Abstract
BACKGROUND
Reported ventricular assist device (VAD) experience in the pediatric congenital heart disease (CHD) population is limited. We sought to describe contemporary use and outcomes of VADs in children with CHD and compare outcomes to children without CHD.
METHODS
Patients enrolled in Pedimacs between September 19, 2012 through June 30, 2017 were included. CHD was classified as biventricular vs single ventricle (stage 1, 2 or 3). Outcomes were compared between groups and multivariable analysis was used to identify factors associated with mortality on device.
RESULTS
Among 471 patients enrolled, 108 (24%) had CHD (45 biventricular and 63 single ventricle). CHD patients were younger (5.7 years ± 5.7 vs 9.8 years ± 6.5; p<0.0001) and smaller (0.8 m2 ± 0.5 vs 1.2 m2 ± 0.7; p<0.0001) compared to non-CHD patients. CHD patients were more likely to receive a paracorporeal continuous VAD (36.1% vs 12.9%; p<0.0001) and less likely to receive an implantable continuous VAD (27.8% vs 55.0%; p<0.0001) compared to non-CHD patients. After six months on VAD, CHD patients had higher mortality (36.4% vs 12.1%) and lower percent transplanted (29.1% vs 59.9%) than non-CHD patients (p<0.0001). In multivariable analysis, CHD was the factor most strongly associated with mortality on VAD (HR 2.9, p<0.0001) while implantable continuous device and high volume center were protective (HR 0.3, p<0.0001; HR 0.6, p=0.02).
CONCLUSIONS
VAD use in children with CHD is associated with increased mortality and decreased transplant rates compared to children without CHD. For the subgroup of children with CHD who received implantable continuous flow VADs, survival rates were higher and comparable to children without CHD. Increased experience is correlated with better survival in pediatric VADs.
BACKGROUND
Congenital heart disease (CHD) is the most common etiology of heart failure in hospitalized children and accounts for approximately 40% of all pediatric heart transplants.1, 2 CHD is one of the strongest risk factors associated with waiting list mortality for children listed for heart transplant.3, 4 Despite the overall growth and progress made in supporting pediatric heart failure patients with ventricular assist devices (VADs), VAD use in CHD is limited and outcomes reported, thus far, have been suboptimal.5-8 There is no consensus as to whether, when and how VADs should be utilized in this complex and high-risk population.9 The specific aims of this study were to (1) describe the contemporary use, characteristics and outcomes of children with CHD implanted with VADs and (2) compare VAD outcomes of children with and without CHD.
METHODS
Pedimacs
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) is a national prospective database of >20,000 patients supported on devices.10 The Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs), the pediatric component of INTERMACS, began enrolling pediatric patients with pediatric-specific data elements on September 19, 2012. Pedimacs contains data on all devices used in pediatric patients (age <19 years at device implantation) and was collecting data from 45 centers at the time of this analysis. The registry is a collaboration between the Society of Thoracic Surgery (STS), National Heart, Lung, and Blood Institute (NHLBI), the Food and Drug Administration, the Centers for Medicare and Medicaid Services, industry and implanting centers.
Between September 19, 2012 and June 30, 2017, patients prospectively enrolled in Pedimacs provided the study cohort. Patients with prior heart transplant who underwent VAD implant for graft failure were excluded. Patients were enrolled at the time of their implantation and follow-up was collected at specified intervals. Patients were censored when they met a study endpoint defined as death, transplant, recovery, or cessation of support.
Definitions
Patients with CHD were identified by searching the following Pedimacs variables: “primary diagnosis”; “secondary diagnosis”; “previous cardiac operation”; “previous congenital cardiac surgery”; and “concomitant surgery.” CHD was classified as biventricular or single ventricle. Single ventricle patients were further grouped into stage 1 (e.g. unrepaired, banded, or shunted), stage 2 (e.g. status post superior cavopulmonary anastomosis, “Glenn”) or stage 3 (e.g. status post total cavopulmonary anastomosis, “Fontan”). For this study, VAD denotes a device implanted into the systemic ventricle (or adjacent atrium) regardless of the underlying morphology. RVAD denotes a device implanted into the subpulmonary ventricle (or right atrium). Each study patient was reviewed by pediatric cardiologists (DMP and SJK) to ensure that the diagnoses were accurate. If there were incongruities or missing data, the inputting center was contacted to clarify data. Previously reported Pedimacs adverse event definitions were used.11 With respect to center volume, “high volume” was defined as enrolling ≥15 patients and “low volume” was defined as enrolling <15 patients during the study period.
Statistical Analysis
Baseline characteristics for pediatric patients were presented as mean ± standard deviation or count (percent). Comparisons were made using chi-square test for categorical variables or Fisher’s exact test where appropriate and one-way ANOVA for continuous variables. Survival after device implantation among groups was compared using Kaplan-Meier survival analysis. The mutually exclusive patient outcomes of death, transplant, or alive on a device were analyzed using competing outcomes methods. Adverse event rates were calculated within 3 months (“early”) and post 3 months (“late”) after implant. Risk factors for death on device were examined using Cox proportional hazard. Covariables for the multivariable analysis were chosen a priori by the authors based on clinical experience and they included: age, gender, race, body surface area, patient profile, device classification, device strategy, albumin, bilirubin, sodium, blood urea nitrogen, creatinine, estimated glomerular filtration rate (eGFR), white blood cell count, platelet count, CHD, single ventricle CHD, any previous ECMO, ECMO during implant hospitalization, pulmonary disease, history of malnutrition, and high volume center. Data were analyzed using statistical software SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC). All statistical tests were two-sided and a p-value <0.05 was considered statistically significant.
RESULTS
Study population and contemporary practice
Among the 471 patients enrolled in Pedimacs during the study period, 21 were excluded with graft failure. In the remaining 450 patients, 108 had CHD and 342 did not have CHD. Of the 108 CHD patients, 45 had biventricular CHD and 63 had single ventricle CHD. There were 23 stage 1 patients, 21 stage 2 patients, and 19 stage 3 patients.
Compared with non-CHD patients at the time of VAD implant, CHD patients were younger (5.7 years ± 5.7 vs 9.8 years ± 6.5; p<0.0001), more likely to be male (65.7% vs 54.7%; p=0.04), smaller (0.8 m2 ± 0.5 vs 1.2 m2 ± 0.7; p<0.0001) and more likely to have had previous cardiac operation (93.5% vs 25.7%; p<0.0001). CHD patients had higher hemoglobin (12.8 g/dL ± 2.1 vs 11.4 g/dL ± 1.9; p<0.0001), bilirubin (2.9 mg/dL ± 4.9 vs 1.5 ± 2.0; p<0.0001), sodium (139.7 mEq/L ± 7.8 vs 137.0 mEq/L ± 6.1; p=0.0002) and INR (1.6 ± 0.9 vs 1.4 ± 0.5; p=0.0008). Of note, CHD and non-CHD patients had similar renal function, prior mechanical circulatory support use, INTERMACS patient profile and device strategy (bridge to transplant, bridge to candidacy, destination therapy, etc.) CHD patients were more likely to receive a paracorporeal continuous device (36.1% vs 12.9%; p<0.0001) and less likely to receive an implantable continuous device (27.8% vs 55.0%; p<0.0001) compared to non-CHD patients. Over the last several years, fewer paracorporeal pulsatile devices and more implantable continuous devices have been used in CHD patients (Supplemental Table 1). Complete pre-implant characteristics for CHD and non-CHD patients are shown in Table 1a.
Table 1a:
Patient Characteristics for CHD vs. Non-CHD Patients (n=450). Pedimacs Patients, September 19, 2012 to June 30, 2017
| Baseline Characteristics | CHD Patients (n=108) |
non-CHD Patients (n=342) |
p-value |
|---|---|---|---|
| Age (y) | 5.7 +/− 5.7 (n= 108) | 9.8 +/− 6.5 (n= 342) | <.0001 |
| Age (y) | <.0001 | ||
| < 1 | 35 (32.4) | 55 (16.1) | |
| 1-5 | 31 (28.7) | 65 (19.0) | |
| 6-10 | 20 (18.5) | 44 (12.9) | |
| 11-19 | 22 (20.4) | 178 (52.0) | |
| Female | 37 (34.3) | 155 (45.3) | 0.04 |
| Race | 0.9 | ||
| White | 63 (58.3) | 203 (59.4) | |
| African American | 22 (20.4) | 73 (21.3) | |
| Other | 23 (21.3) | 66 (19.3) | |
| Body Surface Area (m2) | 0.8 +/− 0.5 (n= 106) | 1.2 +/− 0.7 (n= 334) | <.0001 |
| Blood Urea Nitrogen (mg/dL) | 28.6 +/− 17.8 (n= 108) | 25.1 +/− 16.4 (n= 342) | 0.06 |
| Sodium (mEq/L) | 139.7 +/− 7.8 (n= 108) | 137.0 +/− 6.1 (n= 342) | 0.0002 |
| Potassium (mEq/L) | 3.8 +/− 0.7 (n= 108) | 3.8 +/− 0.6 (n= 342) | 1.0 |
| Aspartate Aminotransferase (u/L) | 214.7 +/− 1012.3 (n= 101) | 223.8 +/− 845.7 (n= 334) | 0.9 |
| Alanine Aminotransferase (u/L) | 183.2 +/− 897.2 (n= 101) | 203.5 +/− 618.4 (n= 336) | 0.8 |
| Brain Natriuretic Peptide (pg/mL) | 1998.3 +/− 1722.2 (n= 44) | 2121.5 +/− 1672.0 (n= 162) | 0.7 |
| Pro Brain Natriuretic Peptide (pg/mL) | 12783 +/− 13909 (n= 19) | 12996 +/− 11596 (n= 95) | 0.9 |
| Albumin (g/dL) | 3.4 +/− 0.8 (n= 104) | 3.4 +/− 0.7 (n= 334) | 1.0 |
| Pre-Albumin (mg/L) | 163.8 +/− 83.8 (n= 27) | 182.8 +/− 71.5 (n= 116) | 0.2 |
| White Blood Cell Count (x103/μL) | 12.0 +/− 6.6 (n= 107) | 11.1 +/− 4.8 (n= 341) | 0.1 |
| Hemoglobin (g/L) | 127.8 +/− 21.2 (n= 108) | 113.5 +/− 19.3 (n= 341) | <.0001 |
| Platelet Count (x103/μL) | 199.8 +/− 115.2 (n= 107) | 229.4 +/− 113.7 (n= 338) | 0.02 |
| INR (international units) | 1.6 +/− 0.9 (n= 96) | 1.4 +/− 0.5 (n= 312) | 0.0008 |
| Uric Acid (mg/dL) | 7.4 +/− 4.0 (n= 20) | 7.8 +/− 3.2 (n= 98) | 0.6 |
| Lymphocyte Count (%) | 17.1 +/− 12.5 (n= 79) | 23.7 +/− 14.0 (n= 253) | 0.0002 |
| Creatinine (mg/dL) | 0.6 +/− 0.4 (n= 108) | 0.8 +/− 0.5 (n= 341) | 0.009 |
| eGFR (mL/min/1.73m2) | 83.9 +/− 39.6 (n= 106) | 87.5 +/− 48.0 (n= 336) | 0.5 |
| Bilirubin (mg/dL) | 2.9 +/− 4.9 (n= 95) | 1.5 +/− 2.0 (n= 304) | <.0001 |
| Previous Cardiac Operation | 101 (93.5) | 88 (25.7) | <.0001 |
| Previous ECMO | 22 (20.4) | 47 (13.7) | 0.1 |
| Previous MCSD | 4 (3.7) | 16 (4.7) | 0.7 |
| Patient Profile | 0.2 | ||
| 1. Critical Cardiogenic Shock | 23 (37.1) | 17 (38.6) | |
| 2. Progressive Decline | 33 (53.2) | 19 (43.2) | |
| 3. Stable but Inotrope Dependent | 6 (9.7) | 5 (11.4) | |
| 4.−7. Resting Symptoms or Less Sick | 3 (6.8) | ||
| Pre-Implant Device Strategy | / | 0. 3 | |
| Bridge to Transplant - Listed | 37 (58.7) | 21 (46.7) | |
| Bridge to Candidacy | 15 (23.8) | 18 (40.0) | |
| Destination Therapy | 1 (1.6) | ||
| Bridge to Recovery | 6 (9.5) | 5 (11.1) | |
| Other | 4 (6.3) | 1 (2.2) | |
| Device Classification | <.0001 | ||
| Implantable Continuous | 30 (27.8) | 188 (55.0) | |
| Paracorporeal Continuous | 39 (36.1) | 44 (12.9) | |
| Paracorporeal Pulsatile | 30 (27.8) | 93 (27.2) | |
| Percutaneous | 7 (6.5) | 14 (4.1) | |
| TAH | 2 (1.9) | 3 (0.9) | |
| Pre-Implant Device Type | 0.0004 | ||
| LVAD | 95 (88.0) | 290 (84.8) | |
| BiVAD | 11 (10.2) | 48 (14.0) | |
| RVAD | 1 (0.3) | ||
| TAH | 2 (1.9) | 3 (0.9) |
eGFR = Estimated Glomerular Filtration Rate; ECMO = Extracorporeal Membrane Oxygenation; MCSD = Mechanical Circulatory Support Device; TAH = Total Artificial Heart; LVAD = Left Ventricular Assist Device; RVAD = Right Ventricular Assist Device; BiVAD = Biventricular Assist Device
Within the CHD group, single ventricle patients were younger (3.8 years ± 4.6 vs 8.4 years ± 6.2; p<0.0001), were smaller (0.6 m2 ± 0.4 vs 1.0 m2 ± 0.6; p<0.0001), had higher hemoglobin (13.6 g/dL ± 1.9 vs 11.6 g/dL ± 1.9; p<0.0001), and had worse renal function (74.7 mL/min/1.73m2 ± 34.9 vs 96.4 mL/min/1.73m2 ± 42.5; p=0.005) compared with biventricular CHD patients at baseline. Single ventricle patients received more paracorporeal continuous devices (50.8% vs 15.6%; p=0.003) and fewer implantable continuous devices (20.6% vs 37.8%; p=0.003) than the biventricular group. Pre-implant characteristics divided by single vs biventricular CHD are listed in Table 1b. Among single ventricle patients, stage 1 patients were most likely to have had previous ECMO (30.4% vs 14.3% vs 0%; p=0.03) and be in critical cardiogenic shock (59.1% vs 28.6% vs 21.1%; p=0.01) compared with stage 2 and 3 patients. There were no statistically significant differences in device strategy across the CHD patient subgroups. Table 1c details the pre-implant characteristics of single ventricle patients divided by palliative stage. There were 35 CHD patients <1 year old and 26 of them had single ventricles. Their baseline characteristics are summarized in Table 1d.
Table 1b:
Patient Characteristics for Single Ventricle vs. Biventricular CHD Patients (n=108). Pedimacs Patients, September 19, 2012 to June 30, 2017
| Baseline Characteristics | Single Ventricle CHD (n=63) |
Biventricular CHD (n=45) |
p-value |
|---|---|---|---|
| Age (y) | 3.8 +/− 4.6 (n= 63) | 8.4 +/− 6.2 (n= 45) | <.0001 |
| Age (y) | 0.0005 | ||
| < 1 | 26 (41.3) | 9 (20.0) | |
| 1-5 | 22 (34.9) | 9 (20.0) | |
| 6-10 | 10 (15.9) | 10 (22.2) | |
| 11-19 | 5 (7.9) | 17 (37.8) | |
| Female | 24 (38.1) | 13 (28.9) | 0.3 |
| Race | 0.3 | ||
| White | 33 (52.4) | 30 (66.7) | |
| African American | 15 (23.8) | 7 (15.6) | |
| Other | 15 (23.8) | 8 (17.8) | |
| Body Surface Area (m2) | 0.6 +/− 0.4 (n= 61) | 1.0 +/− 0.6 (n= 45) | <.0001 |
| Blood Urea Nitrogen (mg/dL) | 32.1 +/− 19.1 (n= 63) | 23.9 +/− 14.8 (n= 45) | 0.02 |
| Sodium (mEq/L) | 139.1 +/− 7.9 (n= 63) | 140.5 +/− 7.7 (n= 45) | 0.4 |
| Potassium (mEq/L) | 3.8 +/− 0.7 (n= 63) | 3.8 +/− 0.7 (n= 45) | 0.5 |
| Aspartate Aminotransferase (u/L) | 123.4 +/− 275.3 (n= 57) | 332.9 +/− 1503.1 (n= 44) | 0.3 |
| Alanine Aminotransferase (u/L) | 87.2 +/− 259.9 (n= 57) | 307.6 +/− 1325.1 (n= 44) | 0.2 |
| Brain Natriuretic Peptide (pg/mL) | 2051.2 +/− 1668.3 (n= 27) | 1914.2 +/− 1853.6 (n= 17) | 0.8 |
| Pro Brain Natriuretic Peptide (pg/mL) | 13389 +/− 12327 (n= 9) | 12237 +/− 15848 (n= 10) | 0.9 |
| Albumin (g/dL) | 3.3 +/− 0.9 (n= 61) | 3.5 +/− 0.7 (n= 43) | 0.2 |
| Pre-Albumin (mg/L) | 153.0 +/− 54.9 (n= 14) | 175.5 +/− 108.0 (n= 13) | 0.5 |
| White Blood Cell Count (x103/μL) | 11.3 +/− 3.5 (n= 62) | 13.0 +/− 9.3 (n= 45) | 0.2 |
| Hemoglobin (g/L) | 136.0 +/− 18.7 (n= 63) | 116.4 +/− 19.3 (n= 45) | <.0001 |
| Platelet Count (x103/gL) | 218.4 +/− 126.8 (n= 62) | 174.1 +/− 92.1 (n= 45) | 0.05 |
| INR (international units) | 1.9 +/− 1.1 (n= 52) | 1.3 +/− 0.3 (n= 44) | 0.001 |
| Uric Acid (mg/dL) | 7.7 +/− 3.1 (n= 11) | 7.1 +/− 5.1 (n= 9) | 0.8 |
| Lymphocyte Count (%) | 18.6 +/− 13.7 (n= 42) | 15.5 +/− 11.0 (n= 37) | 0.3 |
| Creatinine (mg/dL) | 0.6 +/− 0.5 (n= 63) | 0.6 +/− 0.4 (n= 45) | 1.0 |
| eGFR (mL/min/1.73m2) | 74.7 +/− 34.9 (n= 61) | 96.4 +/− 42.5 (n= 45) | 0.005 |
| Bilirubin (mg/dL) | 2.9 +/− 4.1 (n= 55) | 2.9 +/− 5.9 (n= 40) | 1.0 |
| Previous Cardiac Operation | 61 (96.8) | 40 (88.9) | 0.1 |
| Previous ECMO | 10 (15.9) | 12 (26.7) | 0.2 |
| Previous MCSD | 2 (3.2) | 2 (4.4) | 0.7 |
| Patient Profile | 0.2 | ||
| 1. Critical Cardiogenic Shock | 23 (37.1) | 17 (38.6) | |
| 2. Progressive Decline | 33 (53.2) | 19 (43.2) | |
| 3. Stable but Inotrope Dependent | 6 (9.7) | 5 (11.4) | |
| 4.−7. Resting Symptoms or Less Sick | 3 (6.8) | ||
| Pre-Implant Device Strategy | 0. 3 | ||
| Bridge to Transplant - Listed | 37 (58.7) | 21 (46.7) | |
| Bridge to Candidacy | 15 (23.8) | 18 (40.0) | |
| Destination Therapy | 1 (1.6) | ||
| Bridge to Recovery | 6 (9.5) | 5 (11.1) | |
| Other | 4 (6.3) | 1 (2.2) | |
| Device Classification | 0.003 | ||
| Implantable Continuous | 13 (20.6) | 17 (37.8) | |
| Paracorporeal Continuous | 32 (50.8) | 7 (15.6) | |
| Paracorporeal Pulsatile | 15 (23.8) | 15 (33.3) | |
| Percutaneous | 3 (4.8) | 4 (8.9) | |
| TAH | 2 (4.4) | ||
| Pre-Implant Device Type | 0.0004 | ||
| LVAD | 62 (98.4) | 33 (73.3) | |
| BiVAD | 1 (1.6) | 10 (22.2) | |
| TAH | 2 (4.4) |
eGFR = Estimated Glomerular Filtration Rate; ECMO = Extracorporeal Membrane Oxygenation; MCSD = Mechanical Circulatory Support Device; TAH = Total Artifical Heart; LVAD = Left Ventricular Assist Device; BiVAD = Biventricular Assist Device;
Table 1c:
Patient Characteristics for Single Ventricle Patients Divided by Stage of Palliation (n=63). Pedimacs Patients, September 19, 2012 to June 30, 2017
| Baseline Characteristics | Stage 1 (n=23) |
Stage 2 (n=21) |
Stage 3 (n=19) |
p-value |
|---|---|---|---|---|
| Age (y) | 1.1 +/− 2.4 (n= 23) | 2.6 +/− 3.1 (n= 21) | 8.3 +/− 4.7 (n= 19) | <.0001 |
| Age (y) | <.0001 | |||
| < 1 | 17 (73.9) | 9 (42.9) | ||
| 1-5 | 5 (21.7) | 9 (42.9) | 8 (42.1) | |
| 6-10 | 1 (4.3) | 2 (9.5) | 7 (36.8) | |
| 11-19 | 1 (4.8) | 4 (21.1) | ||
| Female | 8 (34.8) | 10 (47.6) | 6 (31.6) | 0. 5 |
| Race | 0.3 | |||
| White | 15 (65.2) | 9 (42.9) | 9 (47.4) | |
| African American | 3 (13.0) | 5 (23.8) | 7 (36.8) | |
| Other | 5 (21.7) | 6 (23.8) | 3 (15.8) | |
| Body Surface Area (m2) | 0.3 +/− 0.2 (n= 23) | 7 (23.8) | 1.0 +/− 0.4 (n= 18) | <.0001 |
| Blood Urea Nitrogen (mg/dL) | 34.4 +/− 19.0 (n= 23) | 8 (23.8) | 28.8 +/− 21.8 (n= 19) | 0.6 |
| Sodium (mEq/L) | 141.3 +/− 5.9 (n= 23) | 9 (23.8) | 134.8 +/− 8.6 (n= 19) | 0.02 |
| Potassium (mEq/L) | 3.7 +/− 0.6 (n= 23) | 10 (23.8) | 3.8 +/− 0.6 (n= 19) | 0.8 |
| Aspartate Aminotransferase (u/L) | 91.0 +/− 114.1 (n= 21) | 11 (23.8) | 195.0 +/− 464.5 (n= 18) | 0.4 |
| Alanine Aminotransferase (u/L) | 43.3 +/− 41.2 (n= 21) | 12 (23.8) | 173.8 +/− 452.9 (n= 18) | 0.2 |
| Brain Natriuretic Peptide (pg/mL) | 910.8 +/− 713.1 (n= 4) | 13 (23.8) | 2134.2 +/− 1631.6 (n= 13) | 0.3 |
| Pro Brain Natriuretic Peptide (pg/mL) | 20320 +/− 12284 (n= 5) | 14 (23.8) | 614.0 +/− 643.5 (n= 2) | 0.1 |
| Albumin (g/dL) | 3.1 +/− 1.3 (n= 22) | 15 (23.8) | 3.5 +/− 0.4 (n= 18) | 0.5 |
| Pre-Albumin (mg/L) | 152.0 +/− 53.0 (n= 4) | 16 (23.8) | 125.2 +/− 48.0 (n= 5) | 0.3 |
| White Blood Cell Count (x103/μL) | 10.9 +/− 4.0 (n= 22) | 17 (23.8) | 10.1 +/− 3.0 (n= 19) | 0.03 |
| Hemoglobin (g/L) | 133.0 +/− 17.3 (n= 23) | 18 (23.8) | 140.0 +/− 18.1 (n= 19) | 0.5 |
| Platelet Count (x103/μL) | 155.7 +/− 103.6 (n= 22) | 19 (23.8) | 258.6 +/− 102.8 (n= 19) | 0.01 |
| INR (international units) | 1.6 +/− 0.7 (n= 18) | 20 (23.8) | 2.6 +/− 1.4 (n= 16) | 0.008 |
| Uric Acid (mg/dL) | 7.7 +/− 4.2 (n= 5) | 21 (23.8) | 7.3 +/− 1.7 (n= 3) | 1.0 |
| Lymphocyte Count (%) | 13.6 +/− 9.2 (n= 11) | 22 (23.8) | 17.7 +/− 10.0 (n= 16) | 0.2 |
| Creatinine (mg/dL) | 0.5 +/− 0.3 (n= 23) | 23 (23.8) | 0.8 +/− 0.4 (n= 19) | 0.06 |
| eGFR (mL/min/1.73m2) | 63.5 +/− 30.2 (n= 23) | 24 (23.8) | 76.6 +/− 29.7 (n= 18) | 0.1 |
| Bilirubin (mg/dL) | 4.6 +/− 6.0 (n= 22) | 25 (23.8) | 2.1 +/− 1.0 (n= 15) | 0.03 |
| Previous Cardiac Operation | 21 (91.3) | 26 (23.8) | 19 (100) | 0.2 |
| Previous ECMO | 7 (30.4) | 27 (23.8) | 0 (0.0) | 0.03 |
| Previous MCSD | 1 (4.3) | 28 (23.8) | 1 (5.3) | 0.6 |
| Patient Profile | 0.01 | |||
| 1. Critical Cardiogenic Shock | 13 (59.1) | 6 (28.6) | 4 (21.1) | |
| 2. Progressive Decline | 5 (22.7) | 14 (66.7) | 14 (73.7) | |
| 3. Stable but Inotrope Dependent | 4 (18.2) | 1 (4.8) | 1 (5.3) | |
| Pre-Implant Device Strategy | 0.2 | |||
| Bridge to Transplant - Listed | 13 (56.5) | 11 (52.4) | 13 (68.4) | |
| Bridge to Candidacy | 7 (30.4) | 5 (23.8) | 3 (15.8) | |
| Destination Therapy | 1 (4.8) | |||
| Bridge to Recovery | 3 (13.0) | 3 (14.3) | ||
| Other | 1 (4.8) | 3 (15.8) | ||
| Device Classification | 0.0006 | |||
| Implantable Continuous | 1 (4.3) | 2 (9.5) | 10 (52.6) | |
| Paracorporeal Continuous | 18 (78.3) | 10 (47.6) | 4 (21.1) | |
| Paracorporeal Pulsatile | 4 (17.4) | 7 (33.3) | 4 (21.1) | |
| Percutaneous | 2 (9.5) | 1 (5.3) | ||
| TAH | 2 (4.4) | |||
| Pre-Implant Device Type | 0. 4 | |||
| LVAD | 22 (95.7) | 21 (100) | 19 (100) | |
| BiVAD | 1 (4.3) |
eGFR = Estimated Glomerular Filtration Rate; ECMO = Extracorporeal Membrane Oxygenation; MCSD = Mechanical Circulatory Support Device; LVAD = Left Ventricular Assist Device; BiVAD = Biventricular Assist Device;
Table 1d:
Patient Characteristics for CHD Patients <1 Year of Age (n=35). Pedimacs Patients, September 19, 2012 to June 30,
| Baseline Characteristics | CHD Patients < 1 Year (n=35) |
|---|---|
| Age (y) | 0.4 +/− 0.3 (n= 35) |
| Female | 11 (31.4) |
| Race | |
| White | 21 (60.0) |
| African American | 6 (17.1) |
| Other | 8 (22.9) |
| Body Surface Area (m2) | 0.3 +/− 0.1 (n= 35) |
| Blood Urea Nitrogen (mg/dL) | 27.6 +/− 18.7 (n= 35) |
| Sodium (mEq/L) | 142.3 +/− 6.6 (n= 35) |
| Potassium (mEq/L) | 3.8 +/− 0.9 (n= 35) |
| Aspartate Aminotransferase (u/L) | 87.6 +/− 114.3 (n= 32) |
| Alanine Aminotransferase (u/L) | 49.8 +/− 56.7 (n= 32) |
| Brain Natriuretic Peptide (pg/mL) | 1907.6 +/− 1884.2 (n= 9) |
| Pro Brain Natriuretic Peptide (pg/mL) | 14841 +/− 6138.4 (n= 2) |
| Albumin (g/dL) | 3.2 +/− 1.1 (n= 34) |
| Pre-Albumin (mg/L) | 176.0 +/− 73.2 (n= 3) |
| White Blood Cell Count (x103/μL) | 11.4 +/− 4.3 (n= 34) |
| Hemoglobin (g/L) | 12.9 +/− 2.0 (n= 35) |
| Platelet Count (x103/μL) | 190.6 +/− 127.3 (n= 34) |
| INR (international units) | 1.4 +/− 0.4 (n= 28) |
| Uric Acid (mg/dL) | 4.6 +/− 1.9 (n= 4) |
| Lymphocyte Count (%) | 18.9 +/− 11.1 (n= 20) |
| Creatinine (mg/dL) | 0.5 +/− 0.5 (n= 35) |
| eGFR (mL/min/1.73m2) | 74.3 +/− 38.2 (n= 35) |
| Bilirubin (mg/dL) | 2.6 +/− 3.3 (n= 33) |
| Previous Cardiac Operation | 30 (85.7) |
| Previous ECMO | 12 (34.3) |
| Previous MCSD | - |
| Patient Profile | |
| 1. Critical Cardiogenic Shock | 15 (42.9) |
| 2. Progressive Decline | 15 (42.9) |
| 3. Stable but Inotrope Dependent | 5 (14.3) |
| 4.−7. Resting Symptoms or Less Sick | - |
| Pre-Implant Device Strategy | |
| Bridge to Transplant - Listed | 17 (48.6) |
| Bridge to Candidacy | 12 (34.3) |
| Destination Therapy | 1 (2.9) |
| Bridge to Recovery | 4 (11.4) |
| Other | 1 (2.9) |
| Device Classification | |
| Implantable Continuous | - |
| Paracorporeal Continuous | 21 (60.0) |
| Paracorporeal Pulsatile | 13 (37.1) |
| Percutaneous | 1 (2.9) |
| TAH | - |
| Pre-Implant Device Type | |
| LVAD | 32 (91.4) |
| BiVAD | 3 (8.6) |
| RVAD | - |
| TAH | - |
eGFR = Estimated Glomerular Filtration Rate; ECMO = Extracorporeal Membrane Oxygenation; MCSD = Mechanical Circulatory Support Device; TAH = Total Artificial Heart; LVAD = Left Ventricular Assist Device; RVAD = Right Ventricular Assist Device; BiVAD = Biventricular Assist Device
A total of 45 centers enrolled patients. Ten of the 45 (22%) centers were high volume and enrolled 275 patients (61%) of the study cohort. The remaining 175 patients (39%) were enrolled at low volume centers. Between the two groups there were no significant differences with respect to CHD diagnosis, age, size or patient profile (Supplemental Table 2). However, high volume centers used proportionally more implantable continuous and paracorporeal pulsatile devices and fewer paracorporeal pulsatile devices (p=0.002).
Outcomes
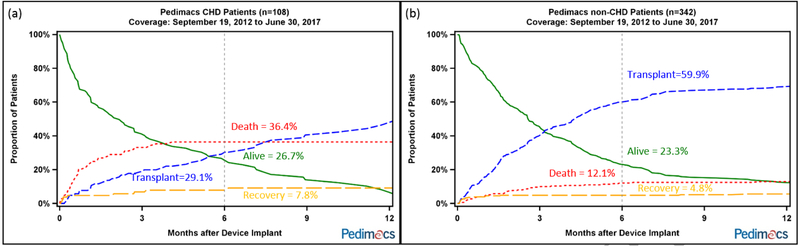
In competing outcomes analysis, CHD patients were more likely to have died (36.4% vs 12.1%, p <0.0001) and less likely to be transplanted (29.1% vs 59.9%, p<0.0001) than non-CHD patients after six months on device (Figure 1). In analyzing only bridge-to-transplant patients, the disparity in percent transplanted at six months was even more pronounced in favor of non-CHD patients (32.0% vs 74.0%,p<0.0001).
Figure 1.
Competing outcomes analysis, including alive with device in place, death before transplant, transplant and explant to recovery after VAD implant for (a) CHD and (b) non-CHD patients.
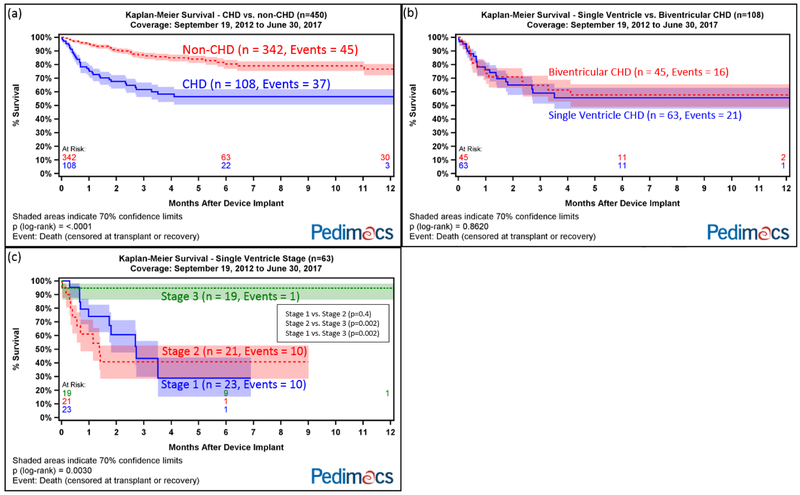
Overall, CHD patients had higher mortality than non-CHD patients (p<0.0001, Figure 2a). There was no difference in survival between single ventricle CHD and biventricular CHD patients (p=0.86, Figure 2b). Among single ventricle patients, stage 3 patients had significantly higher survival compared with stage 1 and 2 patients (p=0.003, Figure 2c). Comparing stage 1 vs. 2, 2 vs. 3, and 1 vs. 3, the p-values were 0.4, 0.002, and 0.002, respectively. Excluding all stage 1 and 2 patients, the difference in survival between CHD and non-CHD patients narrowed but remained statistically significant (p=0.01, Supplemental Figure 1).
Figure 2.
Kaplan-Meier survival after VAD implant divided by (a) CHD vs non-CHD patients; (b) single ventricle CHD vs biventricular CHD; (c) single ventricle stage.
We performed similar analyses excluding patients with critical cardiogenic shock (Supplemental Figure 2), any previous ECMO (Supplemental Figure 3), ECMO during VAD implantation hospitalization (Supplemental Figure 4), bilirubin >2 mg/dL (Supplemental Figure 5), implanted with a non-LVAD (BIVAD, TAH, other, Supplemental Figure 6) and low volume centers (Supplemental Figure 7) and compared survival between CHD and non-CHD patients. After excluding each of these factors, the difference in survival between CHD and non-CHD patients remained statistically significant.
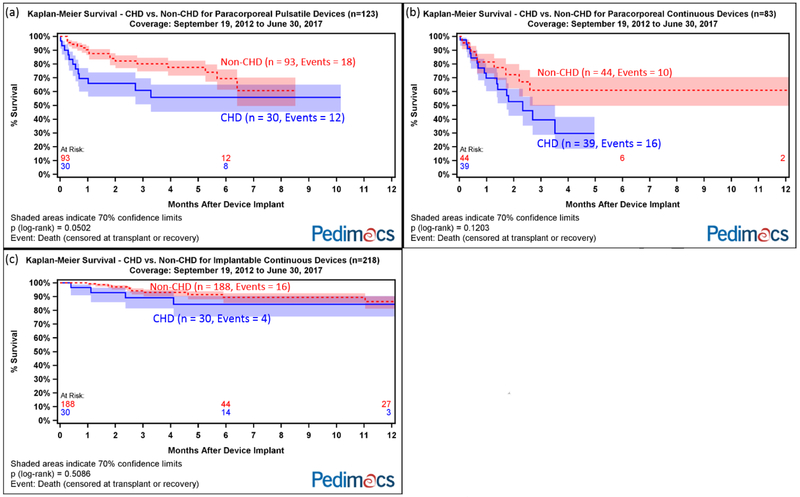
Compared with non-CHD patients, CHD patients had worse survival with paracorporeal pulsatile devices (p=0.05) and similar survival with implantable continuous devices (p=0.5) and paracorporeal continuous devices (p=0.1, Figure 3).
Figure 3.
Kaplan-Meier survival after VAD implant for CHD vs non-CHD patients for (a) paracorporeal pulsatile devices; (b) paracorporeal continuous devices); (c) implantable continuous devices.
Eleven out of 26 (42%) single ventricle CHD infants (<1 year old) died during the study period (Table 2). Of the 20 single ventricle infants on paracorporeal continuous device support, 13 (65%) achieved a favorable outcome. Four of the 5 single ventricle infants supported with paracorporeal pulsatile devices died (Table 2).
Table 2:
Outcomes for CHD Patients <1 year of age as of June 30, 2017 (n=35). Pedimacs Patients, September 2012 through June 2017
| Outcome | SV (Paracorporeal Continous) |
SV (Paracorporeal Pulsatile) |
SV (Percutaneous) |
BV (Paracorporeal Continuous |
BV (Paracorporeal Pulsatile) |
|---|---|---|---|---|---|
| Alive | 4 (20%) | 1 (20%) | |||
| Transplanted | 4 (20%) | 1 (100%) | 1 (100%) | 6 (75%) | |
| Death | 7 (35%) | 4 (80%) | 1 (12%) | ||
| Recovery | 5 (25%) | 1(12%) |
SV = single ventricle; BV = biventricular
CHD patients had more early respiratory failure compared with non-CHD patients (p=<0.001 Table 3). In subgroup analyses of device classifications, CHD patients also had higher rates of “other” serious adverse events on implantable continuous devices (p=0.009) and paracorporeal continuous devices (p=0.001) though the number of events was small overall (Supplemental Tables 3-5). No statistically significant differences in rates of bleeding, infection, and neurological dysfunction were detected between CHD and non-CHD patients.
Table 3:
Adverse Events for CHD vs. Non-CHD Patients. Pedimacs Patients, September 19, 2012 to June 30, 2017
| Pedimacs CHD |
Pedimacs Non-CHD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Perioda | Events | Patient Count |
Patient Percent |
Rateb | Events | Patient Count |
Patient Percent |
Rateb | Rate Ratioc |
p-valued |
| Arterial Non-CNS Thromboembolism | Early | 3 | 3 | 1% | 0.5 | ||||||
| Bleeding | Early | 33 | 24 | 22% | 17.9 | 131 | 89 | 26% | 19.9 | 0.9 | 0.6 |
| Late | 5 | 4 | 4% | 3.2 | 12 | 10 | 3% | 1.4 | 2.3 | 0.1 | |
| Cardiac Arrhythmia | Early | 11 | 8 | 7% | 6 | 30 | 24 | 7% | 4.6 | 1.3 | 0.4 |
| Late | 2 | 2 | 1% | 0.2 | |||||||
| Infection | Early | 33 | 23 | 21% | 17.9 | 93 | 70 | 20% | 14.1 | 1.3 | 0.2 |
| Late | 9 | 6 | 6% | 5.8 | 38 | 26 | 8% | 4.5 | 1.3 | 0.5 | |
| Neurological Dysfunction | Early | 33 | 25 | 23% | 17.9 | 87 | 71 | 21% | 13.2 | 1.4 | 0.1 |
| Late | 4 | 4 | 4% | 2.6 | 20 | 12 | 4% | 2.3 | 1.1 | 0.9 | |
| Other SAE | Early | 33 | 22 | 20% | 17.9 | 113 | 68 | 20% | 17.2 | 1 | 0.8 |
| Late | 6 | 5 | 5% | 3.8 | 19 | 14 | 4% | 2.2 | 1.7 | 0.2 | |
| Pericardial Drainage | Early | 5 | 5 | 5% | 2.7 | 18 | 16 | 5% | 2.7 | 1 | 1.0 |
| Psychiatric Episode | Early | 3 | 3 | 3% | 1.6 | 21 | 20 | 6% | 3.2 | 0.5 | 0.3 |
| Late | 1 | 1 | 1% | 0.6 | 2 | 2 | 1% | 0.2 | 2.7 | 0.4 | |
| Renal Dysfunction | Early | 12 | 11 | 10% | 6.5 | 23 | 23 | 7% | 3.5 | 1.9 | 0.08 |
| Late | 1 | 1 | 1% | 0.6 | 2 | 1 | 0% | 0.2 | 2.7 | 0.4 | |
| Respiratory Failure | Early | 35 | 23 | 21% | 19 | 41 | 34 | 10% | 6.2 | 3 | <.001 |
| Late | 2 | 2 | 2% | 1.3 | 3 | 3 | 1% | 0.4 | 3.6 | 0.1 | |
| Venous Thromboembolism | Early | 6 | 6 | 2% | 0.9 | ||||||
| Wound Dehiscence | Early | 1 | 1 | 1% | 0.5 | 2 | 2 | 1% | 0.3 | 1.8 | 0.6 |
| Late | 1 | 1 | 0% | 0.1 | |||||||
Early = within three months of device implant, Late = more than three months after device implant
Rates are reported per 100 patient months
Rate Ratio is comparing CHD rates to non-CHD rates for the given time period
p-value is comparing CHD rates to non-CHD rates for the given time period
In multivariable modeling for risk of death on device for all patients, CHD, female gender and decreased eGFR were associated with increased mortality. Implantable continuous devices and high volume center were independently associated with improved survival (Table 4).
Table 4:
Multivariable model for mortality on a device (n=450). Pedimacs Patients, September 19, 2012 to June 30, 2017
| Pre-Implant Characteristics | Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|---|
| Implantable Continuous Device | 0.3 | 0.2 | 0.5 | <0.0001 |
| CHD | 2.9 | 1.8 | 4.5 | <0.0001 |
| eGFR (20 unit increase) | 0.9 | 0.8 | 1.0 | 0.01 |
| Female | 1.7 | 1.1 | 2.6 | 0.02 |
| High Volume Center | 0.6 | 0.4 | 0.9 | 0.02 |
CHD = Congenital Heart Disease; eGFR = Estimated Glomerular Filtration Rate;
High Volume Center is defined as enrolling ≥ 15 patients in Pedimacs.
DISCUSSION
In this Pedimacs analysis, 24% of children supported with VADs had CHD. CHD was associated with worse outcomes overall. CHD was found to be an independent risk factor for mortality after VAD implant and CHD patients were less likely to receive a transplant compared with non-CHD patients. Implantable continuous devices and high volume centers were independently associated with better survival. Compared with non-CHD, CHD patients who received implantable continuous devices had similarly high survival.
To date, this report is the largest analysis of VAD use in children with CHD. Approximately one in four children who received VADs had CHD in this study. This is an increase from previous pediatric reports where the percent of VADs with CHD ranged from 16-17.5%.5, 12 We found that paracorporeal continuous devices were commonly used in CHD, especially in smaller single ventricle patients. Unadjusted mortality was highest for this device class but this is likely confounded by the young age, small size, acuity and complexity of these patients. In this high-risk population, recent outcomes have most likely improved with paracorporeal continuous flow devices in comparison to the very poor survival previously reported with paracorporeal pulsatile support.7, 13, 14 Still, the mortality remains high and more studies and experience are needed to determine optimal support strategies for the smaller single ventricle population.
Stage 2 patients present a unique challenge for VAD support for many reasons including their dichotomous systemic venous return, collateral burden and hypoxemia. Given the superior outcomes in stage 3 patients, stage 2 patients with severe systolic dysfunction should be considered for concomitant Fontan operation and VAD implantation (i.e. “mechanically assisted Fontan completion”) which has been reported previously.15 Further study is warranted to see if this approach can improve VAD outcomes for stage 2 patients.
Fewer CHD patients received implantable continuous devices presumably due to their smaller size and complex anatomy. However, the CHD patients who did receive implantable continuous devices had similarly high survival as compared with non-CHD patients, which is consistent with the favorable implantable continuous VAD outcomes in the adult CHD population.16 In the multivariable model of the overall cohort, implantable continuous devices were significantly associated with improved survival. This finding likely reflects improving VAD technology but is also confounded by the characteristics of the patients that did, and did not, receive implantable continuous devices.
CHD was found to be an independent risk factor for mortality, along with lower eGFR and female gender. The gender disparity in outcomes has also been observed in large adult studies and warrants further investigation in the pediatric population.10, 17 CHD and worse renal function have been previously linked with higher mortality in pediatric VAD studies.7, 8, 18 Our analysis does not explain exactly why CHD patients are at higher risk for mortality despite attempts to control for known risk factors. The reasons for worse survival are certainly multifactorial. There was no difference in overall survival between biventricular and single ventricle which demonstrates that the increased risk was not unique to the single ventricle patients. Anatomic complexity, chronic circulatory abnormalities, previous surgeries, and related end-organ effects may all contribute to the worse VAD outcomes seen in CHD.
Of note, we found that CHD patients were much less likely to have been transplanted at 6 months compared with non-CHD patients. The explanation for these findings likely includes differences in age and wait times, early post-VAD mortality, sensitization and clinical status post-VAD implant the two groups.
We were not surprised to find high volume center associated with better survival. Morales and colleagues reported similar findings and also showed that high volume centers had lower associated costs.19 In our analysis comparing low and high volume centers, there were detectable differences in how devices were utilized. These findings support the idea that there is a steep learning curve in pediatric VAD therapy and that experience and expertise can improve outcomes. In response, the pediatric heart failure and VAD community has recently launched Action (the Advanced Cardiac Therapies Improving Outcomes Network) to foster more active sharing and collaboration between centers and to ultimately drive improvement in critical outcomes collectively.
Limitations
There are important limitations to this study. Pedimacs only captures patients from participating centers and there is likely center-to-center variability in the accuracy and completeness of the reporting. Due to the relatively small number of patients, the analysis was mostly descriptive in nature and only the strongest associations were detectable in multivariable modeling. Because of the limited numbers of patients, heterogeneity and inability to adequately decouple the effects of age, size and device, we did not perform analysis for risk factors for mortality within the CHD group and subgroups. In the future, this analysis will be possible if Pedimacs enrollment continues to increase over time. Due to significant inconsistencies in the data, we were unable to accurately analyze and compare device malfunction/pump thrombosis and rehospitalizations in this study.
In conclusion, children with CHD experience higher mortality and a lower rate of transplant compared with children without CHD after VAD implant. Still, CHD patients supported with implantable continuous devices have demonstrated good survival. The experience and expertise gained at larger volume centers have resulted in improved outcomes. Further collaboration and study are warranted to better identify and support the CHD population at highest risk.
Supplementary Material
Acknowledgments
Funding and Conflict of Interest Statement
Early financial support for this analysis was provided by The National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services under Contract No. HHSN268201100025C. The analysis was completed with funding from The Society of Thoracic Surgeons. J.K.K. received partial salary support as Director of Intermacs Registry Data Center and served as chair of DSMB for clinical trial involving Xeltis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rossano JW, Kim JJ, Decker JA, Price JF, Zafar F, Graves DE, Morales DL, Heinle JS, Bozkurt B, Towbin JA, Denfield SW, Dreyer WJ and Jefferies JL. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J Card Fail. 2012;18:459–70. [DOI] [PubMed] [Google Scholar]
- 2.Rossano JW, Dipchand AI, Edwards LB, Goldfarb S, Kucheryavaya AY, Levvey Rn BJ, Lund LH, Meiser B, Yusen RD, Stehlik J, International Society for H and Lung T. The Registry of the International Society for Heart and Lung Transplantation: Nineteenth Pediatric Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016;35:1185–1195. [DOI] [PubMed] [Google Scholar]
- 3.Almond CS, Thiagarajan RR, Piercey GE, Gauvreau K, Blume ED, Bastardi HJ, Fynn-Thompson F and Singh TP. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009;119:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zafar F, Castleberry C, Khan MS, Mehta V, Bryant R 3rd, Lorts A, Wilmot I, Jefferies JL, Chin C and Morales DL. Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. J Heart Lung Transplant. 2015;34:82–8. [DOI] [PubMed] [Google Scholar]
- 5.Blume ED, Rosenthal DN, Rossano JW, Baldwin JT, Eghtesady P, Morales DL, Cantor RS, Conway J, Lorts A, Almond CS, Naftel DC and Kirklin JK. Outcomes of children implanted with ventricular assist devices in the United States: First analysis of the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS). J Heart Lung Transplant. 2016;35:578–84. [DOI] [PubMed] [Google Scholar]
- 6.Wehman B, Stafford KA, Bittle GJ, Kon ZN, Evans CF, Rajagopal K, Pietris N, Kaushal S and Griffith BP. Modern Outcomes of Mechanical Circulatory Support as a Bridge to Pediatric Heart Transplantation. Ann Thorac Surg. 2016;101:2321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales DLS, Zafar F, Almond CS, Canter C, Fynn-Thompson F, Conway J, Adachi I and Lorts A. Berlin Heart EXCOR use in patients with congenital heart disease. J Heart Lung Transplant. 2017;36:1209–1216. [DOI] [PubMed] [Google Scholar]
- 8.Blume ED, VanderPluym C, Lorts A, Baldwin JT, Rossano JW, Morales DLS, Cantor RS, Miller MA, St Louis JD, Koehl D, Sutcliffe DL, Eghtesady P, Kirklin JK, Rosenthal DN and Pedimacs I. Second annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) report: Pre-implant characteristics and outcomes. J Heart Lung Transplant. 2018;37:38–45. [DOI] [PubMed] [Google Scholar]
- 9.Ross HJ, Law Y, Book WM, Broberg CS, Burchill L, Cecchin F, Chen JM, Delgado D, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Martin CM, Murphy AM, Singh G, Spray TL and Stout KK. Transplantation and Mechanical Circulatory Support in Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circulation. 2016;133:802–20. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB and Naftel DC. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal DN, Almond CS, Jaquiss RD, Peyton CE, Auerbach SR, Morales DR, Epstein DJ, Cantor RS, Kormos RL, Naftel DC, Butts RJ, Ghanayem NS, Kirklin JK and Blume ED. Adverse events in children implanted with ventricular assist devices in the United States: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS). J Heart Lung Transplant. 2016;35:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villa CR, Khan MS, Zafar F, Morales DLS and Lorts A. United States Trends in Pediatric Ventricular Assist Implantation as Bridge to Transplantation. Asaio j. 2017;63:470–475. [DOI] [PubMed] [Google Scholar]
- 13.Lorts A, Eghtesady P, Mehegan M, Adachi I, Villa C, Davies R, Gossett JG, Kanter K, Alejos J, Koehl D, Cantor RS and Morales DLS. Outcomes of children supported with devices labeled as "temporary" or short term: A report from the Pediatric Interagency Registry for Mechanical Circulatory Support. J Heart Lung Transplant. 2018;37:54–60. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein S, Bello R, Pizarro C, Fynn-Thompson F, Kirklin J, Guleserian K, Woods R, Tjossem C, Kroslowitz R, Friedmann P and Jaquiss R. The use of the Berlin Heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg. 2014;147:697–704; discussion 704-5. [DOI] [PubMed] [Google Scholar]
- 15.Adachi I, Williams E, Jeewa A, Elias B and McKenzie ED. Mechanically assisted Fontan completion: A new approach for the failing Glenn circulation due to isolated ventricular dysfunction. J Heart Lung Transplant. 2016;35:1380–1381. [DOI] [PubMed] [Google Scholar]
- 16.VanderPluym CJ, Cedars A, Eghtesady P, Maxwell BG, Gelow JM, Burchill LJ, Maltais S, Koehl DA, Cantor RS and Blume ED. Outcomes following implantation of mechanical circulatory support in adults with congenital heart disease: An analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). J Heart Lung Transplant. 2018;37:89–99. [DOI] [PubMed] [Google Scholar]
- 17.McIlvennan CK, Lindenfeld J and Kao DP. Sex differences and in-hospital outcomes in patients undergoing mechanical circulatory support implantation. J Heart Lung Transplant. 2017;36:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetzer R, Kaufmann F and Delmo Walter EM. Paediatric mechanical circulatory support with Berlin Heart EXCOR: development and outcome of a 23-year experience. Eur J Cardiothorac Surg. 2016;50:203–10. [DOI] [PubMed] [Google Scholar]
- 19.Morales DL, Zafar F, Rossano JW, Salazar JD, Jefferies JL, Graves DE, Heinle JS and Fraser CD Jr. Use of ventricular assist devices in children across the United States: analysis of 7.5 million pediatric hospitalizations. Ann Thorac Surg. 2010;90:1313–8; discussion 1318-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.