman CE, Seidman JG, Wallerstein R, Moran E, Sutphen R, Campbell CE, Jorde LB, 1999. The spectrum of mutations in TBX3: Genotype/Phenotype relationship in ulnar-mammary syndrome. American journal of human genetics 64, 1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Lin RC, Law DJ, Watkins WS, Krakowiak PA, Moore ME, Franceschini P, Lala R, Holmes LB, Gebuhr TC, Bruneau BG, Schinzel A, Seidman JG, Seidman CE, Jorde LB, 1997. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet 16, 311–315. [DOI] [PubMed] [Google Scholar]
- Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE, 1997. Mutations in human TBX5 cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet 15, 30–35. [DOI] [PubMed] [Google Scholar]
- Beresford B, 1983. Brachial muscles in the chick embryo: the fate of individual somites. J Embryol Exp Morphol 77, 99–116. [PubMed] [Google Scholar]
- Bhate M, Martin FJ, 2012. Unilateral inferior rectus hypoplasia in a child with Axenfeld-Rieger syndrome. J AAPOS 16, 304–306. [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C, 1995. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376, 768–771. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Müller TS, Wilting J, Christ B, Birchmeier C, 1996. Scatter factor/hepatocyte growth factor (SF/HGF) induces emigration of myogenic cells at interlimb level in vivo. Dev Biol 179, 303–308. [DOI] [PubMed] [Google Scholar]
- Brent AE, Braun T, Tabin CJ, 2005. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 132, 515–528. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ, 2003. A somitic compartment of tendon progenitors. Cell 113, 235–248. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ, 2004. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131, 3885–3896. [DOI] [PubMed] [Google Scholar]
- Bryson-Richardson RJ, Currie PD, 2008. The genetics of vertebrate myogenesis. Nature Reviews Genetics 9, 632–646. [DOI] [PubMed] [Google Scholar]
- Buckingham M, 2006. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev 16, 525–532. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nowicki JL, 2003. A new view of patterning domains in the vertebrate mesoderm. Developmental Cell 4, 159–165. [DOI] [PubMed] [Google Scholar]
- Burrows AM, 2008. The facial expression musculature in primates and its evolutionary significance. Bioessays 30, 212–225. [DOI] [PubMed] [Google Scholar]
- Carmona R, Cañete A, Cano E, Ariza L, Muñoz-Chápuli R, 2016. Conditional deletion of WT1 in the septum transversum mesenchyme causes congenital diaphragmatic hernia in mice. eLife 19, e16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier D, 1990. Activity of the hypaxial muscles during walking in the lizard Iguana iguana. J Exp Biol 152, 453–470. [DOI] [PubMed] [Google Scholar]
- Caruso N, Herberth B, Bartoli M, Puppo F, Dumonceaux J, Zimmermann A, Denadai S, Lebossé M, Roche S, Geng L, Magdinier F, Attarian S, Bernard R, Maina F, Levy N, Helmbacher F, 2013. Deregulation of the protocadherin hene FAT1 alters muscle shapes: implications for the pathogenesis of facioscapulohumeral dystrophy. PLoS Genet 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier A, 1975. Rôle du mésoderme somitique dans le développement de la cage thoracique de I’embryon d’oiseau. I. Origine du segment sternal et mécanismes de la différentiation des côtes. J Embryol Exp Morphol 33, 291–311. [PubMed] [Google Scholar]
- Chevallier A, Kieny M, Mauger A, 1977. Limb-somite relationship: origin of the limb musculature. J Embryol Exp Morphol 41, 245–258. [PubMed] [Google Scholar]
- Christ B, Jacob HJ, Jacob M, 1977. Experimental analysis of the origin of the wing musculature in avian embryos. Anat Embryol (Berl) 150, 171–186. [DOI] [PubMed] [Google Scholar]
- Christ B, Jacob M, Jacob HJ, 1983. On the origin and development of the ventrolateral abdominal muscles in the avian dmbryo - an experimental and ultrastructural-study. Anat Embryol 166, 87–101. [DOI] [PubMed] [Google Scholar]
- Cinnamon Y, Kahane N, Kalcheim C, 1999. Characterization of the early development of specific hypaxial muscles from the ventrolateral myotome. Development 126, 4305–4315. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Iseki S, Birjandi AA, Adel Al-Lami H, Thauvin-Robinet C, Xavier GM, Liu KJ, 2018. How to make a tongue: Cellular and molecular regulation of muscle and connective tissue formation during mammalian tongue development. Semin Cell Dev Biol. [DOI] [PubMed] [Google Scholar]
- Colasanto MP, Eyal S, Mohassel P, Bamshad M, Bonnemann CG, Zelzer E, Moon AM, Kardon G, 2016. Development of a subset of forelimb muscles and their attachment sites requires the ulnar-mammary syndrome gene Tbx3. Dis Model Mech 9, 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM, 1993. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117, 409–429. [DOI] [PubMed] [Google Scholar]
- Denetclaw WF, Christ B, Ordahl CP, 1997. Location and growth of epaxial myotome precursor cells. Development 124, 1601–1610. [DOI] [PubMed] [Google Scholar]
- Deries M, Gonçalves AB, Vaz R, Martins GG, Rodrigues G, Thorsteinsdóttir S, 2012. Extracellular matrix remodeling accompanies axial muscle development and morphogenesis in the mouse. Dev Dyn 241, 350–364. [DOI] [PubMed] [Google Scholar]
- Deries M, Schweitzer R, Duxson MJ, 2010. Developmental fate of the mammalian myotome. Dev Dyn 239, 2898–2910. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C, 1999. The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126, 1621–1629. [DOI] [PubMed] [Google Scholar]
- Diogo R, Abdala V, Lonergan N, Wood BA, 2008. From fish to modern humans— comparative anatomy, homologies and evolution of the head and neck musculature. J Anat 213, 391–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R, Johnston P, Molnar JL, Esteve-Altava B, 2016. Characteristic tetrapod musculoskeletal limb phenotype emerged more than 400 MYA in basal lobe-finned fishes. Sci Rep 6, 37592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R, Kelly RG, Christiaen L, Levine M, Ziermann JM, Molnar JL, Noden DM, Tzahor E, 2015. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520, 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Kronenberg Z, Infante CR, Vickrey AI, Stringham SA, Bruders R, Guernsey MW, Park S, Payne J, Beckstead RB, Kardon G, Menke DB, Yandell M, Shapiro MD, 2016. Molecular shifts in limb identity underlie development of feathered feet in two domestic avian species. eLife 5, e12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durland JL, Sferlazzo M, Logan M, Burke AC, 2008. Visualizing the lateral somitic frontier in the Prx1Cre transgenic mouse. J Anat 212, 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgeworth FH, 1935. The Cranial Muscles of Vertebrates. Cambridge University Press, London. [Google Scholar]
- Eng D, Ma H-Y, Xu J, Shih HP, Gross MK, Kiouss C, 2012. Loss of abdominal muscle in Pitx2 mutants associated with altered axial specification of lateral plate mesoderm. PLoS ONE 7, e42228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA, 2005. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol 280, 396–406. [DOI] [PubMed] [Google Scholar]
- Ericsson R, Cerny R, Falck P, Olsson L, 2004. Role of cranial neural crest cells in visceral arch muscle positioning and morphogenesis in the Mexican axolotl, Ambystoma mexicanum. Dev Dyn 231, 237–247. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ, 2005. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet 14, 3347–3359. [DOI] [PubMed] [Google Scholar]
- Evans DJR, 2003. Contribution of somitic cells to the avian ribs. Dev Biol 256, 115–127. [DOI] [PubMed] [Google Scholar]
- Farmer CG, 2015. The Evolution of Unidirectional Pulmonary Airflow. Physiology (Bethesda) 30, 260–272. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, 1987. A review of the organization and evolution of motoneurons innervating the axial musculature of vertebrates. Brain Res 434, 243–280. [DOI] [PubMed] [Google Scholar]
- Friel JP, Wainwright PC, 1997. A model system of structural duplication: homologies of adductor mandibulae muscles in tetraodontiform fishes. Syst Biol 46, 441–463. [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T, 2005. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci 46, 4200–4208. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh HY, Camper SA, 1999. Dosage requirement of Pitx2 for development of multiple organs. Development 126, 4643–4651. [DOI] [PubMed] [Google Scholar]
- Goodrich ES, 1958. Studies on the structure and development of vertebrates. Dover publications, New York. [Google Scholar]
- Gorlin RJ, Cohen MM, Blackburn LD, 1990. Syndromes of the head and neck, 3rd ed. Oxford University Press, New York. [Google Scholar]
- Grenier J, Teillet M-A, Grifone R, Kelly RG, Duprez D, 2009. Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE 4, e4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim M, Wachtler F, 1991. Muscle morphogenesis in the absence of myogenic cells. Anat Embryol (Berl) 183, 67–70. [DOI] [PubMed] [Google Scholar]
- Gros J, Scaal M, Marcelle C, 2004. A two-step mechanism for myotome formation in chick. Dev Cell 6, 875–882. [DOI] [PubMed] [Google Scholar]
- Haas A, 2001. Mandibular arch musculature of anuran tadpoles, with comments on homologies of amphibian jaw muscles. J Morphol 247, 1–33. [DOI] [PubMed] [Google Scholar]
- Hacker A, Guthrie S, 1998. A distinct developmental programme for the cranial paraxial mesoderm in the chick embryo. Development 125, 3461–3472. [DOI] [PubMed] [Google Scholar]
- Han A, Zhao H, Li J, Pelikan R, Chai Y, 2014. ALK5-mediated transforming growth factor β signaling in neural crest cells controls craniofacial muscle development via tissue-tissue interactions. Mol Cell Biol 34, 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Zhao H, Parada C, Hacia JG, Bringas P, Chai Y, 2012. A TGFβ-Smad4-Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development 139, 1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel I, Maezawa Y, Avraham R, Rinon A, Ma H-Y, Cross JW, Leviatan N, Hegesh J, Roy A, Jacob-Hirsch J, Rechavi G, Carvajal J, Tole S, Kioussi C, Quaggin S, Tzahor E, 2012. Pharyngeal mesoderm regulatory network controls cardiac and head muscle morphogenesis. Proc Natl Acad Sci USA 109, 18839–18844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson P, Del Buono J, Logan MPO, 2007. Tbx5 is dispensable for forelimb outgrowth. Development 134, 85–92. [DOI] [PubMed] [Google Scholar]
- Hasson P, DeLaurier A, Bennett M, Grigorieva E, Naiche LA, Papaioannou VE, Mohun TJ, Logan MPO, 2010. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Developmental Cell 18, 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmbacher F, 2018. Tissue-specific activities of the Fat1 cadherin cooperate to control neuromuscular morphogenesis. PLoS Biol 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heude E, Bouhali K, Kurihara Y, Kurihara H, Couly G, Janvier P, Levi G, 2010. Jaw muscularization requires Dlx expression by cranial neural crest cells. Proc Natl Acad Sci U S A 107, 11441–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heude E, Rivals I, Couly G, Levi G, 2011. Masticatory muscle defects in hemifacial microsomia: a new embryological concept. Am J Med Genet A 155A, 1991–1995. [DOI] [PubMed] [Google Scholar]
- Heude E, Tesarova M, Sefton EM, Jullian E, Adachi N, Grimaldi A, Zikmund T, Kaiser J, Kardon G, Kelly R, Tajbakhsh S, 2018. Unique morphogenetic signatures define mammalian neck muscles and associated connective tissues. BioRxiv. [DOI] [PMC free article] [PubMed]
- Heymann S, Koudrova M, Arnold H, Köster M, Braun T, 1996. Regulation and function of SF/HGF during migration of limb muscle precursor cells in chicken. Dev Biol 180, 566–578. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Fujimoto S, Kuratani S, 2015. Expansion of the neck reconstituted the shoulder-diaphragm in amniote evolution. Dev Growth Differ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa T, Kuratani S, 2013. A new scenario of the evolutionary derivation of the mammalian diaphragm from shoulder muscles. J Anat 222, 504–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitachi K, Kondow A, Danno H, Nishimura Y, Okabayashi K, Asashima M, 2009. Molecular analyses of Xenopus laevis Mesp-related genes. Integrative zoology 4, 387–394. [DOI] [PubMed] [Google Scholar]
- Holland LZ, 1996. Muscle development in Amphioxus: morphology, biochemistry, and molecular biology. Israel Journal of Zoology 42 (sup1), S235–246. [Google Scholar]
- Holliday CM, Witmer LM, 2007. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. J Morphol 268, 457–484. [DOI] [PubMed] [Google Scholar]
- Hollway G, Currie P, 2005. Vertebrate myotome development. Birth Defects Res C Embryo Today 75, 172–179. [DOI] [PubMed] [Google Scholar]
- Horigome N, Myojin M, Ueki T, Hirano S, Aizawa S, Kuratani S, 1999. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Developmental Biology 207, 287–308. [DOI] [PubMed] [Google Scholar]
- Horstadius S, Sellman S, 1946. Experimentelle Untersuchungen uber die Determination des knorpeligen Kopfskelettes bei Urodelen. Nov. Act. Reg. Soc. Scient. Ups. Ser. IV 13. [Google Scholar]
- Hosokawa R, Oka K, Yamaza T, Iwata J, Urata M, Xu X, Bringas P, Nonaka K, Chai Y, 2010. TGF-beta mediated FGF10 signaling in cranial neural crest cells controls development of myogenic progenitor cells through tissue-tissue interactions during tongue morphogenesis. Developmental Biology 341, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DA, Zhao J, Merrell A, Haldar M, Kardon G, 2009. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes Dev 23, 997–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J, Suzuki A, Pelikan RC, Ho TV, Chai Y, 2013. Noncanonical transforming growth factor beta (TGFbeta) signaling in cranial neural crest cells causes tongue muscle developmental defects. J Biol Chem 288, 29760–29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob HJ, Christ B, 1980. On the formation of muscle pattern in the chick limb, Teratology of the Limbs. Walter de Gruyter and Co., Berlin, pp. 89–97. [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM, 2010. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim C, Cinnamon Y, Kahane N, 1999. Myotome formation: a multistage process. Cell Tissue Res 296, 161–173. [DOI] [PubMed] [Google Scholar]
- Kardon G, 1998. Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019–4032. [DOI] [PubMed] [Google Scholar]
- Kardon G, Ackerman KG, McCulley DJ, Shen Y, Wynn J, Shang L, Bogenschutz E, Sun X, Chung WK, 2017. Congenital diaphragmatic hernias: from genes to mechanisms to therapies. Disease models & mechanisms 10, 955–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon G, Campbell JK, Tabin CJ, 2002. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev Cell 3, 533–545. [DOI] [PubMed] [Google Scholar]
- Kardon G, Harfe BD, Tabin CJ, 2003. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell 5, 937–944. [DOI] [PubMed] [Google Scholar]
- Keller C, Hansen MS, Coffin CM, Capecchi MR, 2004. Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: implications for alveolar rhabdomyosarcoma cell of origin. Genes Dev 18, 2608–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M, 1999. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 126, 5749–5758. [DOI] [PubMed] [Google Scholar]
- Lally KP, 2016. Congenital diaphragmatic hernia - the past 25 (or so) years. J Pediatr Surg 51, 695–698. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C, 1988. The somitic level of origin of embryonic chick hindlimb muscles. Dev Biol 126. [DOI] [PubMed] [Google Scholar]
- Landacre F, 1921. The fate of the neural crest in the head of the urodeles. Journal of Comparative Neurology 33, 1–43. [Google Scholar]
- Lanser ME, Fallon JF, 1987. Development of wing-bud-derived muscles in normal and wingless chick embryos: a computer-assisted three-dimensional reconstruction study of muscle pattern formation in the absence of skeletal elements. Anat Rec 217, 61–78. [DOI] [PubMed] [Google Scholar]
- Le Lièvre CS, Le Douarin NM, 1975. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 34, 125–154. [PubMed] [Google Scholar]
- Lepper C, Partridge TA, Fan CM, 2011. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescroart F, Hamou W, Francou A, Théveniau-Ruissy M, Kelly RG, Buckingham M, 2015. Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium. Proc Natl Acad Sci USA 112, 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescroart F, Kelly RG, Le Garrec J-F, Nicolas J-F, Meilhac SM, Buckingham M, 2010. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137, 3269–3279. [DOI] [PubMed] [Google Scholar]
- Li QY, Newbury-Ecob RA, Terrett JA, Wilson DI, Curtis AR, Yi CH, Gebuhr T, Bullen PJ, Robson SC, Strachan T, Bonnet D, Lyonnet S, Young ID, Raeburn JA, Buckler AJ, Law DJ, Brook JD, 1997. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet 15, 21–29. [DOI] [PubMed] [Google Scholar]
- Lines MA, Kozlowski K, Kulak SC, Allingham RR, He on E, Ritch R, Levin AV, Shields MB, Damji KF, Newlin A, Walter MA, 2004. Characterization and prevalence of PITX2 microdeletions and mutations in Axenfeld-Rieger malformations. Invest Ophthalmol Vis Sci 45, 828. [DOI] [PubMed] [Google Scholar]
- Lipton BH, 1977. Collagen synthesis by normal and bromodeoxyuridine-modulated cells in myogenic culture. Dev Biol 61, 153–165. [DOI] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ, 2002. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77–80. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF, 1999. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature 401, 276–278. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G, 2011. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138, 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, Mcmahon AP, Koentges G, 2005. Neural crest origins of the neck and shoulder. Nature 436, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell AJ, Ellis BJ, Fox ZD, Lawson JA, Weiss JA, Kardon G, 2015. Muscle connective tissue controls development of the diaphragm and is a source of congenital diaphragmatic hernias. Nat Genet 47, 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell AJ, Kardon G, 2013. Development of the diaphragm -- a skeletal muscle essential for mammalian respiration. FEBS J 280, 4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Marcotty P, Weisschuh N, Dressler P, Hartmann J, Stellzig-Eisenhauer A, 2008. Morphology of the sella turcica in Axenfeld-Rieger syndrome with PITX2 mutation. J Oral Pathol Med 37, 504–510. [DOI] [PubMed] [Google Scholar]
- Michailovici I, Eigler T, Tzahor E, 2015. Craniofacial Muscle Development. Curr Top Dev Biol 115, 3–30. [DOI] [PubMed] [Google Scholar]
- Millington G, Elliott KH, Chang Y-T, Chang C-F, Dlugosz A, Brugmann SA, 2017. Cilia-dependent GLI processing in neural crest cells is required for tongue development. Dev Biol 424, 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Kardon G, 2011. Origin of vertebrate limb muscle: the role of progenitor and myoblast populations. Curr Top Dev Biol 96, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G, 2011. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE, 2007. Tbx4 is not required for hindlimb identity or post-bud hindlimb outgrowth. Development 134, 93–103. [DOI] [PubMed] [Google Scholar]
- Nassari S, Duprez D, Fournier-Thibault C, 2017. Non-myogenic contribution to muscle development and homeostasis: the role of connective tissues. Front Cell Dev Biol 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury-Ecob RA, Leanage R, Raeburn JA, Young ID, 1996. Holt-Oram syndrome: a clinical genetic study. J Med Genet 33, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM, 1975. Analysis of migratory behavior of avian cephalic neural crest cells. Dev Biol 42, 106–130. [DOI] [PubMed] [Google Scholar]
- Noden DM, 1983a. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat 168, 257–276. [DOI] [PubMed] [Google Scholar]
- Noden DM, 1983b. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol 96, 144–165. [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P, 2006. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn 235, 1194–1218. [DOI] [PubMed] [Google Scholar]
- Noden DM, Trainor PA, 2005. Relations and interactions between cranial mesoderm and neural crest populations. J Anat 207, 575–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki J, Burke A, 2000. Hox genes and morphological identity: axial versus lateral patterning in the vertebrate mesoderm. Development 127, 4265–4275. [DOI] [PubMed] [Google Scholar]
- Nowicki JL, Takimoto R, Burke AC, 2003. The lateral somitic frontier: dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mechanisms of development 120, 227–240. [DOI] [PubMed] [Google Scholar]
- Olson LE, Soriano P, 2009. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson L, Falck P, Lopez K, Cobb J, Hanken J, 2001. Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Dev Biol 237, 354–367. [DOI] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM, 1992. Two myogenic lineages within the developing somite. Development 114, 339–353. [DOI] [PubMed] [Google Scholar]
- Pan B, Grünewald B, Nguyen T, Farah M, Polydefkis M, McDonald J, Schramm LP, Toyka KV, Höke A, Griffin JW, 2012. The lateral thoracic nerve and the cutaneous maximus muscle—a novel in vivo model system for nerve degeneration and regeneration studies. Exp Neurol 236, 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris ND, Coles GL, Ackerman KG, 2015. Wt1 and β-catenin cooperatively regulate diaphragm development in the mouse. Dev Biol 407, 40–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Kim HG, Heo H, Park YG, 2009. Anomalous scleral insertion of superior oblique in Axenfeld-Rieger syndrome. Korean J Ophthalmol 23, 62–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RV 2nd, Scherz PJ, Campbell JK, Tabin CJ, 2007. A cellular lineage analysis of the chick limb bud. Dev Biol 310, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SF, Similowski T, Klein W, Codd JR, 2010. The evolutionary origin of the mammalian diaphragm. Respir. Physiol. Neurobiol 171, 1–16. [DOI] [PubMed] [Google Scholar]
- Piekarski N, Gross JB, Hanken J, 2014. Evolutionary innovation and conservation in the embryonic derivation of the vertebrate skull. Nat Commun 5, 5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski N, Olsson L, 2007. Muscular derivatives of the cranialmost somites revealed by long-term fate mapping in the Mexican axolotl (Ambystoma mexicanum). Evol Dev 9, 566–578. [DOI] [PubMed] [Google Scholar]
- Pu Q, Abduelmula A, Masyuk M, Theiss C, Schwandulla D, Hans M, Patel K, Brand-Saberi B, Huang R, 2013. The dermomyotome ventrolateral lip is essential for the hypaxial myotome formation. BMC Dev Biol 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M, 2005. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435, 948–953. [DOI] [PubMed] [Google Scholar]
- Rinon A, Lazar S, Marshall H, Büchmann-Møller S, Neufeld A, Elhanany-Tamir H, Taketo MM, Sommer L, Krumlauf R, Tzahor E, 2007. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development 134, 3065–3075. [DOI] [PubMed] [Google Scholar]
- Romer AS, Parsons TS, 1986. The Vertebrate Body. Saunders College Publishing, Fort Worth. [Google Scholar]
- Saberi M, Pu Q, Valasek P, Norizadeh-Abbariki T, Patel K, Huang R, 2017. The hypaxial origin of the epaxially located rhomboid muscles. Ann Anat 214, 15–20. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Kuratani S, Tajbakhsh S, 2011. An eye on the head: the development and evolution of craniofacial muscles. Development 138, 2401–2415. [DOI] [PubMed] [Google Scholar]
- Sampath SC, Sampath SC, Millay DP, 2018. Myoblast fusion confusion: the resolution begins. Skelet Muscle 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J, 2004. The extracellular matrix, in: Engel A, Franzini-Armstrong C (Eds.), Myology. McGraw-Hill, New York, pp. 471–487. [Google Scholar]
- Scaal M, 2016. Early development of the vertebral column. Semin Cell Dev Biol 49, 83–91. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Sandri M, Murgia M, 2007. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 22, 269–278. [DOI] [PubMed] [Google Scholar]
- Schilling N, Carrier DR, 2010. Function of the epaxial muscles in walking, trotting and galloping dogs: implications for the evolution of epaxial muscle function in tetrapods. J Exp Biol 213, 1490–1502. [DOI] [PubMed] [Google Scholar]
- Sefton EM, Bhullar B, Mohaddes Z, Hanken J, 2016. Evolution of the head-trunk interface in tetrapod vertebrates. eLife 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton EM, Gallardo M, Kardon G, 2018. Developmental origin and morphogenesis of the diaphragm, an essential mammalian muscle. Dev Biol 440, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward W, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC, 1996. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 14, 392–399. [DOI] [PubMed] [Google Scholar]
- Stricker S, Brieske N, Haupt J, Mundlos S, 2006. Comparative expression pattern of Odd-skipped related genes Osr1 and Osr2 in chick embryonic development. Gene Expr Patterns 6, 826–834. [DOI] [PubMed] [Google Scholar]
- Stumm J, Vallecillo-Garcia P, Vom Hofe-Schneider S, Ollitrault D, Schrewe H, Economides AN, Marazzi G, Sassoon DA, Stricker S, 2018. Odd skipped-related 1 (Osr1) identifies muscle-interstitial fibro-adipogenic progenitors (FAPs) activated by acute injury. Stem Cell Res 32, 8–16. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Eberhart J, Pasquale EB, Krull CE, 2001. EphA4/ephrin-A5 interactions in muscle precursor cell migration in the avian forelimb. Development 128, 4669–4680. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Nguyen V, Mccarthy NQ, Eberhart JK, 2012. Hh signaling regulates patterning and morphogenesis of the pharyngeal arch-derived skeleton. Dev Biol 369, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinehart IT, Schlientz AJ, Quintanilla CA, Mortlock DP, Wellik DM, 2013. Hox11 genes are required for regional patterning and integration of muscle, tendon and bone. Development 140, 4574–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis S, Patel K, Valasek P, Otto A, Pu Q, Harel I, Tzahor E, Tajbakhsh S, Christ B, Huang R, 2010. The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development 137, 2961–2971. [DOI] [PubMed] [Google Scholar]
- Tokita M, Nakayama T, Schneider RA, Agata K, 2013. Molecular and cellular changes associated with the evolution of novel jaw muscles in parrots. Proc Biol Sci 280, 20122319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita M, Schneider RA, 2009. Developmental origins of species-specific muscle pattern. Dev Biol 331, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA, 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3, 349–363. [DOI] [PubMed] [Google Scholar]
- Trainor PA, 2010. Craniofacial birth defects: The role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. Am J Med Genet A 152A, 2984–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA, Tan SS, Tam PP, 1994. Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in mouse embryos. Development 120, 2397–2408. [DOI] [PubMed] [Google Scholar]
- Tzahor E, 2009. Heart and craniofacial muscle development: a new developmental theme of distinct myogenic fields. Dev Biol 327, 273–279. [DOI] [PubMed] [Google Scholar]
- Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB, 2003. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17, 3087–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K, 2010. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12, 143–152. [DOI] [PubMed] [Google Scholar]
- Vallecillo-García P, Orgeur M, vom Hofe-Schneider S, Stumm J, Kappert V, Ibrahim DM, Börno ST, Hayashi S, Relaix F, Hildebrandt K, Sengle G, Koch M, Timmermann B, Marazzi G, Sassoon DA, Duprez D, Stricker S, 2017. Odd skipped-related 1 identifies a population of embryonic fibro-adipogenic progenitors regulating myogenesis during limb development. Nature Commun 8, 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasyutina E, Stebler J, Brand-Saberi B, Schulz S, Raz E, Birchmeier C, 2005. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev 19, 2187–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venters SJ, Ordahl CP, 2002. Persistent myogenic capacity of the dermomyotome dorsomedial lip and restriction of myogenic competence. Development 129, 3873–3885. [DOI] [PubMed] [Google Scholar]
- von Scheven G, Alvares L, Mootoosamy R, Dietrich S, 2006. Neural tube derived signals and Fgf8 act antagonistically to specify eye versus mandibular arch muscles. Development 133, 2731–2745. [DOI] [PubMed] [Google Scholar]
- Wagner G, 1959. Untersuchungen an Bombinator-Triton-chimaeren : das skelett larvaler Triton-köpfe mit Bombinator-Mesektoderm. Wilhelm Roux Arch Entwickl Mech Org 151, 136–158. [DOI] [PubMed] [Google Scholar]
- Walker SF, Homberger DG, 1992. Vertebrate Dissection, 8th edition ed. Harcourt Brace College Publishers, Fort Worth. [Google Scholar]
- Webster EL, Hudson PE, Channon SB, 2014. Comparative functional anatomy of the epaxial musculature of dogs (Canis familiaris) bred for sprinting vs. fighting. J Anat 225, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbottom R, 1974. Descriptive Synonymy of Striated Muscles of Teleostei. P Acad Nat Sci Phila 125, 225–317. [Google Scholar]
- Wosczyna MN, Rando TA, 2018. A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration. Dev Cell 46, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotton KR, Schubert FR, Dietrich S, 2015. Hypaxial muscle: controversial classification and controversial data? Results Probl Cell Differ 56, 25–48. [DOI] [PubMed] [Google Scholar]
- Zacharias AL, Lewandoski M, Rudnicki MA, Gage PJ, 2011. Pitx2 is an upstream activator of extraocular myogenesis and survival. Dev Biol 349, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermann JM, Diogo R, Noden DM, 2018. Neural crest and the patterning of vertebrate craniofacial muscles. Genesis, e23097. [DOI] [PubMed] [Google Scholar]
- Zou Y, Zhang R-Z, Sabatelli P, Chu M-L, Bonnemann CG, 2008. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol 67, 144–154. [DOI] [PubMed] [Google Scholar]