Abstract
Learning and memory are fundamental processes that are disrupted in many neurological disorders including Alzheimer’s disease and epilepsy. The hippocampus plays an integral role in these functions, and modulation of synaptic transmission mediated by γ-aminobutyric acid (GABA) type-A receptors (GABAARs) impacts hippocampus-dependent learning and memory. The protein diazepam binding inhibitor (DBI) differentially modulates GABAARs in various brain regions, including hippocampus, and changes in DBI levels may be linked to altered learning and memory. The effects of genetic loss of DBI signaling on these processes, however, have not been determined. In these studies, we examined male and female constitutive DBI knockout mice and wild-type littermates to investigate the role of DBI signaling in modulating multiple forms of hippocampus-dependent spatial learning and memory. DBI knockout mice did not show impaired discrimination of objects in familiar and novel locations in an object location memory test, but did exhibit reduced time spent exploring the objects. Multiple parameters of Barnes maze performance, testing the capability to utilize spatial reference cues, were disrupted in DBI knockout mice. Furthermore, whereas most wild-type mice adopted a direct search strategy upon learning the location of the target hole, knockout mice showed higher rates of using an inefficient random strategy. In addition, DBI knockout mice displayed typical levels of contextual fear conditioning, but lacked a sex difference observed in wild-type mice. Together, these data suggest that DBI selectively influences certain forms of spatial learning and memory, indicating novel roles for DBI signaling in modulating hippocampus-dependent behavior in a task-specific manner.
Keywords: Barnes maze, contextual fear conditioning, diazepam binding inhibitor, hippocampus, knockout mouse, object location memory, sex differences
Graphical Abstract
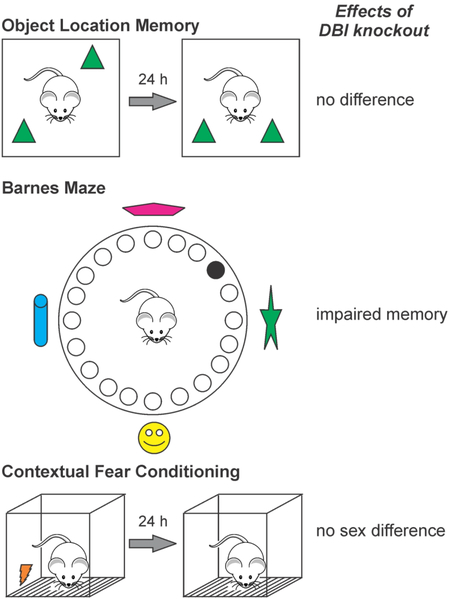
Diazepam binding inhibitor knockout mice and wild-type littermates were tested on three assays of hippocampus-dependent spatial and contextual memory. No differences were observed in object location memory, but knockout mice displayed impaired Barnes maze learning and lacked a sex difference in contextual fear conditioning performance that was observed in wild-types.
1. Introduction
The processes of learning and memory are indispensable to proper cognitive function and are impaired in a variety of neurological disorders, including Alzheimer’s disease, other forms of dementia, and epilepsy. Decades of research has established that the hippocampus plays imperative roles in these functions (Scoville and Milner, 1957; Zola et al., 2000; Burgess et al., 2002; Ergorul and Eichenbaum, 2004). Although learning and memory have been rigorously studied over the years, elucidation of the myriad molecular players involved in hippocampus-dependent learning and memory remains incomplete.
The γ-aminobutyric acid (GABA) neurotransmitter system is critically involved in hippocampal function, and manipulations of GABA transmission have been linked to alterations in learning and memory. For example, injections of muscimol, a GABAA receptor (GABAAR) agonist, into dorsal hippocampus blocks long-term memory of object location (Haettig et al., 2011), and mice with a genetic deletion of the GABAAR α5 subunit display enhanced performance in the Morris water maze task of spatial memory (Collinson et al., 2006). Treatment with benzodiazepines, which exert effects via allosteric modulation of GABAARs, impacts multiple measures of spatial memory (Kant et al., 1996; Hogan et al., 2005; Joksimović et al., 2013; Timić et al., 2013). Furthermore, mice with a point mutation that renders α5-containing GABAARs insensitive to benzodiazepines show improved performance in trace fear conditioning (Crestani et al., 2002). These findings suggest that modulatory actions on GABAARs, and specifically at the benzodiazepine binding site, can have profound impacts on hippocampal learning and memory.
Diazepam binding inhibitor (DBI), also known as acyl-CoA binding protein, is a 10-kDa protein that was first identified for its ability to displace diazepam from GABAAR benzodiazepine binding sites (Guidotti et al., 1983). DBI protein immunoreactivity is present in several brain areas, including the hippocampus (Ball et al., 1989; Ferrarese et al., 1989). Importantly, DBI can modulate GABAARs in various brain regions. Negative allosteric modulation of GABAARs by DBI was seen in cultured spinal neurons (Bormann, 1991) and in transit-amplifying cells of the subventricular zone (Alfonso et al., 2012). In the thalamic reticular nucleus, however, DBI acts as a positive allosteric GABAAR modulator (Christian et al., 2013; Christian and Huguenard, 2013), indicating that the modulatory effects of DBI are region-specific. In this regard, our lab recently demonstrated hippocampal subregion-specific alterations in GABAAR-mediated transmission in DBI knockout (DBI−/−) mice (Courtney and Christian, 2018), suggesting that DBI may play a role in hippocampus-dependent learning and memory processes. DBI may also modulate GABA transmission via mechanisms independent of actions at GABAAR benzodiazepine binding sites. For example, DBI may upregulate biosynthesis of neurosteroids, another class of GABAAR allosteric modulators, by acting as an endogenous ligand of the mitochondrial benzodiazepine receptor (also known as 18kDa translocator protein, TSPO) (Papadopoulos et al., 1991; Korneyev et al., 1993). Furthermore, DBI could modulate neuronal and/or glial function through effects on lipid metabolism and other intracellular actions (Bouyakdan et al., 2015; Neess et al., 2015), and the Dbi gene also has ubiquitous housekeeping functions (Mandrup et al., 1992). DBI is thus capable of exerting multiple biological actions that are poised to have impacts on neural cellular and synaptic physiology and thus modulate complex behaviors.
Interactions between DBI and GABAARs may influence many behavioral abnormalities (Costa and Guidotti, 1991). Notably, cerebrospinal fluid levels of DBI are elevated in patients with depression, anxiety, and hepatic encephalopathy (Barbaccia et al., 1986; Rothstein et al., 1989). Furthermore, studies of animal behavior suggest a role for DBI in various hippocampus-dependent tasks. For example, a DBI-overexpressing transgenic mouse line displays impaired learning performance on the Morris water maze (Siiskonen et al., 2007). Additionally, intracerebroventricular injection of DBI reverses a corticotropin-releasing hormone receptormediated enhancement in contextual fear conditioning (Sherrin et al., 2009). However, investigations into the role of DBI in hippocampus-dependent cognitive tasks using mice with genetic removal of DBI are lacking. Therefore, the primary purpose of the present studies was to identify the behavioral phenotypes of a constitutive DBI knockout mouse (Neess et al., 2011) to determine roles for DBI signaling in a variety of hippocampus-dependent behaviors. We hypothesized that mice with a genetic knockout of DBI would display impaired performance compared with wild-type mice with functional DBI signaling. We employed three well-validated assays of hippocampal learning and memory: object location memory (OLM) (Sharma et al., 2010; Vogel‐Ciernia and Wood, 2014); Barnes maze (Barnes, 1979; Holmes et al., 2002); and contextual fear conditioning (Curzon et al., 2009). These tasks were selected to assess multiple forms of hippocampal-dependent spatial and contextual learning and memory. Our results indicate that mice with a genetic loss of DBI display differential performance across the tasks compared with control mice, providing novel evidence of a role for DBI in modulating specific forms of hippocampusdependent learning and memory, most notably spatial navigation memory.
2. Materials and Methods
2.1). Mice
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign. DBI heterozygous (DBI+/−) and homozygous knockout (DBI−/−) founder mice on the C57BL/6BomTac background (Neess et al., 2011) were acquired from Dr. Susanne Mandrup (University of Southern Denmark). Re-derivation of the colony and backcrossing onto the C57BL/6J background were described previously (Ujjainwala et al., 2018). Breeding pairs of DBI+/− males and females yielded DBI+/+, DBI+/−, and DBI−/− pups, and genotypic identities were confirmed via PCR as previously described (Neess et al., 2011). Mice used in the present experiments were produced in the fifth and sixth filial generations of this colony after backcrossing was completed. Mice were bred and housed on a 14:10 h light:dark cycle with food and water available ad libitum. At weaning, mice were group housed (up to 5 mice per cage) with littermates of the same sex. A total of 34 mice were used for all three behavioral tests, consisting of 16 males (DBI+/+ n=9, DBI−/− n=7) and 18 females (DBI+/+ n=9, DBI−/− n=9). An additional 12 mice (males: DBI+/+ n=3, DBI−/− n=3; females DBI+/+ n=3, DBI−/− n=3) were used as unshocked controls in fear conditioning. For female mice, the estrous cycle stage was recorded by examination of vaginal cytology (Li et al., 2017) on each first day of testing for OLM, Barnes maze, and contextual fear conditioning. A Fisher’s exact test demonstrated that the proportions of the estrous cycle stages were not significantly different between DBI+/+ and DBI−/− mice on any of the three tests (OLM: odds ratio=4, p=0.33; Barnes maze: odds ratio=0.64, p=1; contextual fear conditioning: odds ratio=1, p=1), so all females tested were included in all analyses. Mice were postnatal days (P) 50–250 old at the time of testing. OLM testing was performed when mice were P57–199, Barnes maze testing was performed when mice were P85–230, and contextual fear conditioning was performed when mice were P92–248 (Table 1). Two-way ANOVA of age with genotype and sex as factors showed a main effect of sex on all three tests (P<0.025 for all tests), in which female mice were older than male mice by an average of 40.5 days.
Table 1: Age of mice on individual behavioral tasks.
Postnatal age (days) at which each individual mouse started each behavioral task, broken down by genotype and sex. Mouse identities indicate cage number and ear tag.
| Age at Testing | |||
|---|---|---|---|
| Group | Object Location Memory | Barnes Maze | Fear Conditioning |
| DBI+/+ Males | |||
| 666 L | P199 | P214 | P229 |
| 690 R | P193 | P224 | P243 |
| 708 R | P189 | P219 | P232 |
| 780 N | P113 | P149 | P155 |
| 780 L | P113 | P149 | P155 |
| 780 R | P113 | P149 | P155 |
| 808 N | P92 | P134 | P164 |
| 808 L | P92 | P134 | P164 |
| 820 2R | P126 | P140 | P148 |
| DBI+/+ Females | |||
| 645 L | P189 | P196 | P234 |
| 642 R | P187 | P216 | P231 |
| 657 L | P190 | P215 | P230 |
| 676 N | P182 | P197 | P212 |
| 688 N | P187 | P218 | P244 |
| 706 N | P189 | P219 | P232 |
| 706 L+R | P189 | P219 | P232 |
| 809 N | P92 | P140 | P164 |
| 862 N | P84 | P86 | P100 |
| DBI−/− Males | |||
| 647 R | P187 | P216 | P231 |
| 654 N | P190 | P221 | P229 |
| 654 R | P190 | P221 | P229 |
| 690 L+R | P193 | P224 | P243 |
| 855 L | P73 | P85 | P108 |
| 855 R | P73 | P85 | P108 |
| 861 N | P57 | P87 | P95 |
| DBI−/− Females | |||
| 645 N | P189 | P196 | P234 |
| 657 R | P190 | P222 | P230 |
| 655 N | P191 | P229 | P249 |
| 655 L | P191 | P229 | P249 |
| 665 L | P192 | P214 | P228 |
| 665 R | P192 | P208 | P228 |
| 688 R | P187 | P218 | P244 |
| 688 L+R | P187 | P218 | P244 |
| 688 2L+R | P187 | P225 | P244 |
2.2). Behavioral testing
Experiments were performed between 10 a.m. and 2 p.m., relative to 7 p.m. lights off, in accordance with other studies testing hippocampus-dependent behavior (Paylor et al., 1994; Patil et al., 2009; Kleschevnikov et al., 2012). For all tasks, mice were tested in arenas with ambient light levels between 0.1–0.2 mW. Animals were tested in order of litter date of birth. Although the mice were not intentionally randomized for testing, the heterozygote breeding strategy created a nearly random mixture of genotype and sex combinations. All mice were tested on all behavioral tests described (excluding the unshocked control animals for contextual fear conditioning), performed in the following order: (1) OLM; (2) Barnes maze; and (3) contextual fear conditioning, with 1 to 30 days in between tests (Table 1). This order was chosen so that the task with the least complexity was performed first (OLM), followed by the more complex Barnes Maze. Contextual fear conditioning was performed last to prevent the possibility of retained fear behavior influencing performance on the other tasks. This serial testing paradigm significantly diminishes the interindividual variation that would likely arise if the order of testing had been counterbalanced.
2.3). OLM
The following procedure was adapted from previous reports (Bevins and Besheer, 2006; Roozendaal et al., 2010; Barrett et al., 2011; McQuown et al., 2011; Kleschevnikov et al., 2012; Vogel-Ciernia et al., 2013; Bui et al., 2018). On the day of testing, mice were brought to the testing room, weighed, and placed individually into clean cages with fresh bedding to habituate for 30 minutes. A clear, glass-walled 25 cm × 25 cm terrarium without bedding was used as the testing chamber. Prior to the start of each phase and experiment, the testing chamber and objects were cleaned with 70% ethanol. Each test consisted of two phases: the acquisition phase and the testing phase. The testing area was an enclosed space isolated from the rest of the laboratory room by opaque curtains hanging from the ceiling. Each mouse was tested individually, and mice were left undisturbed for the duration of the testing. Prior to the acquisition phase, two identical plastic objects were placed diagonally from each other in opposite corners, approximately 6 cm away from each wall, and secured into place using Velcro. The objects used were triangular in shape, with each side 4.5 cm in length.
Following acclimation to the testing room, mice began the acquisition phase. Each mouse was individually placed in the center of the testing chamber with the two objects present and allowed to explore freely for 10 minutes. The activity of the mouse was recorded using a video camera. Mice were then returned to the home cage and colony. The testing phase occurred 24 hours following the acquisition phase. Prior to beginning the testing phase, one object was moved to the corner vertically opposite from its initial location, while the other object remained in its former location. The moved object was counterbalanced across subjects. During the testing phase, mice were placed into the center of the testing chamber and allowed to explore the chamber for 3 minutes. The percentage of time spent exploring either object was recorded and scored from the video. Interaction with an object was defined as nose sniffing and head orientation within <1.0 cm of an object, without any further interaction with the object (e.g., biting or climbing). The discrimination index (DI) was computed as DI(%)=((Tnew-Told)/(Tnew+Told))*100, Tnew representing the amount of time spent interacting with the moved object and Told representing the time spent interacting with the unmoved object. Side preference for the acquisition phase was calculated using the same DI equation as above, replacing Tnew with interaction with the object on the left and Told with interaction with the object on the right. For analyses, all video identities were coded and randomized, and scorers were blinded to genotype and sex.
2.4). Barnes maze
The following procedure was adapted from previous reports (Harrison et al., 2006; Patil et al., 2009; Rosenfeld and Ferguson, 2014). In the Barnes maze test, mice use extra-maze cues located around the testing room to learn how to escape from the open maze to a dark “escape box” located underneath the platform. The custom-built Barnes maze consisted of a circular grey, type 1 PVC sheet 91.44 cm in diameter and 91.44 cm from the ground, with 20 equally spaced holes (each 5.08 cm in diameter) located 2.85 cm from the edge of the maze. Each hole was left open during each trial with exception of the target hole, under which was a black cast acrylic escape box (5.08 × 10.16 × 4.76 cm) to which mice had direct access. Because the escape box was located directly under the maze, mice could not visually identify the location of the escape hole from most points on the maze. Prior to each trial, the escape box was cleaned with 70% ethanol and filled with approximately 2.5 cm of fresh bedding. Three extra-maze cues were placed approximately 38.1 cm away from the edges of the maze in the cardinal directions surrounding the maze. Extramaze cues were similar in size yet distinctive from one another, and included a patterned throw pillow, a stuffed animal toy, and a colorful printed image. The experimenter acted as the fourth cue during the trials by sitting in a chair in a consistent place.
Prior to testing, each mouse was brought to the testing room, weighed, and individually placed in a clean cage with fresh bedding to habituate for 30 minutes. Directly before the start of each trial, mice that were not immediately undergoing testing were moved from the testing room to a different laboratory room (“waiting room”) to prevent them from being prematurely exposed to the buzzer (see below). At the conclusion of the 4 trials, the test mouse was returned to the waiting room, and the next mouse to undergo testing was moved from the waiting room into the testing room. Mice were transported in covered cages at all times.
For the pre-training trial (day 0), the mouse was placed in the center of the maze in a black opaque cylindrical start chamber (10.2 cm in diameter), and a buzzer tone (85 dB) and light (positioned directly above the maze) were turned on. The buzzer was emitted via two speakers positioned near the maze. After 10 seconds elapsed, the start chamber was lifted and the mouse was gently guided into the escape box. After the mouse entered the escape box, the buzzer and light were turned off and a square lid was placed over the hole. The mouse remained in the hole for 2 minutes and was returned to the habituation cage following the trial.
On days 1–4, training trials began with the mouse in the center of the maze under the same start chamber that was used during the pre-training trial. 10 seconds following the onset of the light and buzzer, the chamber was lifted and the mouse was allowed to freely explore the maze. The trial ended after the mouse entered the escape box, or if 3 minutes elapsed. After all four paws of the mouse entered the escape box, the light and buzzer were immediately turned off and the mouse was allowed to stay in the box for 1 minute. If the mouse left the escape box, it was gently guided back in. Mice underwent 4 trials per day for 4 days; each trial was separated by a 15-minute inter-trial interval. During the inter-trial interval of one mouse, up to two more mice would be run. The maze was cleaned with 70% ethanol following each trial, and the maze was rotated after each mouse had undergone a trial in order to eliminate any odor cues still present on the maze. The target box was subsequently moved to compensate for the maze rotation.
On day 5, mice underwent one probe trial. The purpose of this trial was to evaluate the short-term retention memory the subjects had for the target hole. During the probe trial, the escape box under the target hole was removed. The probe trial began similarly to the pre-training and training trials and the mouse was allowed to explore the maze for 90 seconds under the buzzer and light aversive stimuli.
Trials were recorded using TopScan video tracking software (CleverSys, Reston, VA). During analysis of videos, scorers were blinded to mouse genotype and sex. From the training trials, latency to enter the escape tunnel, primary latency, distance travelled, search strategy, and number of total errors were analyzed. Errors were identified as nose pokes over any hole that was not defined as the target hole. The definition for primary latency, the first uninterrupted 3-second interaction with the target hole, was adapted from a previous report (Harrison et al., 2006). The search strategy was divided into 3 categories: 1) direct, navigating directly to the target or adjacent hole with 3 or fewer errors without crossing the center of the maze more than once; 2) serial, searching consecutive holes in a clockwise or counterclockwise manner starting at least 2 holes away from the target hole; 3) random, unorganized search that involves crossing through the center of the maze. Each mouse was classified into a single strategy for each trial. For analysis of the probe trial, the number of nose pokes into each hole for the duration of the trial was calculated.
2.5). Contextual fear conditioning
The following procedure was adapted from a previous report (Clark et al., 2008). Prior to testing, each mouse was brought to the testing room, weighed, and individually placed in a clean cage with fresh bedding to habituate to the room for 30 minutes. Mice that were not being tested were separated from the testing area by an opaque curtain, and were placed on a different table than that holding the fear conditioning apparatus. The contextual fear conditioning experiment took place over the span of 2 days: the training phase (day 1) and the testing phase (day 2). On day 1, mice were individually placed in a fear conditioning chamber with a metal grid floor that delivers shock stimuli under the control of a digital timer (Med Associates, St. Albans, VT) (Clark et al., 2008; Kohman et al., 2012). Mice were allowed to explore the chamber for 3 minutes. During the training phase, mice received one footshock (0.5 mA, 2-second duration) (Clark et al., 2008; Curzon et al., 2009) at 120 seconds and a second shock at 150 seconds. 30 seconds after the final shock, mice were returned to the habituation cage, then returned to the home cage and colony. 24 hours later, mice were placed in the same chamber as above for a 3-minute test in the absence of a shock. Control animals were not shocked on either day. Between days and test subjects, the chamber, grid, and the area under the grid were cleaned with 70% ethanol.
Trials were recorded using TopScan video tracking software. The total percentage of time spent freezing and distance traveled were recorded for each day. Recorders were blinded to genotype and sex of the test subjects for analysis.
2.6). Statistical analysis
In the OLM task, both the percentage of time spent sniffing and the DI were analyzed using a three-way repeated measures analysis of variance (ANOVA), with day as the repeated withinsubjects factor and sex and genotype as between-subjects factors. For situations in which a significant interaction involving day was detected, the analysis was further broken down for each individual day using two-way ANOVA with sex and genotype as between-subjects factors.
In the Barnes Maze, latency, primary latency, distance traveled, and number of errors (averaged across 4 trials per day) over 4 consecutive days were analyzed using three-way repeated measures ANOVA with day as the repeated within-subjects factor and sex and genotype as between-subjects factors. In cases where sex or the interaction between sex and other variables were insignificant, 2-way repeated measures ANOVAs (with day and genotype as factors) were applied. Search strategy was assessed using a logistic regression with sex and genotype as between-subjects factors and day as a within-subjects factor. The probabilities of using the direct, serial or random strategy were analyzed separately. For these analyses, the deviance is reported instead of the F statistic. Comparisons of the probability of using each search strategy between genotypes within days, or within genotypes between day 1 to day 4, were made using Fisher’s exact tests. For the probe trial, a three-way repeated measures ANOVA was performed with hole as the repeated within-subjects factor and sex and genotype as between-subjects factors. When required, all pairwise comparisons were made using Fisher’s least significant difference (LSD) post hoc tests for all Barnes maze parameters.
For the contextual fear conditioning task, freezing behavior and total distance traveled were analyzed using three-way repeated measures ANOVA with day as the repeated measure and sex and genotype as between-subjects factors. Due to significant interactions involving day, both freezing behavior and total distance were subsequently analyzed independently by day using twoway ANOVA with sex and genotype as between-subjects factors. Fisher’s LSD post hoc tests were used for pairwise comparisons of means.
Age was included as a covariate in all statistical analyses due to the large age range. In all parametric analyses, if skewness was outside the range of −1 to 1, the data were square root- or log-transformed, depending on which method produced skewness nearest zero, to improve the normality assumption. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC), OriginPro2016 (OriginLab, Northampton, MA), or R software. P<0.05 was considered statistically significant.
Results
3.1). Genetic loss of DBI does not impact contextual OLM
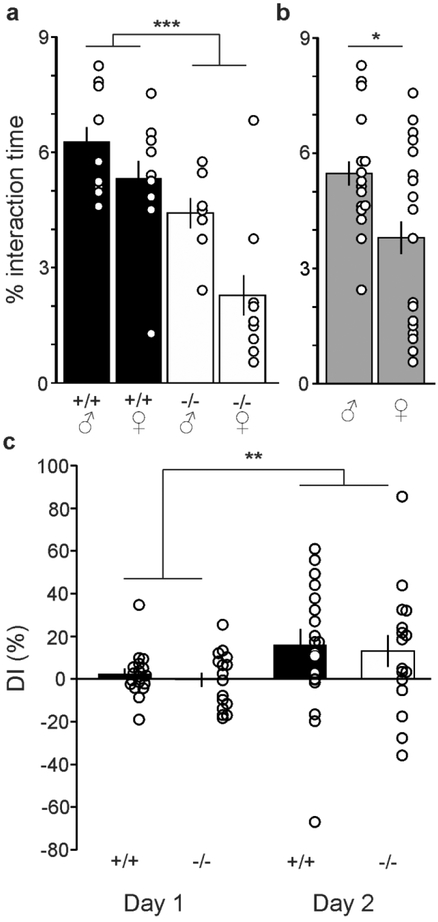
DBI+/+ and DBI−/− mice were first tested on the OLM test. Animals were evaluated for the total percentage of time spent interacting with both objects, as well as the DI between the two objects. No effect of age was detected for either variable. For the percentage of time spent interacting with both objects, a three-way repeated measures ANOVA showed a main effect of genotype (F1,29=19.0, P<0.0002) (Figure 1A), in which DBI−/− mice spent less time interacting with the objects than did DBI+/+ mice. Additionally, a main effect of sex was detected (F1,29=6.3, P=0.02) (Figure 1B), in which male mice spent more time investigating the objects than did females, independent of genotype. No main effect of day and no interactions were significant, indicating that the effects were similar on both days and that the main effects of genotype and sex were independent of each other.
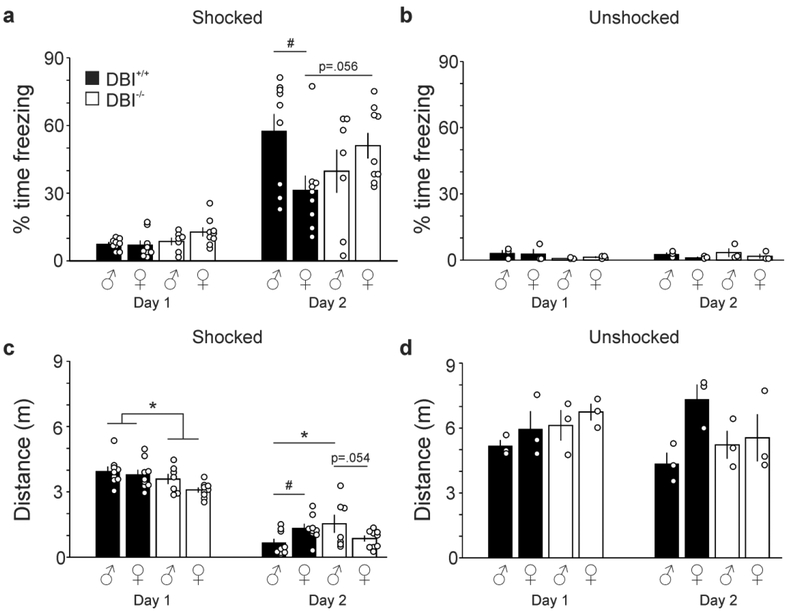
Figure 1: DBI−/− mice show diminished investigation of objects, but no alterations in OLM.
a, Mean ± SEM of percentage time sniffing both objects on days 1 and 2 in male and female DBI+/+ and DBI−/− mice. Black bars: DBI+/+ mice; open bars, DBI−/− mice. Open circles represent individual data values. b, Percentage time sniffing both objects on days 1 and 2, with data for DBI+/+ and DBI−/− mice combined. c, Discrimination index (DI) on days 1 and 2, with one object moved to a novel location on day 2. *, **, *** (P < 0.05, P < 0.01, P < 0.001 respectively)
We next sought to determine if DBI+/+ and DBI−/− mice displayed differences in the ability to identify an object in a novel location on day 2 vs. day 1. To see if either genotype displayed an inherent side preference on day 1, paired t-tests were performed to see if the DI of each mouse was statistically different from 0 (with 0 indicating no side preference). Neither DBI+/+ mice (P=0.35) nor DBI−/− mice (P=0.91) displayed a side preference on day 1. Next, three-way repeated measures ANOVA of the DI yielded a significant main effect of day (F1,30=8.5, P=0.007); no other significant main effects or interactions were present (Figure 1C). These results indicate that the animals learned the task, and that no differences were observed between genotypes or sex in the magnitude of the DI. Overall, these data suggest that the lack of DBI signaling may lead to a decrease in overall object investigation levels, but does not lead to a deficit in OLM.
3.2). Genetic loss of DBI impairs long-term spatial navigation memory in the Barnes maze
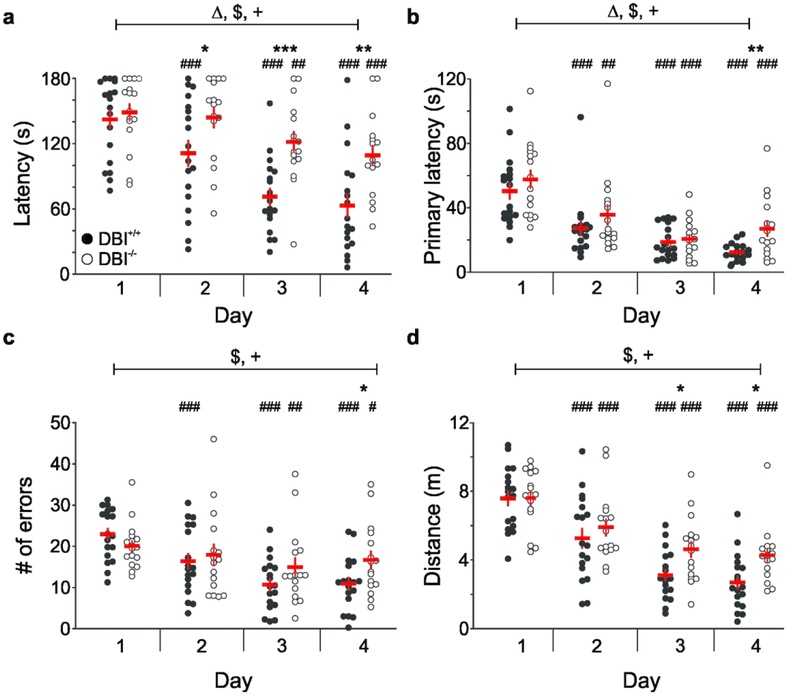
The mice were next tested on the Barnes maze to determine if the loss of DBI signaling impairs spatial navigation memory. Although the Morris water maze is more commonly used to test spatial relational memory, the Barnes maze assay was chosen because it is less stressful than the Morris water maze and recapitulates ethological features relevant to terrestrial rodents (Harrison et al., 2009). Furthermore, we observed that DBI−/− mice have difficulty swimming and are unable to reliably complete the Morris water maze (unpublished observations), prompting our use of a land-based maze. For all Barnes maze outcome variables, no main effects of sex or interactions involving sex were found. Therefore, all analyses were collapsed across sex to examine the effects of genotype. For latency to reach the target hole, two-way repeated measures ANOVA showed main effects of day (F3,96=46.43, P<0.0001), genotype (F1,31=8.3, P=0.007), and a day-by-genotype interaction (F3,96=5.77, P=0.001) (Figure 2A). Pairwise post hoc comparisons using Fisher’s LSD showed significantly increased latency for DBI−/− mice on days 2 (P<0.02), 3 (P<0.001), and 4 (P=0.001) compared with DBI+/+ mice (Figure 2A). Notably, some mice would reach the target hole as expected, but would not physically enter the hole, a phenomenon previously observed by other groups (Harrison et al., 2006; Patil et al., 2009). Therefore, in addition to latency, we also analyzed the primary latency, defined as the time needed to first reach the target hole and remain at the target for at least 3 seconds. The primary latency data displayed a positively skewed residual distribution, so a log transformation was used to improve normality. As with latency, two-way repeated measures ANOVA of primary latency showed main effects of day (F3,96=45.87, P<0.0001) and genotype (F1,31=4.18, P=0.049), as well as a day-by-genotype interaction (F3,96=2.76, P=0.047) (Figure 2B). Fisher’s LSD comparisons showed that DBI−/− mice displayed increased latency on day 4 (P=0.008) compared with DBI+/+ mice. No effect of age was seen for either latency or primary latency. Together, the latency and primary latency data revealed that DBI−/− mice took significantly longer to reach the target hole than did DBI+/+ mice across the four days, suggesting impaired spatial memory.
Figure 2: DBI−/− mice display impaired performance on multiple parameters of the Barnes maze.
Analysis of values from DBI+/+ (closed circles) and DBI−/− mice (open circles) over the course of 4 days (each circle represents the average of 4 trials for an individual mouse). Red lines represent Mean ± SEM. a, Latency to enter escape box. b, Primary latency to interact with target hole. The data were log-transformed to improve normality for statistical analysis; the raw data are presented here. c, Number of errors made. The data were square root-transformed to improve normality for statistical analysis; the raw data are presented here. d, Total distance traveled during duration of test. □□ significant effect of genotype; $, significant effect of day; +, significant dayby-genotype interaction; see Results for exact p-values for each parameter. *,**,***, significant difference between DBI+/+ and DBI−/− mice on given days (P < 0.05, P < 0.01, P < 0.001, respectively); #, ##, ###, significant difference compared with day 1 within the same group (P < 0.05, P < 0.01, P < 0.001, respectively)
We next investigated whether DBI+/+ and DBI−/− mice differed in the number of errors made during the task, defined as the number of visits to holes other than the target hole. The residual distribution was slightly positively skewed, so a square root transformation was used to improve normality. An age effect was detected, in which older animals displayed significantly fewer errors than younger animals (F1,31=7.4, P=0.01). After correcting for age, two-way repeated measures ANOVA of the transformed data revealed a main effect of day (F3,96=26.14, P<0.0001) and a day-by-genotype interaction (F3,96=5.24, P<0.003), indicating that DBI−/− mice had a higher rate of errors as the days progressed than did DBI+/+ mice. Fisher’s LSD comparisons revealed that DBI/- mice made significantly more errors on day 4 than did DBI+/+ mice (P<0.02) (Figure 2C). We also analyzed the total distance that the mice traveled on the maze. Two-way repeated measures ANOVA showed a significant effect of day (F3,96=92.55, P<0.0001) and a day-by-genotype interaction (F3,96=3.56, P<0.02), demonstrating that DBI+/+ and DBI−/− mice traveled different distances in the maze as the days progressed (Figure 2D). Fisher’s LSD comparisons showed that DBI−/− mice traveled a greater distance than DBI+/+ mice did on days 3 (P<0.02) and 4 (P<0.02). No effect of age was detected. These data are in agreement with the increased latency, primary latency, and number of errors displayed by the DBI−/− mice. Altogether, the phenotypic differences observed in these four parameters of Barnes maze testing provide evidence for impaired spatial navigation memory in mice with a genetic loss of DBI signaling.
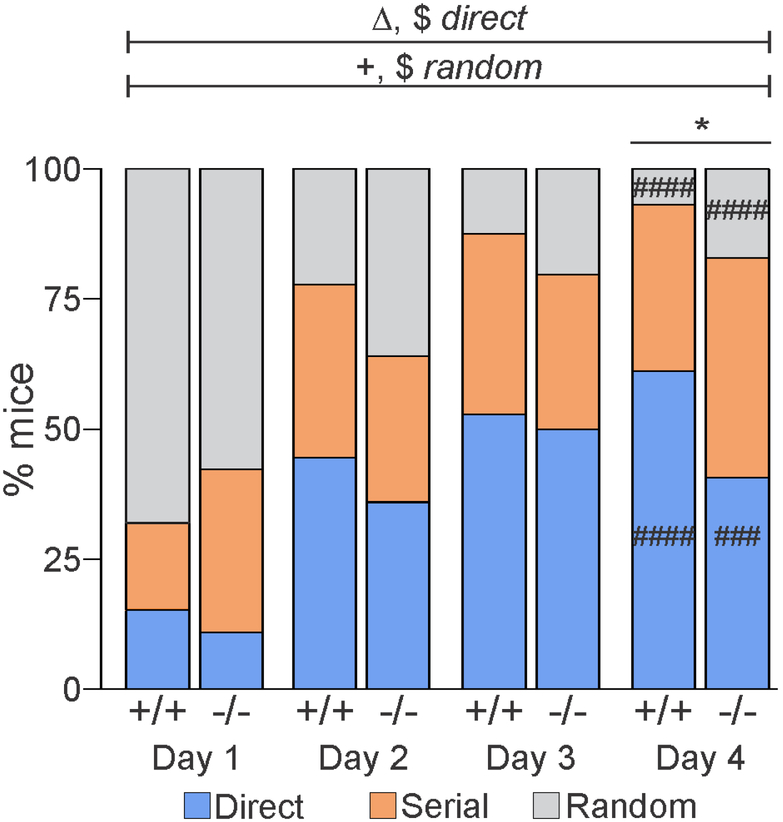
We next investigated whether the genetic loss of DBI signaling impacted the search strategy used by the mice to exit the maze (Figure 3). Search strategy is an important indicator of the ability of mice to use the external visual cues to solve the task, thereby signaling intact longterm spatial memory (Patil et al., 2009). All individual trials for each mouse were analyzed post hoc and scored for one of three possible search strategies: direct, serial, or random (see Materials and Methods for a complete description of strategy criteria). No effect of age was detected for any of the three strategies. Logistic regression analysis yielded main effects of day for the direct and random search strategies (Deviance3=62.3, P<0.0001 and Deviance3,=105.4, P<0.0001, respectively) (Figure 3), indicating that the mice shifted their search strategy as the days progressed independent of genotype. Specifically, the probability of using the direct strategy increased and the probability of using the random strategy decreased across days. To confirm this, Fisher’s exact tests were run on days 1 and 4 for both direct and random search strategies. For the direct search strategy, both DBI+/+ (P<0.0001) and DBI−/− mice (P=0.0002) displayed an increase in the probability of using this strategy on day 4 compared with day 1. Concomitantly, both DBI+/+ (P<0.0001) and DBI−/− mice (P<0.0001) displayed a decrease in the probability of using the random search strategy across days, suggesting that both genotypes learned and used the external visual cues by switching from a random to direct strategy. However, logistic regression analysis also yielded a main effect of genotype (Deviance1=4.65, P=0.04) for the direct strategy and a day-bygenotype interaction for the random strategy (Deviance3=8.2, P=0.04) (Figure 3), indicating that differences were present between DBI+/+ and DBI−/− mice in the use of these search strategies over the course of the task. In confirmation of this effect, a Fisher’s exact test revealed that DBI−/− mice used the direct search strategy significantly less than DBI+/+ mice did on day 4 (P<0.03). No significant effects were observed for the serial strategy. Taken together, both genotypes displayed the ability to learn the task, as evidenced by the shift from random to direct search strategies. In comparison to DBI+/+ mice, however, DBI−/− mice displayed a reduced rate of using the visual cues necessary for the direct strategy, providing further support of a role for DBI signaling in spatial navigation memory.
Figure 3: DBI−/− mice show curtailed adoption of direct search strategy by day 4.
Percentage of DBI+/+ and DBI−/− mice using each search strategy (direct: blue bars; serial: orange bars; random: gray bars) over the course of 4 days (each day represents the average of 4 trials). □□ significant effect of genotype (P < 0.05); $, significant effect of day (P < 0.0001); +, significant day-bygenotype interaction (P < 0.05) for the indicated search strategies; *, significant difference in use of direct search strategy between DBI+/+ and DBI−/− mice on day 4 (P < 0.05); ###, ####, significant difference comparing days 1 and 4 for each search strategy within genotype (P < 0.001, P < 0.0001, respectively)
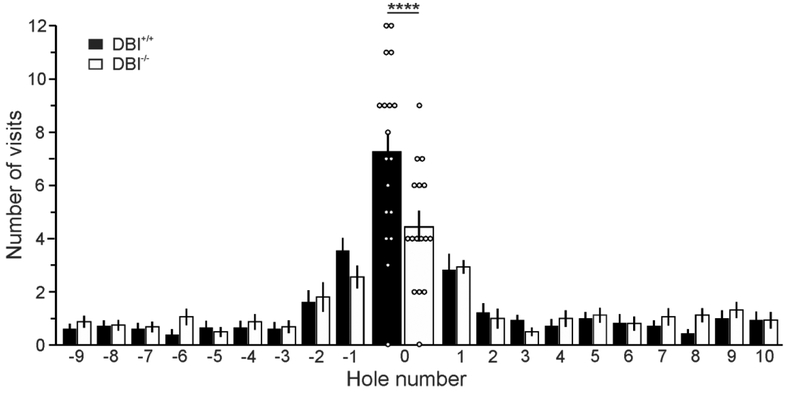
Finally, to investigate long-term spatial memory retention, DBI+/+ and DBI−/− mice were analyzed on a probe trial in which the escape box was removed from the target hole. Three-way repeated measures ANOVA on the number of visits made to each hole showed a significant effect of hole (F19,570=32.83, P<0.0001) and a significant genotype-by-hole interaction (F19,570=1.82, P=0.018). A Fisher’s LSD post hoc test revealed that DBI−/− mice made significantly fewer visits to the target hole during the probe trial than did DBI+/+ mice (P<0.0001) (Figure 4). No effect of age was detected, and no significant differences between genotypes were seen in the number of visits to any of the other 19 holes, further suggesting that DBI−/− mice displayed a specific deficit in long-term spatial memory.
Figure 4: DBI−/− mice display impaired performance in the probe trial of the Barnes maze test.
Mean ± SEM of number of nose pokes (visits) into each hole during probe trial. Hole 0 is the target hole, with positive values progressing clockwise from the target hole. Open circles represent individual data values for number of visits to the target hole by each mouse. ****, significant difference between DBI+/+ and DBI−/− mice (P <0.0001)
In summary, mice with a genetic lack of DBI signaling on average took longer to escape the maze, made more performance errors, traveled a greater distance, displayed decreased aptitude in learning external visual cues, and displayed impaired long-term retention of the target hole location. These data thus provide evidence for a role of DBI signaling in long-term spatial navigation learning and memory.
3.3). Genetic loss of DBI abolishes sex differences in contextual fear conditioning
DBI+/+ and DBI−/− mice were also assessed on a third hippocampus-dependent assay of learning and memory, contextual fear conditioning. To determine if mice associated the environmental context with a subsequent foot shock, mice were placed in a testing chamber on day 1 and footshocked at two separate intervals. On day 2, mice were placed within the same testing chamber and not shocked. Two outcome variables were evaluated: 1) percentage of time spent freezing; and 2) total distance traveled. No effect of age was detected for either variable. For the time spent freezing (Figure 5A), overall three-way repeated measures ANOVA found a significant effect of day (F1,30=118.9, P<0.0001), a significant interaction between sex and genotype (F1,29=5.3 P=0.029) and a day-by-sex-by-genotype interaction (F1,30=6.0, P=0.02); therefore, the data were analyzed separately for each day. On day 1, two-way ANOVA of freezing behavior detected no significant main effects or interactions. On day 2, two-way ANOVA of freezing behavior found no main effect of sex (F1,29=1.42, P=0.07) nor genotype (F1,29=0.00, P=0.96), but there was a significant sex-by-genotype interaction (F1,29=5.3, P=.03). Fisher’s LSD post hoc tests revealed that DBI+/+ males displayed more robust freezing behavior than did DBI+/+ females (P<0.05), but no differences between sexes were observed in the DBI−/− mice. This finding suggests that DBI+/+ males formed stronger associations between the context and the foot-shock than did females, confirming previous results in both mice (Wiltgen et al., 2001; Villasana et al., 2010) and rats (Maren et al., 1994; Kudo et al., 2004). This effect of sex was lost in DBI−/− mice (Fisher’s LSD: P>0.2) (Figure 5A). In addition, although there was not a significant difference in freezing between DBI+/+ and DBI−/− males (P>0.1), the effect between genotypes was borderline significant for female mice (P=0.056), with DBI−/− females showing a trend towards increased freezing.
Figure 5: DBI−/− mice lack sex effect seen in DBI+/+ mice in contextual fear conditioning.
a, Mean ± SEM of percentage time that shocked DBI+/+ (black bars) and DBI−/− mice (open bars) spent freezing on both days of the fear conditioning task. Open circles represent individual data values. b, Percentage time that unshocked DBI+/+ and DBI−/− mice spent freezing on both days of the task. c, Distance traveled by the shocked mice on both days of the fear conditioning task. d, Distance traveled by the unshocked mice on both days of the task. * Significant difference between DBI+/+ and DBI−/− mice (P < 0.05); # Significant difference between sexes within respective genotype (P < 0.05).
It is conceivable that DBI−/− mice may display altered baseline freezing behavior compared with DBI+/+ mice. To investigate this possibility, we tested three additional mice of each genotype and sex combination that did not receive any footshocks on either day. A three-way repeated measures ANOVA of the data from unshocked controls showed no main effects of day, genotype, sex, or any interactions (Figure 5B), indicating that the phenotypic differences seen in the shocked mice on day 2 were not due to a characteristic disparity in baseline freezing behavior.
For the total distance traveled in the chamber, an overall three-way repeated measures ANOVA of the data from shocked mice revealed a significant effect of day (F1,29=322.6, P<0.0001), a significant genotype-by-sex interaction (F1,30=7.0, P=0.013), and a significant dayby-genotype interaction (F1,30=6.5, P=0.016) (Figure 5C). Therefore, the data were analyzed separately for each day. Two-way ANOVA of distance traveled on day 1 did not yield a main effect of sex (F1,29=3.17, P=0.09) nor a sex-by-genotype interaction (F1,29=1.4, P=0.24), but there was a significant main effect of genotype (F1,29=8.8, P=0.006), in which DBI−/− mice traveled a shorter distance than did DBI+/+ mice. On day 2, two-way ANOVA found no main effects of sex (F1,29=0.51, P=0.48) nor genotype (F1,29=0.68, P>0.42), but there was a significant sex-bygenotype interaction (F1,29=7.5, P=0.01). A Fisher’s LSD post hoc test demonstrated greater distance traveled by females than males in the DBI+/+ genotype (P<0.04). However, although the sex difference observed in DBI+/+ mice was lost in DBI−/− mice, it was nearly reversed (Fisher’s LSD: P=0.054), with DBI−/− females showing a trend towards shorter distances traveled compared to males. In addition, within males, the DBI−/− mice traveled a greater distance than DBI+/+ mice (P<0.02), but no differences between genotypes were observed for females, and the trend was in the opposite direction. The data for distance traveled by the shocked mice on both days support the effects seen in freezing behavior. For the unshocked controls, an overall three-way repeated measures ANOVA of distance traveled showed no main effects of day, genotype, sex, or any interactions (Figure 5D), suggesting that DBI−/− mice do not display inherent locomotor impairment. Overall, these results indicate a potential role for DBI in regulating a sexually dimorphic circuit mediating contextual fear conditioning.
4. Discussion
In these studies, we sought to determine the effects of genetic loss of DBI signaling in mice on performance in multiple hippocampus-dependent tasks of spatial and contextual learning and memory. Our data show that the loss of DBI plays a selective role in hippocampus-dependent behaviors, modulating performance in a task-specific manner. We found no significant differences between DBI+/+ and DBI−/− mice in the DI of OLM, although DBI−/− mice showed lower degrees of total object exploration. In the Barnes maze task, we found that DBI−/− mice showed inferior learning of spatial reference cues necessary for the memory of the escape hole location. Furthermore, our results indicate that DBI−/− mice did not simply fail to associate entering the escape box with completion of the trial, as analysis of search strategy demonstrated that these mice showed lower rates of relying on extra-maze cues to locate the escape box. Analysis of the contextual fear conditioning task revealed an intriguing sex-by-genotype interaction on day 2, with a sex effect existing in DBI+/+ mice that was not observed in DBI−/− mice. These results potentially suggest a role for DBI in maintaining and modulating sexually dimorphic fear behavior in mice. Taken together, these results provide evidence of a role for DBI in modulating various hippocampus-dependent assays of learning and memory. These effects are task-specific, showing genotype and sex-dependent deficiencies in some, but not all, behavioral paradigms.
The hippocampus has long been associated with multiple forms of spatial knowledge (O’Keefe and Nadel, 1978; Gray and McNaughton, 1983). Several assays of spatial memory in rodents have been invented with varying degrees of complexity and differential requirements of hippocampal engagement. Each of the paradigms examined in this study require the animal to use the learned information in a distinctive way. For example, spatial relational memory is a hippocampus-demanding form of spatial knowledge that relies on the animal to encode geometric relationships of intra- and extra-maze cues, and to apply this knowledge to locate the target from multiple viewpoints (Eichenbaum, 2000). With a specific goal in place, tasks such as the Barnes maze and the Morris water maze force the animal to use various strategies employing the given cues to solve the maze. Conversely, tasks such as OLM and contextual fear conditioning rely on the subject forming a cognitive map of the environment by investigating cues and forming associations. These representations do not necessarily require a flexible use of the learned information regarding the setting (Sutherland and Rudy, 1989; Moses and Ryan, 2006). Furthermore, tasks involving detection of novelty, such as OLM, are fairly simple and useful in investigating spatial cognitive mapping rather than spatial reference memory (Sharma et al., 2010). In addition, multiple studies have implicated the basolateral amygdala and the retrosplenial cortex in influencing contextual fear conditioning (Phillips and LeDoux, 1992; Sparta et al., 2014), and inactivation of the dorsal hippocampus was shown to lead to only a ~35% impairment in contextual fear conditioning (Desmedt et al., 2003), suggesting that other brain regions play critical roles influencing this task. Therefore, it is reasonable to expect that impairments in some hippocampusdependent tasks do not necessarily correlate to deficits in others.
Recent evidence supports a role for DBI in the regulation of neurogenesis in the dentate gyrus of the hippocampus (Dumitru et al., 2017). Specifically, DBI appears to control the balance between preserving the neural stem cell population and facilitating development of new granule cells. Importantly, disruption of neurogenesis in the dentate gyrus selectively impairs performance in the Morris water maze. This effect, however, was not seen in either contextual fear conditioning or tasks involving identification of a novel environment (Drapeau et al., 2003; Dupret et al., 2008). Therefore, it is possible that the lack of DBI signaling may disrupt hippocampal neurogenesis, thus specifically impacting behavioral tasks that require spatial reference memory over other neurogenesis-independent forms of memory. Our results, demonstrating a specific impairment in mice lacking DBI on performance in the Barnes maze, but not in OLM nor contextual fear conditioning, support this conclusion. In addition, it is noteworthy that the impairment of spatial memory seen here upon genetic removal of DBI signaling is similar to the effect seen previously with transgenic overexpression of DBI (Siiskonen et al., 2007). These results suggest that DBI levels may need to be strictly regulated to ensure proper neural function, and that a disruption of this balance in either direction can lead to cognitive deficits, particularly in spatial memory. Further work is needed to uncover the complex role of DBI in the hippocampus, with a potential focus on how learning and memory may depend on the regulation of hippocampal DBI levels and its subsequent impact on neurogenesis.
We recently reported that DBI−/− mice lack the typical sex difference in social interest seen in DBI+/+ mice, in which males display higher levels of social interest than females, suggesting that DBI may play a role in developing and/or maintaining sexually dimorphic circuits underlying social behavior (Ujjainwala et al., 2018). In the present studies, DBI+/+ male mice exhibited greater freezing levels compared with females in contextual fear conditioning, and this sex effect was diminished and nearly reversed in DBI−/− mice. The current study thus provides further evidence that DBI may play a role in mediating behaviors that show established sex differences. These results may be explained by, for example, DBI impacting the formation and maintenance of critical sexually dimorphic circuits and/or the sex-specific production of certain GABAAR-modulating neurosteroids. In the latter regard, DBI can potentially upregulate steroid hormone biosynthesis through actions at the mitochondrial benzodiazepine receptor TSPO (Papadopoulos et al., 1991; Korneyev et al., 1993), although the role of TSPO in this process has recently come into question (Morohaku et al., 2014). Furthermore, within the hippocampus, testosterone and/or its metabolites generated by 5α-reduction, dihydrotestosterone and 3α-androstanediol, can improve performance in spatial tasks and contextual fear conditioning in rats and modulate hippocampal synaptic spine density (Frye and Lacey, 2001; Isgor and Sengelaub, 2003; Leranth et al., 2003; Edinger et al., 2004). Dbi mRNA expression in at least some areas of the mouse brain is androgen-sensitive (Compère et al., 2006), and seminal vesicle weights, a proxy of circulating testosterone levels, are not different in DBI−/− male mice (Ujjainwala et al., 2018). Therefore, it is also possible that some of the observed effects of genetic loss of DBI represent impaired mediation of certain effects of testosterone in the hippocampus and associated brain structures.
The OLM task relies on the testing groups displaying similar levels of interaction with the objects on both days of the test. In our studies, DBI+/+ and DBI−/− mice did not show similar levels of investigation on either day 1 or day 2. Therefore, it is difficult to ascertain whether the lack of a genotype effect in the DI is a secondary consequence of differences in object interest. It may be useful in future studies to utilize a behavioral assay that does not depend on innate object interest. The reduced levels of interest of DBI−/− mice in the OLM task recapitulate findings we recently reported in an odor discrimination task (Ujjainwala et al., 2018), in which DBI−/− mice could discriminate between social and non-social odors, but showed reduced investigation of cotton swabs containing only water. The reduced level of investigation displayed in these behavioral tests could potentially reflect an overall disinterest in various novel tasks and objects. Mouse models of anhedonia have been shown to exhibit decreased interest across multiple parameters (Nestler and Hyman, 2010; Dedic et al., 2011), and it is possible that the genetic lack of DBI modulates pathways or substrates associated with anhedonia. Interestingly, although a sex difference was present for the percentage of time interacting with the objects during the OLM task, this effect appears to be primarily driven by the low interaction time of the DBI−/− females rather than DBI+/+ females. DBI+/+ females do not differ from DBI+/+ males, and display higher interaction times than DBI−/− males, suggesting that the lack of DBI signaling is likely a more critical factor than sex in overall investigation levels in the OLM task.
Several potential alternative explanations for our results exist. Although extensive backcrossing onto the original C57BL/6BomTac background was performed when this knockout line was first developed (Neess et al., 2011), it is possible that genetic material linked to the knockout region may still exist in DBI−/− mice. However, since heterozygote breeding was utilized during backcrossing onto the C57BL/6J strain in our colony, it is unlikely that the behavioral differences seen between DBI+/+ and DBI−/− mice are due to off-target genetic variations related to the creation of the knockout. It is also possible that the results seen in these tasks may be explained by impaired visual acuity or locomotor impairment in DBI−/− mice. As mentioned previously, DBI−/− mice display difficulty swimming and have demonstrated the inability to consistently complete four consecutive daily trials in the Morris water maze. It should be noted that DBI−/− mice are phenotypically distinguishable by varying degrees of alopecia and distinctly oily fur (Bloksgaard et al., 2012), which may be debilitating to swimming ability, for example by decreasing the ability of the mice to regulate body temperature in an aquatic environment. In previous studies, DBI−/− mice did not display large degrees of locomotor impairment on a rotarod task (Ujjainwala et al., 2018) or in open field tests (Budry et al., 2016), and in the present studies, DBI−/− mice traveled the same distance as DBI+/+ mice did on day 1 of the Barnes maze task, and greater distances on subsequent days. In addition, the overall distance traveled by the unshocked control DBI−/− mice in the contextual fear conditioning task was not statistically different from the values of the unshocked DBI+/+ mice (see Figure 5D). Therefore, these mice do not appear to have a gross locomotor impairment, and it appears more likely that the swimming impairment is related to the distinct skin condition. However, the visual acuity of DBI−/− mice compared to their DBI+/+ counterparts remains untested and may be a target of future experimentation. In the contextual fear conditioning task, in which each mouse received shocks of the same intensity, one possible explanation for the differences seen between groups could be attributed to varying degrees of shock sensitivity. Although we did not test whether DBI−/− mice show altered shock sensitivity, it is a variable to consider when evaluating data in fear conditioning assays. In addition, it should be noted that the unshocked control mice were tested as a separate cohort, and these animals did not previously undergo the OLM or Barnes maze tasks. Although the unshocked mice performed as expected and clearly displayed phenotypic differences compared to the shocked mice, the cohort difference should be considered when making direct comparisons between the two groups.
In summary, the goal of these studies was to determine the effects of genetic loss of DBI in mice on performance in multiple hippocampus-dependent behavioral tasks of spatial memory correlates. Our data indicate that DBI−/− mice show an impairment in Barnes maze learning, but perform similarly to DBI+/+ mice in OLM, suggesting a role for DBI in specific forms of spatial navigation memory. In addition, we observed that the loss of DBI appears to negate an established sex difference in contextual fear conditioning response, providing further evidence of a potential role of DBI in mediating sex differences in certain behaviors. Overall, this work provides novel evidence that DBI is a modulator of hippocampus-dependent learning and memory, and supports further investigation into the roles that DBI plays in cognition.
Significance Statement.
The diazepam binding inhibitor (DBI) peptide is implicated in both normal and pathological functions in the central nervous system. In the hippocampus, DBI modulates GABAAreceptor mediated transmission and may thus be involved in shaping hippocampus-dependent behavioral tasks. However, the impacts of the genetic loss of DBI signaling on hippocampal learning and memory in mice have not been investigated. In these studies, we demonstrate that DBI knockout mice display task-specific impairments in different assays of spatial and contextual learning and memory. These data offer further support for a critical role of DBI in proper learning and memory functions.
Acknowledgements:
This work was supported by the Brain and Behavior Research Foundation (NARSAD Young Investigator Grant 24086, C.A.C.). The National Institute of Neurological Disorders and Stroke also supports C.A.C. through grants R01 NS105825 and R03 NS103029, and supports J.S.R. through grant R21 NS104293. We thank Scott Baker and Jared Bear for making the custombuilt Barnes maze apparatus, and Shivang Chaudhary for assisting with these experiments.
Footnotes
Conflict of Interest Statement:
The authors declare no competing interests.
References:
- Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, Monyer H (2012) Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell 10:76–87. [DOI] [PubMed] [Google Scholar]
- Ball JA, Ghatei MA, Sekiya K, Krausz T, Bloom SR (1989) Diazepam binding inhibitor-like immunoreactivity (51–70): distribution in human brain, spinal cord and peripheral tissues. Brain Res 479:300–305. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Costa E, Ferrero P, Guidotti A, Roy A, Sunderland T, Pickar D, Paul SM, Goodwin FK (1986) Diazepam-binding inhibitor: a brain neuropeptide present in human spinal fluid: studies in depression, schizophrenia, and Alzheimer’s disease. Arch Gen Psychiatry 43:1143–1147. [DOI] [PubMed] [Google Scholar]
- Barnes CA (1979) Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93(1):74–104. [DOI] [PubMed] [Google Scholar]
- Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, Lynch G, Greene RW, Wood MA (2011) Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology 36:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-tosample learning task to study ‘recognition memory’. Nat Protoc 1(3):1306–1311. [DOI] [PubMed] [Google Scholar]
- Bloksgaard M, Bek S, Marcher A-B, Neess D, Brewer J, Hannibal-Bach HK, Helledie T, Fenger C, Due M, Berzina Z (2012) The acyl-CoA binding protein is required for normal epidermal barrier function in mice. J. Lipid Res 53:2162–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J (1991) Electrophysiological characterization of diazepam binding inhibitor (DBI) on GABAA receptors. Neuropharmacology 30:1387–1389. [DOI] [PubMed] [Google Scholar]
- Bouyakdan K, Taib B, Budry L, Zhao S, Rodaros D, Neess D, Mandrup S, Faergeman NJ, Alquier T (2015) A novel role for central ACBP/DBI as a regulator of long-chain fatty acid metabolism in astrocytes. J Neurochem 133:253–265. [DOI] [PubMed] [Google Scholar]
- Budry L, Bouyakdan K, Tobin S, Rodaros D, Marcher A-B, Mandrup S, Fulton S, Alquier T (2016) DBI/ACBP loss-of-function does not affect anxiety-like behaviour but reduces anxiolytic responses to diazepam in mice. Behav Brain Res 313:201–207. [DOI] [PubMed] [Google Scholar]
- Bui AD, Nguyen TM, Limouse C, Kim HK, Szabo GG, Felong S, Maroso M, Soltesz I (2018) Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science 359:787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J (2002) The human hippocampus and spatial and episodic memory. Neuron 35:625–641. [DOI] [PubMed] [Google Scholar]
- Christian CA, Huguenard JR (2013) Astrocytes potentiate GABAergic transmission in the thalamic reticular nucleus via endozepine signaling. Proc Natl Acad Sci USA 110:2027820283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Herbert AG, Holt RL, Peng K, Sherwood KD, Pangratz-Fuehrer S, Rudolph U, Huguenard JR (2013) Endogenous positive allosteric modulation of GABAA receptors by diazepam binding inhibitor. Neuron 78:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS (2008) Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 155:1048–1058. [DOI] [PubMed] [Google Scholar]
- Collinson N, Atack J, Laughton P, Dawson G, Stephens D (2006) An inverse agonist selective for α5 subunit-containing GABA A receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology 188:619–628. [DOI] [PubMed] [Google Scholar]
- Compère V, Ouellet J, Luu-The V, Dureuil B, Tonon M, Vaudry H, Labrie F, Pelletier G (2006) Role of androgens and glucocorticoids in the regulation of diazepam-binding inhibitor mRNA levels in male mouse hypothalamus. Brain Res 1119:50–57. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A (1991) Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci 49:325–344. [DOI] [PubMed] [Google Scholar]
- Courtney CD, Christian CA (2018) Subregion-Specific Impacts of Genetic Loss of Diazepam Binding Inhibitor on Synaptic Inhibition in the Murine Hippocampus. Neuroscience 388:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U (2002) Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA 99:8980–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P, Rustay NR, Browman KE (2009) Cued and contextual fear conditioning for rodents In: Methods of Behavior Analysis in Neuroscience, 2nd Edition pp 19–37. Boca Raton, FL: CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Dedic N, Walser SM, Deussing JM (2011) Mouse models of depression In: Psychiatric Disorders-Trends and Developments, pp 185–222. London, United Kingdom: IntechOpen. [Google Scholar]
- Desmedt A, Marighetto A, Garcia R, Jaffard R (2003) The effects of ibotenic hippocampal lesions on discriminative fear conditioning to context in mice: impairment or facilitation depending on the associative value of a phasic explicit cue. Eur J Neurosci 17:1953–1963. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza P-V, Abrous DN (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA 100:14385–14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru I, Neitz A, Alfonso J, Monyer H (2017) Diazepam binding inhibitor promotes stem cell expansion controlling environment-dependent neurogenesis. Neuron 94:125–137. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest J-M, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV (2008) Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE 3(4):e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger KL, Lee B, Frye CA (2004) Mnemonic effects of testosterone and its 5α-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav 78:559–568. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2000) A cortical–hippocampal system for declarative memory. Nat Rev Neurosci 1(1):41–50. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H (2004) The hippocampus and memory for “what,” “where,” and “when”. Learn. Mem 11:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarese C, Appollonio I, Frigo M, Gaini S, Piolti R, Frattola L (1989) Benzodiazepine receptors and diazepam‐binding inhibitor in human cerebral tumors. Ann Neurol 26:564–568. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH (2001) Posttraining androgens’ enhancement of cognitive performance is temporally distinct from androgens’ increases in affective behavior. Cogn Affect Behav Neurosci 1:172–182. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N (1983) Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev 7:119–188. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Forchetti CM, Corda MG, Konkel D, Bennett CD, Costa E (1983) Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci USA 80:3531–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA (2011) HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem 18:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison F, Hosseini A, McDonald M (2009) Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res 198:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP (2006) Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem 13:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan JB, Hodges DB, Lelas S, Gilligan PJ, McElroy JF, Lindner MD (2005) Effects of CRF 1 receptor antagonists and benzodiazepines in the Morris water maze and delayed nonmatching to position tests. Psychopharmacology 178:410–419. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wrenn C, Harris A, Thayer K, Crawley J (2002) Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav 1:55–69. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR (2003) Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. Dev. Neurobiol 55:179–190. [DOI] [PubMed] [Google Scholar]
- Joksimović S, Divljaković J, Van Linn ML, Varagic Z, Brajković G, Milinković MM, Yin W, Timić T, Sieghart W, Cook JM (2013) Benzodiazepine-induced spatial learning deficits in rats are regulated by the degree of modulation of α1 GABAA receptors. Eur Neuropsychopharmacol 23:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant GJ, Wylie RM, Vasilakis AA, Ghosh S (1996) Effects of triazolam and diazepam on learning and memory as assessed using a water maze. Pharmacol Biochem Behav 53:317–322. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Faizi M, Jacobs LF, Htun K, Shamloo M, Mobley WC (2012) Deficits in cognition and synaptic plasticity in a mouse model of Down syndrome ameliorated by GABAB receptor antagonists. J Neurosci 32:9217–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Clark PJ, DeYoung EK, Bhattacharya TK, Venghaus CE, Rhodes JS (2012) Voluntary wheel running enhances contextual but not trace fear conditioning. Behav Brain Res 226:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneyev A, Pan B, Polo A, Romeo E, Guidotti A, Costa E (1993) Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J. Neurochem 61:1515–1524. [DOI] [PubMed] [Google Scholar]
- Kudo K, Qiao C-X, Kanba S, Arita J (2004) A selective increase in phosphorylation of cyclic AMP response element-binding protein in hippocampal CA1 region of male, but not female, rats following contextual fear and passive avoidance conditioning. Brain Res 1024:233–243. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ (2003) Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci 23:1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kim JS, Abejuela VA, Lamano JB, Klein NJ, Christian CA (2017) Disrupted female estrous cyclicity in the intrahippocampal kainic acid mouse model of temporal lobe epilepsy. Epilepsia Open 2:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup S, Hummel R, Ravn S, Jensen G, Andreasen PH, Gregersen N, Knudsen J, Kristiansen K (1992) Acyl-CoA-binding protein/diazepam-binding inhibitor gene and pseudogenes. A typical housekeeping gene family. J Mol Biol 228:1011–1022. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS (1994) Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 661:25–34. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA (2011) HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, Selvaraj V (2014) Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 155:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SN, Ryan JD (2006) A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus 16:43–65. [DOI] [PubMed] [Google Scholar]
- Neess D, Bek S, Engelsby H, Gallego SF, Faergeman NJ (2015) Long-chain acyl-CoA esters in metabolism and signaling: Role of acyl-CoA binding proteins. Prog Lipid Res. 59:1–25. [DOI] [PubMed] [Google Scholar]
- Neess D, Bloksgaard M, Bek S, Marcher AB, Elle IC, Helledie T, Due M, Pagmantidis V, Finsen B, Wilbertz J, Kruhoffer M, Faergeman N, Mandrup S (2011) Disruption of the acylCoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. J Biol Chem 286:3460–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L (1978) The hippocampus as a cognitive map: Oxford: Clarendon Press. [Google Scholar]
- Papadopoulos V, Berkovich A, Krueger K, Costa E, Guidotti A (1991) Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology 129:1481–1488. [DOI] [PubMed] [Google Scholar]
- Patil SS, Sunyer B, Höger H, Lubec G (2009) Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res 198:58–68. [DOI] [PubMed] [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW (1994) DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci 108(4):810–817. [DOI] [PubMed] [Google Scholar]
- Phillips R, LeDoux J (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106(2):274–285. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA (2010) Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci 30:5037–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Ferguson SA (2014) Barnes maze testing strategies with small and large rodent models. J Vis Exp February 26;(84):e51194. doi: 10.3791/51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, McKhann G, Guarneri P, Barbaccia ML, Guidotti A, Costa E (1989) Cerebrospinal fluid content of diazepam binding inhibitor in chronic hepatic encephalopathy. Ann Neurol 26:57–62. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Rakoczy S, Brown-Borg H (2010) Assessment of spatial memory in mice. Life Sci 87:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrin T, Blank T, Saravana R, Rayner M, Spiess J, Todorovic C (2009) Region specific gene expression profile in mouse brain after chronic corticotropin releasing factor receptor 1 activation: the novel role for diazepam binding inhibitor in contextual fear conditioning. Neuroscience 162:14–22. [DOI] [PubMed] [Google Scholar]
- Siiskonen H, Oikari S, Korhonen V-P, Pitkänen A, Voikar V, Kettunen M, Hakumäki J, Wahlfors T, Pussinen R, Penttonen M (2007) Diazepam binding inhibitor overexpression in mice causes hydrocephalus, decreases plasticity in excitatory synapses and impairs hippocampus-dependent learning. Mol Cell Neurosci 34:199–208. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Smithuis J, Stamatakis AM, Jennings JH, Kantak PA, Ung RL, Stuber GD (2014) Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Front Behav Neurosci 8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Rudy JW (1989) Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology 17:129–144. [Google Scholar]
- Timić T, Joksimović S, Milić M, Divljaković J, Batinić B, Savić MM (2013) Midazolam impairs acquisition and retrieval, but not consolidation of reference memory in the Morris water maze. Behav Brain Res 241:198–205. [DOI] [PubMed] [Google Scholar]
- Ujjainwala AL, Courtney CD, Rhoads SG, Rhodes JS, Christian CA (2018) Genetic loss of diazepam binding inhibitor in mice impairs social interest. Genes Brain Behav 17:e12442. [DOI] [PubMed] [Google Scholar]
- Villasana L, Rosenberg J, Raber J (2010) Sex‐dependent effects of 56Fe irradiation on contextual fear conditioning in C57BL/6J mice. Hippocampus 20:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Matheos DP, Barrett RM, Kramár EA, Azzawi S, Chen Y, Magnan CN, Zeller M, Sylvain A, Haettig J (2013) The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat Neurosci 16(5):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel‐Ciernia A, Wood MA (2014) Examining object location and object recognition memory in mice. Curr Protoc Neurosci October 8;69:8.31.1–17. doi: 10.1002/0471142301.ns0831s69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Behne NS, Fanselow MS (2001) Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav Neurosci 115(1):26–32. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE (2000) Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci 20:451463. [DOI] [PMC free article] [PubMed] [Google Scholar]