Abstract
Objective:
Oral anticoagulation (OAC) prescribed to AF patients for the prevention of cardioembolic complications likely has the added benefit of preventing venous thromboembolism (VTE). We evaluated, among AF patients who are anticoagulated, whether type of OAC was associated with subsequent VTE risk.
Methods:
Non-valvular AF patients prescribed OACs between 2010 and September 2015 were identified via the MarketScan administrative claims databases. OACs included warfarin and direct OACs (DOACs: dabigatran, rivaroxaban and apixaban). Incident VTE was defined by ICD-9-CM codes. Patients were matched on age, sex, CHA2DS2-VASc and high-dimensional propensity scores. The final analysis included 117,912 AF patients.
Results:
1,357 VTE events accrued over a mean follow-up of 484 days. In multivariable-adjusted, propensity score-matched Cox models, relative to new users of warfarin, risk of incident VTE was lower among new users of dabigatran [hazard ratio (95% confidence interval): 0.55 (0.47–0.66)] and apixaban [0.51 (0.39–0.68)], but similar among new users of rivaroxaban [1.01 (0.87–1.19)]. In head-to-head DOAC comparisons, VTE risk was lower among users of dabigatran [0.48 (0.36, 0.64)] and apixaban [0.61 (0.47, 0.78)], versus rivaroxaban. Findings were mostly similar across patient subgroups.
Conclusions:
In this large practice-based population of AF patients prescribed OACs for primary prevention of stroke and systemic embolization, subsequent risk of VTE was lowest among those prescribed apixaban and dabigatran, while risk was similar with prescriptions for warfarin and rivaroxaban. Among AF patients prescribed OACs, lowering risk of VTE may be an additional benefit of apixaban and dabigatran, beyond the reduced bleeding risk observed in randomized clinical trials.
Keywords: anticoagulation, atrial fibrillation, venous thromboembolism, comparative effectiveness
Introduction
Atrial fibrillation (AF) and venous thromboembolism (VTE) are common conditions among older adults.[1] Over their lifetime, approximately 1 in 4 individuals will experience AF,[2] while 1 in 8 will develop VTE.[3] VTE consists of both pulmonary embolism (PE) and deep vein thrombosis (DVT).
Management of non-valvular AF includes rate or rhythm control, as well as oral anticoagulant therapy (OAC) for the prevention of stroke and cardioembolic complications in patients with a CHA2DS2-VASc score ≥2.[4] Historically, OAC choices were limited to vitamin K antagonists such as warfarin in the U.S. In 2010 options for OAC therapy expanded with approval by the U.S. Food and Drug Administration of the first of now several direct oral anticoagulants (DOACs). In randomized clinical trials[5, 6, 7, 8] and large observational comparative effectiveness studies,[9] DOACs (i.e. dabigatran, rivaroxaban, apixaban, edoxaban) have been shown to be as effective as warfarin for the prevention of stroke and cardioembolic complications in non-valvular AF patients.
Although the primary focus of anticoagulation therapy in AF patients is to prevent ischemic stroke and systemic embolism, there are likely additional benefits in regard to preventing VTE. This is especially important since VTE predominantly afflicts the elderly and those with comorbidities, and converging evidence from both pathophysiologic and epidemiologic studies support AF as a potential VTE risk factor.[10, 11, 12] More specifically, AF may lead to PE through right atrial thrombi formation, or to VTE via the procoagulant state which accompanies AF.[13, 14] Among non-valvular AF patients it is likely that anticoagulant therapy lowers risk of VTE, given the proven efficacy of OACs for the secondary prevention of VTE[15, 16] and the primary prevention of VTE after hip or knee arthroplasty.[17] However, the comparative effectiveness of warfarin versus DOACs for VTE risk reduction in AF patients is unknown. Using data from the MarketScan administrative claims database, we tested the hypothesis that, among non-valvular AF patients, DOACs would be more effective than warfarin in preventing VTE. Head-to-head comparisons of individual DOACs were also explored, in order to identify the OAC option which may have the greatest utility for preventing VTE in AF patients.
Methods
Study population
Health claims data obtained from the MarketScan data warehouse (Truven Health Analytics, Inc., Ann Arbor, MI),[18] for the time-period from January 1, 2010 through September 30, 2015, were used to conduct this retrospective cohort study. Specifically, we used data from both the MarketScan Commercial Database, which consists of employer and health plan sourced data, and the Medicare Supplemental database, which includes retirees with Medicare supplemental insurance paid for by employers. Beneficiaries in these databases have detailed inpatient and outpatient medical claims that are linked to outpatient prescription drug claims and person-level enrollment information. The MarketScan databases are de-identified, compliant with the Health Insurance Portability and Accountability Act, and commercially available. As such, the University of Minnesota Institutional Review Board deemed this analysis exempt from review. The present analysis includes individuals aged 45 and older with prevalent non-valvular AF, at least one prescription for oral anticoagulation after their first AF claim, and ≥90 days of continuous enrolment prior to their first oral anticoagulant prescription. AF was defined as having at least one inpatient claim for AF or 2 outpatient claims for AF 7 to 365 days apart [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 427.3, 427.31, and 427.32 in any position]. This definition has a positive predictive value of about 90%, and a sensitivity of approximately 80%.[19] We did not include individuals with ICD-9-CM codes indicating valvular disease, repair or replacement, since DOACs have only received approval for non-valvular AF. For individuals who disenrolled and re-enrolled, only the first enrolment period was considered in the analysis.
The initial sample included 956,884 non-valvular AF patients, aged 18 to 99 years. The analytic sample was 546,214 once we restricted to individuals on OACs between January 1, 2010 and September 30, 2015; 217,962 after requiring ≥3 months of continuous enrolment before the 1st OAC prescription; 212,058 after requiring participants to be aged 45 or older (VTE and AF at younger ages often has atypical etiology and different treatment considerations); and 202,617 after additionally excluding those with VTE prior to or on same date as the first OAC prescription. These 202,617 were eligible for matching.
Anticoagulant use
Prescriptions for warfarin and DOACs were identified from 2010 through September 30, 2015. Validity of warfarin claims in administrative databases is excellent (sensitivity: 94%, positive predictive value (PPV): 99%),[20] and validity for DOACs claims has not yet been established.
The primary analysis focused on ‘new users’ (i.e. individuals without known prior exposure to OACs). Participants were categorized according to the first OAC they were prescribed. In order to mimic the intent-to-treat approach of a randomized clinical trial,[21] participants remained in this initial category for the entire analysis. For secondary analyses we identified ‘switchers’, specifically warfarin users switching to dabigatran, rivaroxaban or apixaban, and initial dabigatran users switching to rivaroxaban. Other switching comparisons were not conducted due to low numbers.
Initial matching
Each DOAC new user was matched with up to 5 warfarin-only new users by age (± 3 years), sex, time since database enrolment (± 90 days) and drug initiation date (± 90 days). Matching was done separately for each individual DOAC versus warfarin, using an automated greedy matching algorithm.[22] The matching process was repeated for each head-to-head DOAC comparison.
For switching analyses, when warfarin-only users were the reference, individuals switching from warfarin to a DOAC were matched with up to 5 warfarin-only users by age (± 3 years), sex, time since database enrolment (± 90 days) and warfarin initiation date (± 90 days). The date the individual switched to the DOAC (the index date) then became the index date for the matched warfarin-only user. Warfarin users must have had ≥90 days of warfarin use before the index date to be considered as a match. A similar process was used for the analysis of dabigatran-only users who switched to rivaroxaban.
VTE ascertainment
Incident VTE cases were identified by having at least 1 inpatient claim for VTE (ICD-9-CM codes 415.1x, 451.1x, 453.2, 453.4x, 453.5x, 453.8, or 453.9 in any position). The validity of ICD-9-CM codes for the identification of VTE has been well established,[23, 24, 25, 26, 27, 28, 29] with a positive predictive value of approximately 85%.
Assessment of pre-specified covariates
Information prior to the OAC initiation date (minimum 90 days) from all data sources in MarketScan (i.e. demographic data, inpatient, outpatient and pharmacy claims) was used to derive pre-specified covariates. Using validated published algorithms,[30, 31] we defined numerous pre-specified comorbidities, prior procedures, and medications (pharmacy prescription fills). These are listed in Table 1, and details of the codes used to derive these covariates are provided in Supplementary Table 1. The CHA2DS2-VASc score[32] was also calculated.
Table 1.
Characteristics* of atrial fibrillation patients by anticoagulant use, MarketScan databases, 2010 to September 30, 2015.
| New Users |
||||
|---|---|---|---|---|
| Warfarin (n=41,592) | Dabigatran (n=28,089) | Rivaroxaban (n=31,119) | Apixaban (n=17,112) | |
| Age, years | 71.9 ± 11.4 | 69.3 ± 11.4 | 69.1 ± 11.4 | 69.9 ± 11.7 |
| Female, % | 39.8 | 36.3 | 39.3 | 40.6 |
| Comorbidities,† % | ||||
| Hypertension | 82.6 | 82.5 | 77.7 | 80.7 |
| Diabetes | 35.0 | 33.0 | 29.6 | 30.8 |
| Myocardial infarction | 12.8 | 10.8 | 8.9 | 9.7 |
| Heart failure | 36.6 | 32.4 | 25.9 | 28.3 |
| Ischemic stroke/TIA | 27.7 | 25.5 | 19.2 | 20.6 |
| Hemorrhagic stroke | 1.5 | 1.1 | 0.80 | 1.03 |
| Peripheral artery disease | 22.3 | 20.7 | 15.3 | 16.2 |
| Dementia | 2.3 | 1.7 | 1.3 | 1.3 |
| Renal Disease | 15.3 | 11.1 | 9.0 | 12.1 |
| Chronic pulmonary disease | 33.4 | 31.4 | 26.4 | 27.3 |
| Liver disease | 6.6 | 7.2 | 5.2 | 5.3 |
| Malignancy | 16.2 | 15.6 | 13.2 | 13.6 |
| Depression | 11.9 | 11.4 | 9.8 | 10.2 |
| Hematological disorders | 16.5 | 13.9 | 10.0 | 10.3 |
| Metastatic cancer | 2.6 | 2.3 | 1.9 | 1.7 |
| Alcohol abuse | 0.65 | 0.64 | 0.52 | 0.44 |
| Gastrointestinal bleed | 10.7 | 12.2 | 7.0 | 6.7 |
| Other bleed | 8.2 | 7.9 | 4.5 | 4.7 |
| CHA2DS2-VASC score | 3.9 ± 2.1 | 3.6 ± 2.1 | 3.3 ± 2.0 | 3.4 ± 2.0 |
| Prior procedures, % | ||||
| Cardiac | 73.9 | 77.7 | 68.0 | 67.7 |
| Vascular | 9.7 | 6.9 | 5.4 | 5.6 |
| Gastrointestinal | 38.9 | 43.7 | 29.9 | 30.1 |
| Neurological | 21.1 | 22.1 | 18.0 | 15.8 |
| Medications, % | ||||
| Digoxin | 15.3 | 16.7 | 8.3 | 8.0 |
| Clopidogrel | 14.4 | 14.4 | 11.2 | 12.4 |
| Antiplatelets | 2.7 | 2.6 | 2.2 | 2.4 |
| Angiotensin-converting enzyme inhibitors | 39.7 | 40.6 | 32.5 | 32.7 |
| Angiotensin receptor blockers | 25.2 | 26.6 | 23.7 | 24.7 |
| Beta-blockers | 69.6 | 72.7 | 60.4 | 64.7 |
| Calcium channel blockers | 43.4 | 44.8 | 36.6 | 38.7 |
| Anti-arrhythmias | 24.4 | 32.1 | 18.1 | 19.2 |
| Statins | 57.3 | 57.4 | 50.8 | 53.3 |
| Diabetes medications | 25.2 | 23.8 | 20.9 | 22.2 |
Values correspond to mean ± standard deviation or percentage.
ICD codes used to define these comorbidities are provided in Supplementary Table 1.
Creation of high-dimensional propensity scores (HDPS) & re-matching
Separate high-dimensional propensity scores[33] were calculated for each one of the main comparisons (individual DOACs vs. warfarin or vs. other DOACs) for both the analyses of new OAC users (6 comparisons) and switchers (4 comparisons). Briefly, the steps to obtain the high dimensional propensity scores are as follows: [33]
Information in MarketScan was categorized into 5 domains: inpatient diagnostic codes, inpatient procedure codes, outpatient diagnostic codes, outpatient procedure codes, and medications. Within each of the 5 domains, we selected the 200 most prevalent conditions. This resulted in 1,000 covariates.
All the covariates in the dimensions listed above were empirically rank ordered based on their potential for controlling confounding (i.e. strength of the covariate-outcome association and prevalence of the covariate).[34] We selected the top 500 covariates based on this ordering.
These 500 covariates, along with the pre-specified covariates mentioned above (also listed in Table 1), were included as covariates in a regression model to calculate the probability of receiving a specific DOAC versus warfarin (or the reference DOAC).
As noted above, anticoagulant users were initially matched by age, sex, enrolment date and anticoagulant initiation date, for the purpose of defining an index date, and to collect covariate information at the time of drug initiation. To make the participant characteristics even more similar at the time of drug initiation, for each comparison we then re-matched patients according to each outcome-specific HDPS. A greedy matching technique with a caliper of 0.25 of a standard-deviation of each HDPS was used to improve exchangeability.[35] Using this caliper approach, participants were matched with up to 3 comparison participants (i.e. users of warfarin, or the reference DOAC).
Estimating magnitudes of effect
Cox proportional hazards regression was used to estimate the association between OACs and risk of incident VTE. Follow-up began at the date of the index drug prescription (i.e. for new user analyses date of first OAC prescription, for switcher analyses date of switching to a different OAC prescription). Person-time accrued until incident VTE, health plan disenrollment, or the end of study follow-up, whichever came first. Cox proportional hazards models were adjusted for age (continuous), sex, CHA2DS2-VASc and HDPS. Separate models also compared OACs by dosage. Multiplicative interactions were evaluated by sex, age (<75 vs. ≥75 years), CHA2DS2-VASc score (<2 vs. ≥2) and time since OAC prescription index date (<90 vs. ≥90 days). We also conducted analyses stratified according to whether the VTE was initially treated in an inpatient or outpatient setting, and evaluated separately the outcomes of PE (with or without DVT) and DVT alone.
Sensitivity analyses were also conducted requiring ≥6 months of continuous enrolment before the 1st OAC prescription (≥3 months was used for the primary analysis). All statistical analyses were performed with SAS v9.3 (SAS Inc, Cary, NC). As the MarketScan data are a commercial data source, the data are not publically available for free; however they can be purchased from Truven Health Analytics.
Results
For the primary ‘new user’ analysis a total of 117,912 AF patients were successfully matched, both initially and also on HDPS. Characteristics of these individuals, according to initial prescribed OAC, are provided in Table 1. There were a total of 41,592 patients prescribed warfarin, 28,089 prescribed dabigatran, 31,119 prescribed rivaroxaban and 17,112 prescribed apixaban. Overall, the AF patients were well-matched across OAC options, though participants prescribed warfarin were slightly older, had modestly higher prevalences of most chronic conditions (e.g. malignancy, heart failure), and higher CHA2DS2-VASc scores. For example, CHA2DS2-VASc scores were 3.9 for patients prescribed warfarin, 3.6 for those prescribed dabigatran, 3.3 for those prescribed rivaroxaban, and 3.4 for those prescribed apixaban.
In our matched sample of 117,912 AF patients, a total of 1,357 incident VTE events occurred over a mean follow-up of 484 ± 404 days [median (IQR): 384 (153, 718)]. Table 2 presents hazard ratios (HRs) and 95% confidence intervals (95% CIs) for incident VTE among new OAC users, by OAC prescription, after adjusting for age, sex, CHA2DS2-VASc score and HDPS. Relative to AF patients prescribed warfarin, risk of incident VTE was lower among those prescribed dabigatran [HR (95% CI): 0.55 (0.47, 0.66)] and apixaban [0.51 (0.39, 0.68)]. There was no difference in VTE risk when comparing rivaroxaban to warfarin [1.01 (0.87, 1.19)]. In head-to-head comparisons of DOACs, as compared to rivaroxaban users, risk of VTE was lower among dabigatran [0.48 (0.36, 0.64)] and apixaban [0. 61 (0.47, 0.78)] users, respectively. Risk of VTE was similar among apixaban and dabigatran users (HRapixaban vs. dabigatran = 1.06 (0.57, 1.95)]. Results were also similar in analyses looking separately at PE and DVT events (Supplemental Table 2).
Table 2.
Adjusted hazard ratios (95% confidence intervals) for incident venous thromboembolism comparing new users of specific oral anticoagulants among patients with non-valvular atrial fibrillation, MarketScan databases, 2010 to September 30, 2015.
| Warfarin | Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|---|
| N | 27,065 | 27,065 | ||
| # VTE events | 413 (1.5%) | 214 (0.79%) | ||
| Person-years | 44,169 | 44,386 | ||
| HR (95% CI) | 1.00 (reference) | 0.55 (0.47–0.66) | ||
| N | 22,581 | 22,581 | ||
| # VTE events | 336 (1.5%) | 332 (1.5%) | ||
| Person-years | 26,086 | 26,532 | ||
| HR (95% CI) | 1.00 (reference) | 1.01 (0.87–1.19) | ||
| N | 11,632 | 11,362 | ||
| # VTE events | 157 (1.4%) | 73 (0.63%) | ||
| Person-years | 9,923 | 9,554 | ||
| HR (95% CI) | 1.00 (reference) | 0.51 (0.39–0.68) | ||
| N | 10,580 | 10,580 | ||
| # VTE events | 76 (0.72%) | 160 (1.5%) | ||
| Person-years | 15,234 | 15,126 | ||
| HR (95% CI) | 0.48 (0.36, 0.64) | 1.0 (reference) | ||
| N | 3100 | 3100 | ||
| # VTE events | 20 (0.65%) | 24 (0.77%) | ||
| Person-years | 2,874 | 3,331 | ||
| HR (95% CI) | 1.0 (reference) | 1.06 (0.57–1.95) | ||
| N | 16,234 | 16,234 | ||
| # VTE events | 175 (1.1%) | 98 (0.60%) | ||
| Person-years | 12,632 | 12,477 | ||
| HR (95% CI) | 1.0 (reference) | 0.61 (0.47–0.78) |
Matched 1:1 on HDPS and adjusted for age, sex, CHA2DS2-VASc score and high-dimensional propensity score.
CI: confidence interval; HDPS: high-dimensional propensity scores; HR: hazard ratios; N: number; VTE: venous thromboembolism.
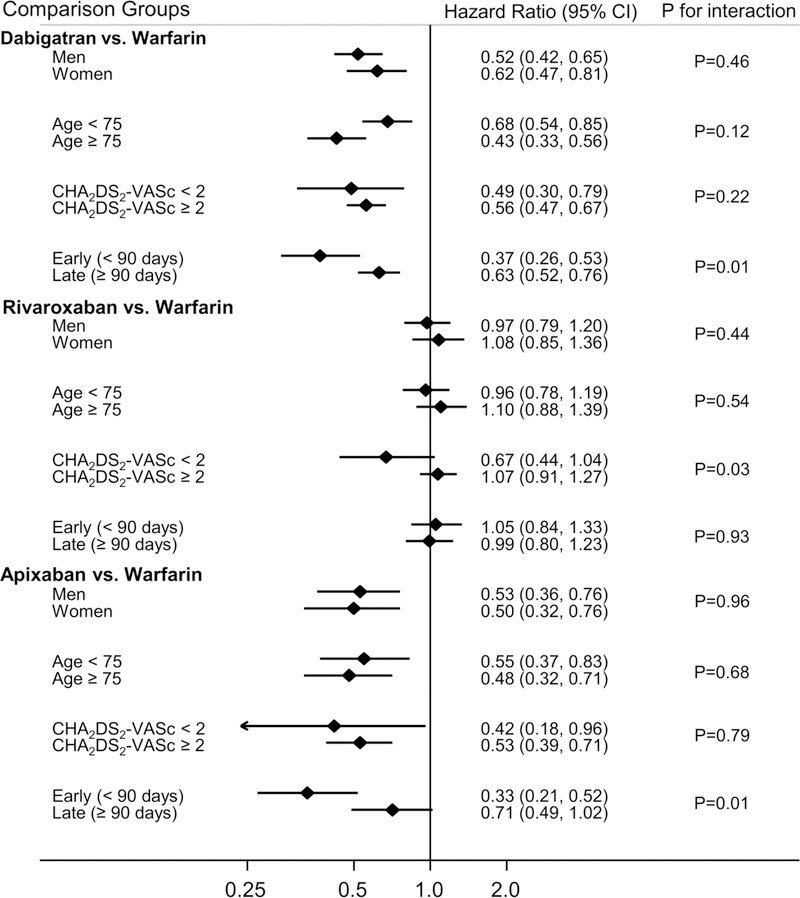
Figure 1 presents findings of pre-defined subgroup analyses, comparing DOACs to warfarin, according to categories of sex, age (<75 vs. ≥ 75 years), CHA2DS2-VASc score <2 vs. ≥2) and dividing follow-up time into early versus late (<90 vs. ≥90 days). The direction of the effect estimate was the same across all subgroups compared; however in some instances the magnitude of the effect was significantly different across subgroups. In comparisons of dabigatran and apixaban vs. warfarin, both of these DOACs appeared more beneficial for preventing VTE in the first 90 days of follow-up vs. follow-up beyond ≥90 days. For rivaroxaban vs. warfarin, there was some evidence that the association was more protective among individuals with a CHA2DS2-VASc <2 Results were also similar when analyses were stratified according to whether the VTE was initially treated in an inpatient or outpatient setting (Supplemental Table 3).
Figure 1.
Risk of incident VTE according to initial OAC therapy with dabigatran, rivaroxaban or apixaban versus warfarin* in patients with non-valvular AF, by patient characteristics, MarketScan databases, 2010 to September 30, 2015.
*Matched on HDPS 1:1
AF: atrial fibrillation; OAC, oral anticoagulant; VTE: venous thromboembolism.
We also explored associations by OAC dose. Of dabigatran users, 90.3% were on the standard 150 mg dosage and 9.7% were on the 75 mg dosage. Among rivaroxaban users 73.4% were on the standard 20 mg dosage, while 7.9% were on the 10 mg dosage and 18.6% on the 15 mg dosage. Of apixaban users, 81.5% were on the standard 5 mg dosage, and 18.5% were on the 2.5 mg dosage. In the analyses stratifying DOACs by initial dosage (Table 3), risk of VTE in AF patients was similar according to dose for both dabigatran vs. warfarin and apixaban vs. warfarin. For rivaroxaban vs. warfarin there were differences; for the standard dose (20 mg) the HR was 0.67 (0.56, 0.82), while for the 15 mg dose it was 2.15 (1.74, 2.66) and for the 10 mg dose 1.33 (0.94, 1.86). In Supplemental Table 4 we explored participant characteristics according to rivaroxaban dose. Those on the 15 mg dose tended to be older, and have more kidney disease and other comorbidities.
Table 3.
Adjusted hazard ratios (95% confidence intervals) for incident venous thromboembolism comparing new users of oral anticoagulants by dose of direct oral anticoagulant to warfarin, among patients with non-valvular AF, MarketScan databases, 2010 to September 30, 2015.
| Warfarin | Dabigatran | |||
|---|---|---|---|---|
| Reduced dose (75 mg) | Standard dose (150 mg) | |||
| N | 27,065 | 2,636 | 24,429 | |
| # VTE events | 413 (1.5%) | 32 (1.2%) | 182 (0.75%) | |
| Person-years | 44,169 | 4,270 | 43,115 | |
| HR (95% CI) | 1.00 (ref) | 0.75 (0.52–1.08) | 0.53 (0.44–0.63) | |
| Warfarin | Rivaroxaban | |||
| Reduced dose (10 mg) | Reduced dose (15 mg) | Standard dose (20 mg) |
||
| N | 22,581 | 1,792 | 4,206 | 16,583 |
| # VTE events | 336 (1.5%) | 37 (2.1%) | 125 (3.0%) | 170 (1.0%) |
| Person-years | 26,086 | 2,265 | 4,456 | 19,810 |
| HR (95% CI) | 1.00 (ref) | 1.33 (0.94–1.86) | 2.15 (1.74–2.66) | 0.67 (0.56–0.82) |
| Warfarin | Apixaban | |||
| Reduced dose (2.5 mg) | Standard dose (5 mg) |
|||
| N | 11,632 | 2,149 | 9,483 | |
| # VTE events | 157 (1.4%) | 13 (0.60%) | 60 (0.63%) | |
| Person-years | 9,923 | 1611 | 7943 | |
| HR (95% CI) | 1.00 (reference) | 0.42 (0.23–0.75) | 0.54 (0.40–0.73) | |
Matched 1:1 on HDPS and adjusted for age, sex, CHA2DS2-VASc score and high-dimensional propensity score.
AF: atrial fibrillation; CI: confidence interval; HDPS: high-dimensional propensity scores; HR: hazard ratios; N: number; VTE: venous thromboembolism.
For the analyses of OAC ‘switchers’ there were 72,851 AF patients successfully matched, both initially and by HDPS. These patients went on to experience 743 VTE events over a mean follow-up of 473 ± 403 days [median (IQR): 368 (152, 685)]. Analyses of AF patients who switched from their initial OAC to a different OAC are shown in Table 4. As compared to patients who remained on warfarin, patients who switched from warfarin to dabigatran and apixaban experienced lower risk of VTE versus staying on warfarin, respectively. There were no others significant differences VTE risk among people who switched OACs. However, as there are fewer switchers the effect estimates are less precise than for the new user analyses.
Table 4.
Adjusted hazard ratios (95% confidence intervals) for incident venous thromboembolism comparing patients with non-valvular AF who switched from their initially prescribed oral anticoagulant to a different oral anticoagulant, MarketScan databases, 2010 to September 30, 2015.
| Warfarin-only | Switchers to Dabigatran | Switchers to Rivaroxaban | Switchers to Apixaban | |
|---|---|---|---|---|
| N | 27,628 | 9,462 | ||
| # VTE events | 355 (1.3%) | 111 (1.2%) | ||
| Person-years | 42,897 | 18,056 | ||
| HR (95% CI) | 1.00 (reference) | 0.80 (0.64–0.99) | ||
| N | 16,993 | 5,773 | ||
| # VTE events | 161 (0.95%) | 65 (1.1%) | ||
| Person-years | 18,571 | 7,047 | ||
| HR (95% CI) | 1.00 (reference) | 1.12 (0.83–1.49) | ||
| N | 7,421 | 2,605 | ||
| # VTE events | 70 (0.94%) | 14 (0.54%) | ||
| Person-years | 5,900 | 2,242 | ||
| HR (95% CI) | 1.00 (reference) | 0.50 (0.28–0.90) | ||
| Dabigatran-only | ||||
| N | 10,722 | 3,850 | ||
| # VTE events | 55 (0.51%) | 31 (0.81%) | ||
| Person-years | 11,904 | 5,520 | ||
| HR (95% CI) | 1.00 (reference) | 1.19 (0.75–1.87) |
Matched 1:3 on HDPS and adjusted for age, sex, CHA2DS2-VASc score, and high-dimensional propensity score.
AF: atrial fibrillation; CI: confidence interval; HDPS: high-dimensional propensity scores; HR: hazard ratios; N: number; VTE: venous thromboembolism.
In sensitivity analyses we required ≥6 months of continuous enrolment before the 1st OAC prescription. Results were similar to those of the primary analysis, which required a ≥3 month “run-in” period (Supplemental Table 5).
Discussion
In this retrospective claims-based analysis of 117,912 AF patients, risk of VTE was lower among patients prescribed dabigatran and apixaban, than among patients prescribed rivaroxaban and warfarin. However, it is important to note that warfarin users were slightly older and had more comorbidities than DOAC users. These patterns were generally consistent across AF patient subgroups, and when we evaluated OAC ‘switchers’. There was some evidence that rivaroxaban performed better among those who had lower CHA2DS2-VASc scores, and that dabigatran and apixaban had the greatest benefit in the initial 90 days after OAC prescription. However, these subgroup analyses were based on smaller groups of AF patients. These findings suggest that within the context of AF, dabigatran and apixaban may be more beneficial than warfarin and rivaroxaban for the prevention of VTE.
A growing body of evidence has linked AF to elevated risk of VTE,[10, 11, 12] and AF patients are commonly prescribed OACs to prevent thromboembolic complications.[4] Yet, to our knowledge this is the first study to compare the effectiveness, in terms of VTE risk, of OACs prescribed for the prevention of stroke in AF. Notably, among VTE patients DOACs are now approved for VTE primary treatment and secondary prevention[36] on the basis of randomized trials demonstrating non-inferiority of DOACs versus warfarin for VTE primary treatment and superiority of DOACs versus placebo for the secondary prevention of recurrent VTE.[37, 38, 39, 40, 41, 42, 43] Findings from the present analysis complement those from the trials of VTE patients, providing additional support for the VTE-protective effects of dabigatran and apixaban. Further, our results are consistent with a protective effect of dabigatran and apixaban vs. warfarin for the prevention of VTE in the context of AF; this is important given the growing appreciation of AF as a VTE risk factor,[10, 11, 12] potentially acting through mechanisms such as right atrial thrombi formation, and the procoagulant state which accompanies AF.[13, 14]
In head-to-head comparisons of DOACs, users of both dabigatran and apixaban had lower rates of VTE than users of rivaroxaban in our study sample. Notably both apixaban and rivaroxaban act by directly inhibiting factor Xa (which acts to cleave prothrombin to thrombin), while dabigatran directly inhibits thrombin.[44] Our analyses of AF patients who switched anticoagulants followed a similar pattern, with dabigatran and apixaban appearing preferential to rivaroxaban and warfarin for preventing VTE. However, effect sizes were smaller than for the new user analyses, and precision was poor for apixaban. It is not clear why rivaroxaban was less effective for preventing VTE in the present analysis. In practice-based head-to-head comparisons of DOACs for the prevention of stroke or thromboembolic events in AF, some studies have suggested poorer performance of rivaroxaban (versus dabigatran)[45] while other studies have shown no difference.[46, 47, 48] From a pharmacokinetic point of view, despite rivaroxaban’s shorter half-life (~5–9 hours in younger healthy individuals; 11–13 hours in elderly patients)[49] it has a once-daily dosing regimen, whereas dabigatran and apixaban have twice daily dosing regimens despite longer half-lives (12–17 hours and 8–15 hours, respectively).[44] As such, poor compliance among rivaroxaban users may be particularly hazardous. In the present analyses, when we evaluated specific doses of rivaroxaban, risk was VTE was substantially higher among AF patients prescribed the 15 mg dose, and comorbidity burden was highest among this group. It is possible that the 15 mg dose is not sufficient for anticoagulation, and that the 20 mg (standard) dose should have been used instead. In an analysis of Optum data, reduced dose rivaroxaban was associated with (nonsignificant) stroke risk compared to warfarin.[50] Interestingly, and similar to our analysis, that pattern was not seen for dabigatran or apixaban. There is a growing body of literature suggesting that inappropriate DOAC dosing is commonplace.[51, 52, 53] Though in the MarketScan data it is not possible to determine appropriateness of rivaroxaban doses prescribed, we speculate that anticoagulation may have been inadequate for some AF patients prescribed the 15 mg dose.
Understanding the comparative effectiveness of DOACs versus warfarin according to patient subgroups is important, since bleeding profiles and the effectiveness of OACs may vary by patient characteristics such as age. A concern frequently raised about results of randomized clinical trials relates to their generalizability to patient subgroups, given that a) the sample size of randomized clinical trials often results in poor precision to detect effects in patient subgroups, and b) the inclusion criteria of randomized clinical trials may result in samples which may not be generalizable to the entire patient population. Given the large number of AF patients in the present analysis, we had capacity to explore associations in patient subgroups. A priori we specified as subgroups of interest sex, categories of age and CHA2DS2-VASc score. We also evaluated associations according to time since initial OAC prescription. Overall, patterns across subgroups of interest and follow-up time were similar to those observed in the full cohort. However, there were a few exceptions, which need to be interpreted cautiously given the smaller sample sizes for subgroup analyses and multiple comparisons. As compared to warfarin, both dabigatran and apixaban were associated with lower VTE risk. However, for both the magnitude of the association varied by time since initiation, with the relative risk of VTE being lower in the initial 90 days of treatment as compared to after longer treatment durations. This is not unexpected since some DOACs (i.e. rivaroxaban and apixaban) are approved for the initial treatment of VTE and therapeutic levels occur within several hours, whereas with warfarin INRs do not stabilize for several weeks.[54] No statistically significant interactions by sex or age category were observed. Results were also similar when we evaluated the endpoints of PE and DVT separately.
The present analysis has several strengths. First, the sample size of OAC users was large, resulting in a sizable number of VTE events, and the ability to evaluate associations by patient subgroups and by DOAC dosage. Second, we conducted comparisons of each DOAC versus warfarin, and head-to-head comparisons of DOACs, something not likely to be done in industry-sponsored research. Third, we evaluated these associations outside of a highly selected randomized clinical trial population. Thus, our results may have higher external validity, being generalizable across the breadth of patient and provider profiles, and representative of routine clinical practice. This analysis also has limitations. Foremost is the possibility of uncontrolled confounding; namely, that warfarin users had more comorbidities than DOAC users and therefore were at higher risk of VTE. Our study period includes the first few years after DOAC approvals. Thus, clinicians may have restricted DOAC prescription to selected patients; as clinicians become more comfortable with prescription of DAOCs, patients profiles may change and, as a consequence, comparative effectiveness could be affected. We attempted to control for confounding through matching on initial patient characteristics (including age and CHA2DS2-VASc), then re-matching on HDPS, which combined information on key pre-specified covariates and the wealth of information available in the MarketScan database. This approach has been shown to be effective at controlling for confounding.[33] Also, this analysis used administrative data, which have known limitations regarding misclassification. However, the AF and VTE algorithms used have high PPVs. Outpatient VTE events were not included in the present analysis, as some studies have shown them to have a low PPV[55] unless there is evidence of OAC therapy prescribed for the indication of VTE.[56] Warfarin also has a high PPV, and DOACs may as well. Additionally, for warfarin time in therapeutic range is unknown. However, it is important to note that the intent of this manuscript was to assess OAC comparative effectiveness in a ‘real world’ setting. In the ‘real world’, patients on warfarin are often outside the therapeutic range. A strength of the present analysis is that it reports risk of VTE according to ‘usual use’ of warfarin and the DOACs, respectively, in a broad patient population. Were the population on warfarin in tight therapeutic control, the results may have differed. Another consideration related to OACs is that patients may switch OACs or cease taking the medication. However, those who switch or have low persistence may be different than those who do not, thus leading to bias. To mitigate this, our analyses were based on OAC initially prescribed, following the intent-to-treat principle, which has been shown to be advantageous in making observational data more closely resemble clinical trial data.[21] Secondarily, we did evaluate risk among switchers versus individuals who remained on their originally prescribed OAC. Also, a variety of patients were on reduced doses of DOACs; given our data the rationale for the reduced doses is unclear. Despite the limitations of administrative data, they represent an efficient way to answer these research questions. Randomized clinical trials, in comparison, would need to be extremely large, and thus prohibitively expensive and time-consuming, to capture this number of VTE events. Non-administrative observational data sources are typically too small to answer the questions posed here. Despite the limitations of administrative data, when used with rigorous analytic methods, these data can provide timely answers about the comparative effectiveness of different treatment strategies.[57] Lastly, edoxaban was not included in the present analysis, as it was only approved by the Food and Drug Administration in January 2015.
In these retrospective claims-based analyses we demonstrated that, among AF patients with health insurance, the use of dabigatran and apixaban over warfarin and rivaroxaban to prevent thromboembolic complications was associated with lower rates of incident VTE. Future studies should evaluate whether dabigatran or apixaban may be preferentially prescribed in AF patients at particularly elevated risk of VTE.
Supplementary Material
Acknowledgements
No assistance in the preparation of this article is to be declared.
Declaration of funding
This work was supported by NIH National Heart Lung and Blood Institute grants R01-HL122200 and R01-HL131579 and American Heart Association grant 16EIA26410001 (Alonso). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Transparency
Declaration of financial/other relationships
No relationships to declare. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017. March 07;135(10):e146–e603. 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004. August 31;110(9):1042–6. 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Bell EJ, Lutsey PL, Basu S, et al. Lifetime Risk of Venous Thromboembolism in Two Cohort Studies. Am J Med 2016. March;129(3):339 10.1016/j.amjmed.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014. December 02;64(21):e1–76. 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361(12):1139–1151. 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365(10):883–891. 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 7.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365(11):981–992. 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 8.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine 2013;369(22):2093–2104. 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 9.Bengtson LGS, Lutsey PL, Chen LY, et al. Comparative effectiveness of dabigatran and rivaroxaban versus warfarin for the treatment of non-valvular atrial fibrillation. J Cardiol 2017. June;69(6):868–876. 10.1016/j.jjcc.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikdeli B, Abou Ziki MD, Lip GY. Pulmonary Embolism and Atrial Fibrillation: Two Sides of the Same Coin? A Systematic Review. Semin Thromb Hemost 2017. February 14 10.1055/s-0036-1598005.; [DOI] [PubMed]
- 11.Enga KF, Rye-Holmboe I, Hald EM, et al. Atrial fibrillation and future risk of venous thromboembolism:the Tromso study. J Thromb Haemost 2015. January;13(1):10–6. 10.1111/jth.12762. [DOI] [PubMed] [Google Scholar]
- 12.Wang CC, Lin CL, Wang GJ, et al. Atrial fibrillation associated with increased risk of venous thromboembolism. A population-based cohort study. Thromb Haemost 2015. January;113(1):185–92. 10.1160/TH14-05-0405. [DOI] [PubMed] [Google Scholar]
- 13.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 2009. January 10;373(9658):155–66. 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki YK, Nishida K, Kato T, et al. Atrial fibrillation pathophysiology: implications for management. Circulation 2011. November 15;124(20):2264–74. 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003. April 10;348(15):1425–34. 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 16.Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003. August 14;349(7):631–9. 10.1056/NEJMoa035422. [DOI] [PubMed] [Google Scholar]
- 17.Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in Orthopedic Surgery Patients. Chest 2012. 2012/02/01/;141(2):e278S–e325S. 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen L The Truven Health MarketScan Databases for life sciences researchers: Truven Health Analytics, IBM Watson Health; 2017. [cited 2017 Aug 24]. Available from: https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf
- 19.Jensen PN, Johnson K, Floyd J, et al. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012. January;21 Suppl 1:141–7. 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg RK, Glazer NL, Wiggins KL, et al. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiology and Drug Safety 2011;20(3):313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments. An application to postmenopausal hormone therapy and coronary heart disease. Epidemiology 2008;19(6):766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergstralh EKJ. GMATCH macro. 2003 Available from: http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros.
- 23.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med 2004. July 01;117(1):19–25. 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 24.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Annals of Internal Medicine 1998. May 1;128(9):737–740. [DOI] [PubMed] [Google Scholar]
- 25.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol 2004. December 15;160(12):1152–8. 10.1093/aje/. [DOI] [PubMed] [Google Scholar]
- 26.Kniffin WD Jr., Baron JA, Barrett J, et al. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 1994. April 25;154(8):861–6. [PubMed] [Google Scholar]
- 27.Birman-Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005. May;43(5):480–5. doi: 00005650-200505000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Tamariz L, Harkins T, Nair V. Mini-Sentinel systematic evaluation of health outcome of interest definitions for studies using administrative data: Venous Thromboembolism Report. Mini-Sentinel. 2011.
- 29.Fang MC, Fan D, Sung SH, et al. Validity of Using Inpatient and Outpatient Administrative Codes to Identify Acute Venous Thromboembolism: The CVRN VTE Study. Medical Care 10.1097/mlr.0000000000000524. [DOI] [PMC free article] [PubMed]
- 30.Cunningham A, Stein CM, Chung CP, et al. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 2011. June;20(6):560–6. 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005. November;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 32.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010. February;137(2):263–72. 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss S, Rassen JA, Glynn RJ, et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20(4):512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bross ID. Spurious effects from an extraneous variable. J Chronic Dis 1966;19:637–647. [DOI] [PubMed] [Google Scholar]
- 35.Bergstralh E, Kosanke J. Locally written SAS macros: GMATCH 2003. [cited 2017 March 15]. Available from: http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros
- 36.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016. February;149(2):315–52. 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism [Article]. N Engl J Med 2009. December 10;361(24):2342–52. 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 38.Einstein Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism [Article]. N Engl J Med 2010. December 23;363(26):2499–510. 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 39.Buller HR, Decousus H, Grosso MA, et al. Edoxaban versus Warfarin for the Treatment of Symptomatic Venous Thromboembolism [Article]. New England Journal of Medicine 2013. October;369(15):1406–1415. 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 40.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism [Article]. N Engl J Med 2013. August 29;369(9):799–808. 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 41.Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014. February 18;129(7):764–72. 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 42.Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013. February 21;368(8):709–18. 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 43.Raskob G, Ageno W, Cohen AT, et al. Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol 2016. May;3(5):e228–36. 10.1016/S2352-3026(16)00023-5. [DOI] [PubMed] [Google Scholar]
- 44.Salem JE, Sabouret P, Funck-Brentano C, et al. Pharmacology and mechanisms of action of new oral anticoagulants. Fundam Clin Pharmacol 2015. February;29(1):10–20. 10.1111/fcp.12091. [DOI] [PubMed] [Google Scholar]
- 45.Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Internal Medicine 2016;176(11):1662–1671. 10.1001/jamainternmed.2016.5954. [DOI] [PubMed] [Google Scholar]
- 46.Chan Y-H, Kuo C-T, Yeh Y-H, et al. Thromboembolic, Bleeding, and Mortality Risks of Rivaroxaban and Dabigatran in Asians With Nonvalvular Atrial Fibrillation. Journal of the American College of Cardiology 2016. 2016/09/27/;68(13):1389–1401. 10.1016/j.jacc.2016.06.062. [DOI] [PubMed] [Google Scholar]
- 47.Noseworthy PA, Yao X, Abraham NS, et al. Direct Comparison of Dabigatran, Rivaroxaban, and Apixaban for Effectiveness and Safety in Nonvalvular Atrial Fibrillation. Chest 2016. 2016/12/01/;150(6):1302–1312. 10.1016/j.chest.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Maura G, Blotière P-O, Bouillon K, et al. Comparison of the Short-Term Risk of Bleeding and Arterial Thromboembolic Events in Nonvalvular Atrial Fibrillation Patients Newly Treated With Dabigatran or Rivaroxaban Versus Vitamin K Antagonists: A French Nationwide Propensity-Matched Cohort Study. Circulation 2015. 09/28;132(13):1252–1260. 10.1161/CIRCULATIONAHA.115.015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueck W, Stampfuss J, Kubitza D, et al. Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban. Clinical Pharmacokinetics 2014. 09/03;53(1):1–16. 10.1007/s40262-013-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulman S, Singer D, Ageno W, et al. NOACs for treatment of venous thromboembolism in clinical practice. Thromb Haemost 2017. April 20 10.1160/TH17-01-0065. [DOI] [PubMed]
- 51.Parks AL, Redberg RF. Dabigatran compared with rivaroxaban vs warfarin—reply. JAMA Internal Medicine 2017;177(5):744–744. 10.1001/jamainternmed.2017.0571. [DOI] [PubMed] [Google Scholar]
- 52.Kaufman JS, MacLehose RF. Which of these things is not like the others? Cancer 2013;119(24):4216–4222. 10.1002/cncr.28359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.