Abstract
Background
Aspirin, an anti‐inflammatory drug, has been widely investigated in the treatment of many cancer types, including colorectal, ovarian, breast, and prostate cancers. MicroRNAs (miRNAs) are the most well studied noncoding RNAs in cancers. In the current study, we were interested in defining the function of aspirin in lung cancer treatment and the related noncoding RNAs involved in this process.
Methods
The function of aspirin in lung cancer growth was evaluated by cell viability and colony formation assays. Screening of miRNAs affected by aspirin was performed through quantitative real‐time PCR. Prediction of miR‐98 targeting WNT1 was performed using online bioinformatics software and was further confirmed by luciferase reporter gene analysis. The levels of miR‐98 and WNT1 were tested through immunoblotting and quantitative real‐time PCR analysis in lung cancer cells under aspirin treatment.
Results
Cell viability was sharply suppressed in lung cancer cells with an increasing dose of aspirin. Aspirin markedly weakened the malignant colony formation ability of lung cancer cells. One out of six tumor suppressor miRNAs could be obviously regulated by aspirin in lung cancer cells. The inhibition of miR‐98 on the luciferase activities of wild‐type 3' untranslated region vectors of WNT1 was clearly revealed in lung cancer cells. Meanwhile, the inhibitor of miR‐98 increased the luciferase activities of wild‐type 3' untranslated region vectors of WNT1. After treatment with aspirin the expression of miR‐98 was induced and then its target gene, WNT1, was depressed in the cells.
Conclusion
Aspirin targets the miR‐98/WNT1 axis to ameliorate lung cancer development.
Keywords: Aspirin, cell proliferation, lung cancer, miR‐98, WNT1
Introduction
Lung cancer is one of the most common causes of cancer‐related death worldwide. Mono‐application or combined‐application chemotherapy are the traditional treatment strategies. However, the efficacy of such strategies for cancer treatment is unsatisfying, yielding poor overall survival. Aspirin, an anti‐inflammatory drug, is widely applied in clinical therapy. Studies have revealed that aspirin can regulate cell proliferation, apoptosis, migration, or other processes by affecting transcription factors.1, 2, 3 In recent years, the anti‐cancer effect of aspirin has been widely explored and applied in the clinical treatment of colorectal cancer.4, 5, 6 Aspirin treatment is associated with reduced risk, overall mortality, or metastasis in colorectal cancer.7 It has also been investigated in ovarian, breast, prostate, and liver cancers.8, 9, 10, 11 Considering the important role of inflammation in lung cancer development, we are interested in examining the role of the anti‐inflammatory drug, aspirin, in the development of lung cancer.
Over the past decade, non‐coding RNAs (ncRNAs) have mainly been implicated in the regulation of gene expression, the cell signaling pathway, and the development and therapy of disease, including cancer.12, 13 As members of ncRNAs, microRNAs (miRNAs) often degrade messenger RNA (mRNA) or inhibit gene translation via interaction with the 3' untranslated region (UTR) of mRNAs in the cells.14 An increasing number of studies have indicated the important role of miRNAs in some aspirin‐involved cancer treatments.15, 16 MiR‐145 can function as a tumor‐suppressive gene in several cancers, including lung, breast, gastric, ovarian, and prostate cancers.17, 18, 19, 20, 21, 22, 23, 24 MiR‐5195 inhibits the progression of lung cancer by directly targeting MYO6. 25 MiR‐98 can bind to TGFBR1 to depress cell proliferation and metastasis in lung cancer.26 In miR‐4317‐restrained lung cancer, FGF9 and CCND2 serve as the key target genes.27 MiR‐886 can play a suppressive role in the development of lung cancer.28 In a mouse model, tumor suppressor let‐7 can destroy the growth of lung cancer.29 In this study we were interested in searching for aspirin‐targeted ncRNAs in lung cancer development.
We aimed to decipher the role of aspirin in lung cancer treatment and the associated underlying mechanism. We reveal that aspirin can effectively inhibit lung cancer growth in vitro. We explored the novel mechanism of aspirin treatment, by which aspirin can induce tumor suppressor miR‐98 and then restrain its target gene WNT1 to suppress lung cancer cell proliferation. Our findings suggest another effective therapeutic strategy for lung cancer.
Methods
Cell lines
A549 and H1299 cell lines were acquired from American Type Culture Collection (ATCC, Rockville, MD, USA). All cell lines were cultivated in 10% fetal bovine serum (Gibco, Rockville, MD, USA) and supplemented with Dulbecco's modified Eagle medium (Gibco) containing penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37°C with 5% CO2.
Cell viability analysis
The cell proliferation ability was determined by methyl‐thiazolyl‐tetrazolium (MTT) assay. Cells were seeded on 96‐well plates with at least three replicates at a density of 3000 cells/well. After 10 hours of incubation to form confluent monolayers, the media were replaced with medium containing aspirin for another 24 hours and 10 μL MTT (5 mg/mL in phosphate buffered saline [PBS]) was then added to each well. Four hours later, the medium was removed and MTT was dissolved in 150 μL dimethyl sulfoxide per well. The absorbance values were measured at optical density 490nm using an absorbance reader.
Colony formation
Cells were seeded in 12‐well plates at a density of 500 cells/well. Twenty‐four hours later, different treatments were administered. The cells were subsequently incubated for another 15–20 days.
RNA extraction and PCR
Total RNA was extracted using TRIzol reagent. For each sample, 1 μg RNA was reverse transcribed into complementary DNA. The mRNA levels were measured by reverse transcription‐PCR and real‐time PCR using SYBR PCR Master Mix (Takara, Dalian, China). The relative quantification of the mRNAs was performed according to the comparative method (2−▵▵Ct, Applied Biosystems User Bulletin no. 2P/N 4303859); the ▵Ct value for each sample is the average of triplicates.
Luciferase reporter gene assay
A549 cells were plated into 24‐well plates at a density of 4 × 104 cells/well. The cells were cotransfected with reporter gene plasmids (pGL3‐WNT1‐wild type [wt] or pGL3‐WNT1‐mutant [mut]) at a dose of 100 ng/well and pRL‐TK plasmid (40 ng/well) (Promega, Madison, WI, USA) and corresponding miRNA and miRNA inhibitors. The cells were collected after 48 hours and the luciferase activity was evaluated according to the manufacturer's instructions. Each experiment was repeated at least three times.
Immunoblotting analysis
Immunoblotting assay was performed as previously reported. Total proteins were extracted after corresponding treatments. The same amount of protein from each sample was analyzed. The primary antibodies, anti‐WNT1 and anti‐β‐actin, were purchased from GeneTex, Inc. (Irvine, CA, USA) and Proteintech (Chicago, IL, USA) and then diluted as recommended by the instructions. Secondary antibodies, such as goat anti‐rabbit or anti‐mouse antibodies (Sigma‐Aldrich, St Louis, MO, USA) were then incubated with the blots and visualized using the Bio‐Rad GelDoc system (Hercules, CA, USA). The band quantification was performed using BandScan software (Glyko Software, Novato, CA, USA).
Statistical analysis
The results were expressed as means ± standard error of the mean. Statistical differences between the two groups were analyzed by Student's t‐test. Non‐significant differences were marked as “ns.” The criteria for statistically significant differences were: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Aspirin stifles the proliferation of lung cancer A549 and H1299 cells
Aspirin is reported to exert an anti‐proliferative effect in multiple cancers, including colorectal and breast cancers, and hepatocellular carcinoma;6, 30, 31, 32 however whether aspirin can disturb lung cancer growth has not yet been clarified. To answer this question, we performed cell viability and colony formation experiments with lung cancer A549 and H1299 cell lines and treated the cells with saline or different doses of aspirin. Administration of an increasing concentration of aspirin (2.5 mM and 5.0 mM) significantly reduced cell viability (Fig 1a,b). Furthermore, colony formation assay showed that aspirin treatment obviously destroyed the ability of A549 cells to develop malignancy (Fig 1c,d). It is worth noting that aspirin actually has the ability to suppress lung cancer cell proliferation.
Figure 1.
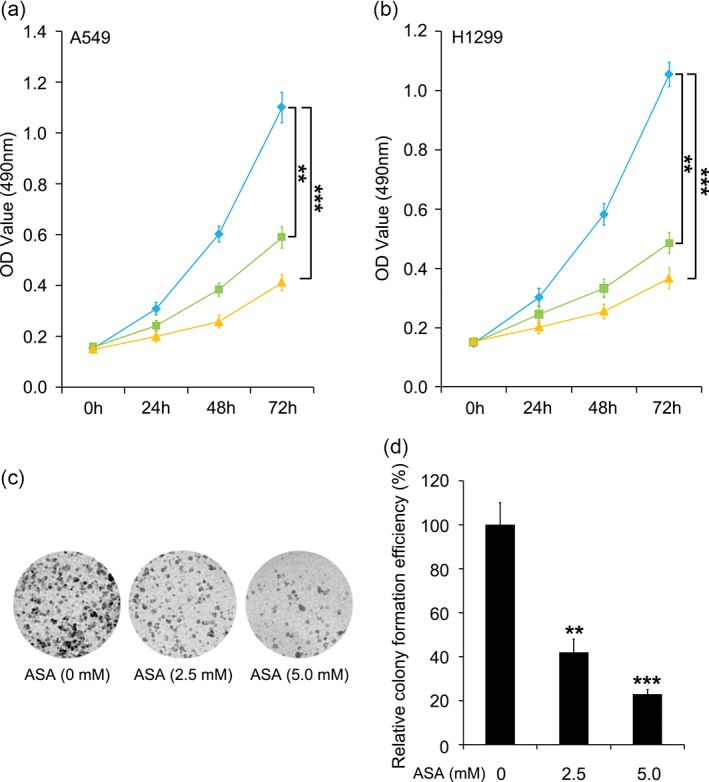
Aspirin (ASA) inhibits the proliferation of lung cancer A549 and H1299 cells. (a,b) Methyl‐thiazolyl‐tetrazolium assay of A549 and H1299 cell lines under different doses was used to evaluate the effect of ASA on cell proliferation. ( ) ASA (0 mM), (
) ASA (0 mM), ( ) ASA (2.5 mM), and (
) ASA (2.5 mM), and ( ) ASA (5.0 mM). (c,d) Colony formation assay and the relative colony formation efficiency of the A549 cell line after treatment with different concentrations of aspirin was used to analyze the function of ASA in the malignancy of lung cancer cells. *** P < 0.001; ** P < 0.01. OD, optical density.
) ASA (5.0 mM). (c,d) Colony formation assay and the relative colony formation efficiency of the A549 cell line after treatment with different concentrations of aspirin was used to analyze the function of ASA in the malignancy of lung cancer cells. *** P < 0.001; ** P < 0.01. OD, optical density.
Screening of microRNAs involved in aspirin‐depressed lung cancer
NcRNAs, including long non‐coding RNAs (lncRNAs) and miRNAs have been thoroughly investigated in many types of cancers. In particular, they are frequently considered as tumor genes or tumor suppressive genes for miRNAs. Reports have revealed that some miRNAs, including miR‐145, miR‐5195, miR‐98, miR‐4317, miR‐886, and let‐7, can inhibit cancer development.18, 25, 26, 27, 28, 29
We were interested in whether these miRNAs are involved in aspirin‐depressed lung cancer. We used 5.0 mM of aspirin for the treatment of lung cancer A549 and H1299 cells and then analyzed the level of these candidate miRNAs through quantitative real‐time PCR assay. We found that compared to saline‐treated cells, all of these tumor suppressor miRNAs were induced by aspirin in the two lung cancer cell lines (Fig 2a,b). One of these six miRNAs, miR‐98, increased most sharply after the cells were treated with aspirin. Overall, our data imply that miR‐98 is involved when aspirin has a treatment effect on lung cancer.
Figure 2.
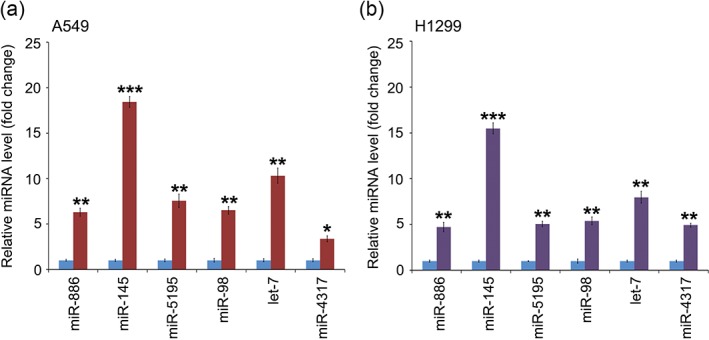
Screening of the microRNAs (miRNAs) involved in aspirin‐depressed lung cancer. (a) Analysis of miRNAs of A549 cell lines after aspirin (ASA) treatment was performed through quantitative real time PCR. (
 ) ASA (0 mM) and (
) ASA (0 mM) and (
 ) ASA (5 mM). (b) Evaluation of miRNA changes in H1299 cell lines after ASA was added to the cells. ***P < 0.001; **P < 0.01; * P < 0.05.
) ASA (5 mM). (b) Evaluation of miRNA changes in H1299 cell lines after ASA was added to the cells. ***P < 0.001; **P < 0.01; * P < 0.05.
As a mediator in aspirin‐reduced lung cancer, miR‐98 directly binds to the 3' untranslated region of WNT1 and depresses its expression
The most extensive function of miRNAs is degrading mRNA or inhibiting gene translation by binding to the 3'UTR of mRNAs.14 We predicted the target genes with a 3'UTR that might be bound by miR‐98 using TargetScan (http://www.targetscan.org/). WNT1 was one of the candidate target genes. Increasing evidence reveals that WNT1 can drive lung cancer progression.33, 34 We hypothesized whether WNT1 is a target gene in aspirin‐induced miR‐98 in lung cancer. Our data showed that there was a putative binding site of miR‐98 within the 3'UTR of WNT1 mRNAs (Fig 3a). We cloned the 3'UTR region of WNT1 (wt or mut) into pGL3‐control plasmid to detect whether miR‐98 targets WNT1 (Fig 3b). As shown in Figure 3c, the luciferase activities of the wt reporter gene gradually decreased along with the elevated dose of miR‐98, while the administration of miR‐98 did not change the luciferase activities of the mut reporter gene. In parallel, the application of anti‐miR‐98 obviously elevated the luciferase activity of pGL3‐WNT1‐wt but not pGL3‐WNT1‐mut (Fig 3d). Notably, the addition of anti‐miR‐98 counteracted the inhibition of pGL3‐WNT1‐wt activity caused by aspirin (Fig 3e). These results indicate that miR‐98 can impede WNT1 expression by targeting the 3'UTR in lung cancer that has been impacted by aspirin treatment.
Figure 3.
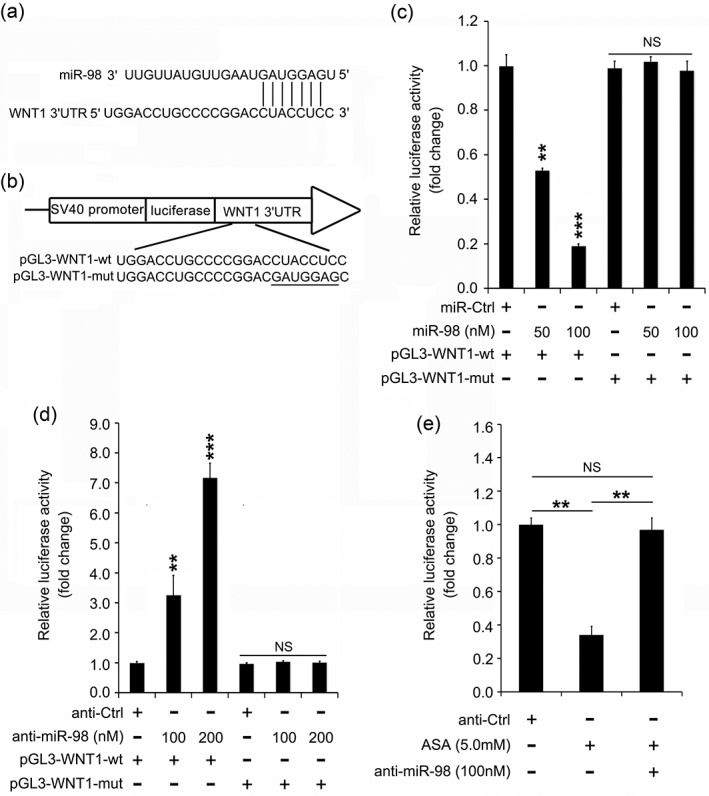
MiR‐98 restrains the level of WNT1 by directly targeting the 3′ untranslated region (3′UTR) of messenger RNAs (mRNAs). (a) Prediction of the targeting of miR‐98 on WNT1 by Targetscan online software. (b) Construction of wild‐type (wt) or mutant (mut) vectors containing binding sites of miR‐98 with 3′UTR of WNT1 mRNA. (c,d) Analysis of miR‐98 (or its inhibitor)‐regulated WNT1 in an A549 cell line by luciferase reporter gene assay. (e) Effect of anti‐miR‐98 and/or aspirin (ASA) on WNT1 3′UTR vector in A549 cell line by luciferase reporter gene assay. *** P < 0.001; ** P < 0.01; ns, not significant.
Aspirin regulates the miR‐98/ WNT1 axis to suppress lung cancer
Based on the observation that miR‐98 targets WNT1 and aspirin can induce miR‐98 in lung cancer, we clarified and confirmed the mechanism by which aspirin inhibits the proliferation of lung cancer cells. After the administration of aspirin (2.5 mM and 5.0 mM), the miR‐98 level was increased 7–16‐fold in A549 cells and at least 5–12‐fold in H1299 cells (Figs 4a, b). In addition, immunoblotting analysis showed that the miR‐98 target gene was dose‐dependently downregulated in both A549 and H1299 cells (Figs 4a, b). Thus, we conclude that aspirin is capable of ameliorating lung cancer by modulating the miR‐98/ WNT1 axis.
Figure 4.
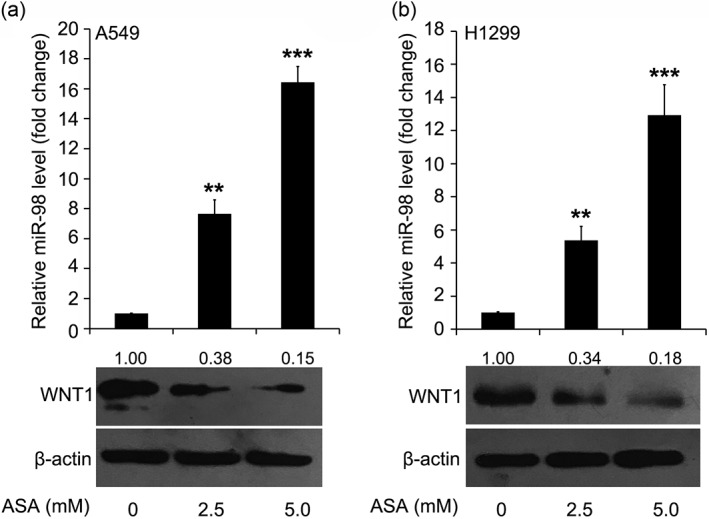
Aspirin (ASA) regulates the miR‐98/WNT1 axis to suppress lung cancer. The level change of miR‐98 and its target gene, WNT1, in (a) A549 or (b) H1299 cells treated with different doses of ASA by quantitative real‐time PCR and immunoblotting assay. BandScan was used to quantify immunoblotting bands. ***P < 0.001; **P < 0.01.
Discussion
Lung cancer remains the leading cause of cancer‐related death worldwide. The development of lung cancer is associated with inflammation and fibrosis. However, the efficacy of current strategies of treatment remains unsatisfactory, with poor overall survival. As a classic anti‐inflammatory drug, aspirin is widely applied in clinical therapy. It has also been well studied in numerous cancers, including ovarian, breast, prostate, and liver cancers.8, 9, 10, 11 In this investigation, we were interested in determining the function of aspirin in lung cancer progression.
We found that increasing concentrations of aspirin inhibited cell viability. We also observed that aspirin administration could destroy the ability of lung cancer cells to develop malignancy. Thus aspirin suppresses lung cancer growth.
In recent years, novel targets, including miRNAs and lncRNAs, have emerged as therapy for cancer.14 Some miRNAs can serve as a tumor suppressor of lung cancer progression. MiR‐145 can function as a tumor‐suppressive gene in several types of cancers, including lung, breast, gastric, ovarian, and prostate cancers.17, 18, 19, 20, 21, 22, 23, 24 MiR‐5195 can inhibit the progression of lung cancer by directly targeting MYO6. 25 MiR‐98 can bind to TGFBR1 to depress cell proliferation and metastasis in lung cancer.26 In miR‐4317‐restrained lung cancer, FGF9 and CCND2 serve as the key target genes.27 MiR‐886 is capable of playing a suppressive role in the development of lung cancer.28 Tumor suppressor let‐7 can destroy the growth of lung cancer in a mouse model.29
In this study we treated lung cancer cells with increasing concentrations of aspirin and evaluated changes in the candidate miRNAs to determine whether miRNAs are involved in aspirin‐inhibited lung cancer. Our findings revealed that one tumor suppressor miRNA, miR‐98, was obviously upregulated by aspirin. We attempted to further decipher the underlying mechanism of the anti‐proliferation effect of aspirin. Sequence analysis and website prediction indicated that miR‐98 might bind to the 3'UTR of WNT1. We therefore conducted mutation of the miR‐98 binding site on 3'UTR of WNT1, and the results from luciferase reporter gene assay indicated that the activity of mutated 3'UTR of WNT1 could not be downregulated by miR‐98 mimics or stimulated by its inhibitor. We then showed that aspirin could induce the level of miR‐98 to decrease the level of WNT1 in lung cancer.
Our findings indicate that aspirin is a novel mechanism to ameliorate lung cancer. Aspirin plays an inhibitory role in the growth of lung cancer. As a tumor suppressor miRNA, miR‐98 can be induced by aspirin treatment in lung cancer. WNT1 serves as a mediator in aspirin/miR‐98‐depressed lung cancer; therefore, aspirin can ameliorate the development of lung cancer by sabotaging the miR‐98/WNT1 axis. Our findings highlight the important role of this newfound mechanism in lung cancer progression as a potential medication for lung cancer.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by the Education Department of Jilin Province (No. JJKH20170849KJ) and the Department of Science and Technology of Jilin Province (No. 20180101164JC).
References
- 1. Huo X, Zhang X, Yu C et al Aspirin prevents NF‐kappaB activation and CDX2 expression stimulated by acid and bile salts in oesophageal squamous cells of patients with Barrett's oesophagus. Gut 2018; 67: 606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benamouzig R, Uzzan B, Martin A et al Cyclooxygenase‐2 expression and recurrence of colorectal adenomas: Effect of aspirin chemoprevention. Gut 2010; 59: 622–9. [DOI] [PubMed] [Google Scholar]
- 3. Galipeau PC, Li X, Blount PL et al NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med 2007; 4: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCowan C, Munro AJ, Donnan PT, Steele RJ. Use of aspirin post‐diagnosis in a cohort of patients with colorectal cancer and its association with all‐cause and colorectal cancer specific mortality. Eur J Cancer 2013; 49: 1049–57. [DOI] [PubMed] [Google Scholar]
- 5. Li P, Wu H, Zhang H et al Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: A meta‐analysis. Gut 2015; 64: 1419–25. [DOI] [PubMed] [Google Scholar]
- 6. Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA 2009; 302: 649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet 2012; 379: 1591–601. [DOI] [PubMed] [Google Scholar]
- 8. Sitia G, Aiolfi R, Di Lucia P et al Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A 2012; 109: E2165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Downer MK, Allard CB, Preston MA et al Regular aspirin use and the risk of lethal prostate cancer in the Physicians' Health Study. Eur Urol 2017; 72: 821–7. [DOI] [PubMed] [Google Scholar]
- 10. Marshall SF, Bernstein L, Anton‐Culver H et al Nonsteroidal anti‐inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst 2005; 97: 805–12. [DOI] [PubMed] [Google Scholar]
- 11. Trabert B, Ness RB, Lo‐Ciganic WH et al Ovarian Cancer Association C: Aspirin, nonaspirin nonsteroidal anti‐inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: A pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst 2014; 106: djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beermann J, Piccoli MT, Viereck J, Thum T. Non‐coding RNAs in development and disease: Background, mechanisms, and therapeutic approaches. Physiol Rev 2016; 96: 1297–325. [DOI] [PubMed] [Google Scholar]
- 13. Mattick JS. The functional genomics of noncoding RNA. Science 2005; 309: 1527–8. [DOI] [PubMed] [Google Scholar]
- 14. Berindan‐Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome genome: A treasure for cancer diagnosis and therapy. CA Cancer J Clin 2014; 64: 311–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yiannakopoulou E. Modulation of lymphangiogenesis: A new target for aspirin and other nonsteroidal anti‐inflammatory agents? A systematic review. J Clin Pharmacol 2012; 52: 1749–54. [DOI] [PubMed] [Google Scholar]
- 16. Dovizio M, Bruno A, Tacconelli S, Patrignani P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res 2013; 191: 39–65. [DOI] [PubMed] [Google Scholar]
- 17. Jiang Y, Wang D, Ren H, Shi Y, Gao Y. MiR‐145‐targeted HBXIP modulates human breast cancer cell proliferation. Thorac Cancer 2019; 10: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan Y, Haiying G, Zhuo L, Ying L, Xin H. Long non‐coding RNA LINC00339 facilitates the tumorigenesis of non‐small cell lung cancer by sponging miR‐145 through targeting FOXM1. Biomed Pharmacother 2018; 105: 707–13. [DOI] [PubMed] [Google Scholar]
- 19. Misono S, Seki N, Mizuno K et al Dual strands of the miR‐145 duplex (miR‐145‐5p and miR‐145‐3p) regulate oncogenes in lung adenocarcinoma pathogenesis. J Hum Genet 2018; 63: 1015–28. [DOI] [PubMed] [Google Scholar]
- 20. Yin Q, Han Y, Zhu D et al miR‐145 and miR‐497 suppress TGF‐beta‐induced epithelial‐mesenchymal transition of non‐small cell lung cancer by targeting MTDH. Cancer Cell Int 2018; 18: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Z, Yan Y, Cao S, Chen Y. Long non‐coding RNA SNHG14 contributes to gastric cancer development through targeting miR‐145/SOX9 axis. J Cell Biochem 2018; 119: 6905–13. [DOI] [PubMed] [Google Scholar]
- 22. Fang CY, Chen PY, Ho DC et al miR‐145 mediates the anti‐cancer stemness effect of photodynamic therapy with 5‐aminolevulinic acid (ALA) in oral cancer cells. J Formos Med Assoc 2018; 117: 738–42. [DOI] [PubMed] [Google Scholar]
- 23. Zhang S, Pei M, Li Z, Li H, Liu Y, Li J. Double‐negative feedback interaction between DNA methyltransferase 3A and microRNA‐145 in the Warburg effect of ovarian cancer cells. Cancer Sci 2018; 109: 2734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iscaife A, Reis ST, Morais DR et al Treating metastatic prostate cancer with microRNA‐145. Apoptosis 2018; 23: 388–95. [DOI] [PubMed] [Google Scholar]
- 25. Yang Q. MicroRNA‐5195‐3p plays a suppressive role in cell proliferation, migration and invasion by targeting MYO6 in human non‐small cell lung cancer. Biosci Biotechnol Biochem 2019; 83: 212–20. [DOI] [PubMed] [Google Scholar]
- 26. Jiang F, Yu Q, Chu Y et al MicroRNA‐98‐5p inhibits proliferation and metastasis in non‐small cell lung cancer by targeting TGFBR1. Int J Oncol 2019; 54: 128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He X, Chen SY, Yang Z et al MiR‐4317 suppresses non‐small cell lung cancer (NSCLC) by targeting fibroblast growth factor 9 (FGF9) and cyclin D2 (CCND2). J Exp Clin Cancer Res 2018; 37: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen J, Zhou W, Bi N et al MicroRNA‐886‐3P functions as a tumor suppressor in small cell lung cancer. Cancer Biol Ther 2018; 19: 1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu C, Hu W, Li LL et al Roles of miR‐200 family members in lung cancer: More than tumor suppressors. Future Oncol 2018; 14: 2875–86. [DOI] [PubMed] [Google Scholar]
- 30. Choi BH, Chakraborty G, Baek K, Yoon HS. Aspirin‐induced Bcl‐2 translocation and its phosphorylation in the nucleus trigger apoptosis in breast cancer cells. Exp Mol Med 2013; 45: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Din FV, Valanciute A, Houde VP et al Aspirin inhibits mTOR signaling, activates AMP‐activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 2012; 142: 1504–15 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sitia G, Iannacone M, Guidotti LG. Anti‐platelet therapy in the prevention of hepatitis B virus‐associated hepatocellular carcinoma. J Hepatol 2013; 59: 1135–8. [DOI] [PubMed] [Google Scholar]
- 33. Gu B, Wang J, Song Y, Wang Q, Wu Q. microRNA‐383 regulates cell viability and apoptosis by mediating Wnt/beta‐catenin signaling pathway in non‐small cell lung cancer. J Cell Biochem 2018. 10.1002/jcb.28069. [DOI] [PubMed] [Google Scholar]
- 34. Ding L, Yao W, Lu J, Gong J, Zhang X. Upregulation of circ_001569 predicts poor prognosis and promotes cell proliferation in non‐small cell lung cancer by regulating the Wnt/beta‐catenin pathway. Oncol Lett 2018; 16: 453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


