Abstract
Background
The purpose of this study was to investigate an association between EGFR mutation status and
18F‐fluorodeoxyglucose positron emission tomography‐computed tomography (18F‐FDG PET‐CT) image features in lung adenocarcinoma.
Methods
Retrospective analysis of the data of 139 patients with lung adenocarcinoma confirmed by surgical pathology who underwent preoperative 18F‐FDG PET‐CT was conducted. Correlations between EGFR mutation status, clinical characteristics, and PET‐CT parameters, including the maximum standardized uptake value (SUVmax), the mean of the SUV (SUVmean), the peak of the SUV (SUVpeak) of the primary tumor, and the ratio of SUVmax between the primary tumor and the mediastinal blood pool (SUVratio), were statistically analyzed. Multivariate logistic regression analysis was performed to identify predictors of EGFR mutation. Receiver operating characteristic curves of statistical quantitative parameters were compared.
Results
EGFR mutations were detected in 74 (53.2%) of the 139 lung adenocarcinomas and were more frequent in non‐smoking patients. Univariate analysis showed that the SUVmax, SUVmean, SUVpeak, and SUVratio were lower in EGFR‐mutated than in wild‐type tumors. The receiver operating characteristic curves showed no significant differences between their diagnostic efficiencies. Multivariate logistic regression analysis showed that being a never smoker was an independent predictor of EGFR mutation.
Conclusion
Quantitative parameters based on 18F‐FDG PET‐CT have modest power to predict the presence of EGFR mutation in lung adenocarcinoma; however, when compared to smoking history, they are not good or significant predictive factors.
Keywords: 18F‐FDG, EGFR mutation, lung cancer, PET/CT
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide and its incidence is steadily increasing in industrialized countries.1 Non‐small cell lung cancer (NSCLC) accounts for more than 80% of lung cancers and adenocarcinoma is the main histological subtype. EGFR mutation status plays an important role in guiding EGFR‐based targeted therapy for NSCLC patients; front‐line EGFR‐tyrosine kinase inhibitor (TKI) therapy is considered the standard of care for advanced NSCLC patients with sensitizing EGFR mutations.2, 3 Thus, determining EGFR mutation status is essential to identify the NSCLC patients who may benefit from treatment with EGFR‐TKIs and, hence, to improve prognosis and the efficacy of EGFR‐TKI therapy.
18F‐fluoro‐2‐deoxy‐glucose positron emission tomography (18F‐FDG‐PET), a functional imaging modality based on glucose metabolism, is widely used for the diagnosis, initial staging, and evaluation of treatment efficacy in lung cancer.4 A previous study showed that EGFR signaling regulates the global metabolic pathway in EGFR‐mutated lung adenocarcinoma cells and EGFR‐TKIs decrease lactate production, glucose consumption, and the glucose‐induced extracellular acidification rate.5 These findings suggest that 18F‐FDG uptake on PET may be a noninvasive biomarker for predicting EGFR mutation.
However, previous data concerning the association between 18F‐FDG uptake and EGFR mutation in lung cancer are conflicting and the correlation has not been satisfactorily evaluated.6, 7, 8, 9, 10, 11 Further studies are needed to validate these results. Therefore, we conducted this retrospective study to investigate whether or not 18F‐FDG PET could be a valuable method for predicting EGFR mutation in lung adenocarcinomas.
Methods
Patients
This retrospective study was approved by our institutional review board and the informed consent requirement was waived. We retrospectively collected data of 560 patients who underwent preoperative PET‐CT and were pathologically diagnosed with lung cancer at our institute between June 2016 and October 2017. The inclusion criteria were as follows: (i) visible lung cancer on preoperative PET‐CT images (diameter > 1 cm); (ii) surgical resection with histopathologically verified lung adenocarcinoma; (iii) patients were not admistered treatment before surgery; and (iv) resected specimens were examined for EGFR mutation. The exclusion criteria were as follows: (i) patients who underwent a biopsy before PET‐CT examination; (ii) patients administered neoadjuvant chemotherapy or radiotherapy before surgery; (iii) lesions displaying as ground‐glass nodules or part‐solid nodules; (iv) FDG uptake similar to adjacent pulmonary parenchyma, which was difficult to measure; and (v) patients without EGFR mutation data. In total, 139 patients met the requirements for the study. Clinical and pathologic information (age, gender, smoking history, tumor location, tumor stage, and EGFR mutation status) were collected from the hospital's electronic medical records system.
18F‐FDG PET‐CT scanning
In this study, PET‐CT scans were performed using a GE Discovery Elite PET/CT scanner (GE Medical Systems, Waukesha, WI, USA). After a six‐hour fast, patients were injected with 4.2 MBq 18F‐FDG/kg body weight. After an hour, a spiral CT scan with ~25 effective mAs, 130 kVp, and a 5 mm slice thickness was taken, followed by a PET emission scan from the distal femur to the top of the skull. The PET scanning time was two minutes per bed position, with increments of 16.2 cm (three‐dimensional [3D] mode), and all patients were scanned in eight bed positions. PET images were reconstructed using iterative algorithms (ordered‐subset expectation maximization, 6 iterations, 8 subsets) to a final pixel size of 5.3 × 5.3 × 2.5 mm. A 6 mm full‐width at half maximum Gaussian filter was applied after the reconstruction.
Image analysis
Two board‐certified nuclear medicine physicians with eight and five years experience in PET‐CT imaging, respectively, reviewed the PET‐CT images side by side and reached a consensus on the findings at the workstation (AW4.6, GE Medical Systems). The tumor was delineated and then three‐dimensionally reconstructed at the AW4.6 workstation using the PET volume computerized assisted reporting (PETVCAR) software (GE Medical Systems, Waukesha, WI, USA). To quantify the uptake, a volume of interest using a 3D sphere was placed over the primary tumor. The maximum voxel uptake, which reflected the maximal uptake of 18F‐FDG within the tumor, was found and its maximum standardized uptake value (SUVmax) was calculated according to the following formula: SUV = tissue radioactivity concentration (becquerels per millilitre)/(injected dose [becquerels]/patient weight [grams]). The mean of the SUV (SUVmean) was determined with a 3D isocontour at 50% of the maximum voxel value and the peak of the SUV (SUVpeak) using a 12 mm diameter spherical volume of interest automatically centred on the tumor area with the maximum uptake. For the mediastinal blood pool, a circular region‐of‐interest (ROI) with a 10 mm diameter was placed centrally within the ascending aorta. SUVratio = SUVmax of the primary tumor/SUVmax of the mediastinal blood pool.
EGFR mutation assessment
Genomic DNA was extracted from frozen lung cancer tissues sampled from surgically resected specimens. EGFR mutations were analyzed using the peptide nucleic acid‐locked nucleic acid PCR clamp method.12 EGFR exons 18, 19, 20, and 21 were tested. Patients were categorized according to the mutation testing as EGFR‐mutated (EGFR+) and wild‐type EGFR (EGFR−).
Statistical analysis
Statistical analysis was performed using two commercially available statistical software packages (SPSS version 19.0, IBM Corp., Armonk, NY, USA; and MedCalc version 15.2.2, Mariakerke, Belgium). Continuous variables were compared using an independent‐sample t or Mann–Whitney U test, while categorical variables were presented as a frequency and were compared using chi‐square or rank sum tests. Receiver operating characteristic (ROC) curves for the significant parameters were constructed and the areas under the curve (AUCs) were calculated with a cutoff value, sensitivity, specificity, and positive and negative likelihood ratios (LR). The differences between the AUCs were then compared. Multivariate logistic regression analysis was performed to identify predictors of EGFR mutation. P < 0.05 was considered to indicate a statistically significant difference.
Results
Association between patient characteristics and EGFR mutation status
A total of 139 patients with 139 lung adenocarcinomas were included in this study. The pathological type of all lesions was adenocarcinoma, including 135 invasive non‐mucinous adenocarcinomas and 4 mixed invasive mucinous/non‐mucinous adenocarcinomas. There were no adenocarcinoma in situ or minimally invasive adenocarcinomas. The patients’ clinicopathological characteristics are summarized in Table 1. EGFR mutations were identified in 74 patients (74/139, 53.2%). Of 139 patients, 62 (62/139, 44.6%) were male and 77 (77/139, 55.4%) were female. Lung cancer with EGFR mutation was more frequently identified in women, but there was no significant difference between women and men (59.5% vs. 50.7%). The median age at the time of surgery was 62.5 years for EGFR+ patients and 63 years for EGFR− patients. In this study, 46 patients (46/139, 33.1%) were classified as current and former smokers and 93 (93/139, 66.9%) were never smokers. Lung cancer with EGFR mutation was more frequently identified in never smokers (77.0%; P < 0.05). The majority of patients enrolled in this study were in clinical stage I (97/139, 69.8%). There were no significant differences in age, tumor location, or tumor stage between EGFR+ and EGFR− patients.
Table 1.
Association between clinicopathological characteristics and EGFR status
| EGFR status | P | |||
|---|---|---|---|---|
| Clinicopathological characteristics | Total | EGFR+ (n = 74) | EGFR− (n = 65) | |
| Age, mean (range) | 62 (28–81) | 61 (33–78) | 62 (28–81) | 0.921 |
| Gender | ||||
| Male | 62 | 30 | 32 | 0.304 |
| Female | 77 | 44 | 33 | |
| Smoking history | ||||
| Never smoker | 93 | 57 | 36 | 0.007 |
| Smoker | 46 | 17 | 29 | |
| Tumor location | ||||
| Right upper lobe | 47 | 26 | 21 | |
| Right middle lobe | 15 | 10 | 5 | |
| Right lower lobe | 27 | 14 | 13 | |
| Left upper lobe | 32 | 17 | 15 | |
| Left lower lobe | 18 | 7 | 11 | |
| Stage | ||||
| I or II | 111 | 59 | 52 | 0.968 |
| III or IV | 28 | 15 | 13 | |
Association between 18F‐FDG uptake and EGFR mutation status
Table 2 shows that the SUVmax, SUVmean, SUVpeak, and SUV ratio of EGFR+ tumors were significantly lower than those of EGFR− tumors. There were significant differences between EGFR+ and EGFR− tumors (P < 0.05).
Table 2.
Comparisons of quantitative parameters based on FDG uptake measurements between EGFR+ and EGFR− groups
| EGFR status | P | ||
|---|---|---|---|
| Parameters | EGFR+ (n) | EGFR− (n) | |
| SUVmax† | 7.70 ± 3.93 | 10.18 ± 5.67 | 0.004 |
| SUVmean† | 4.76 ± 2.49 | 6.36 ± 3.59 | 0.003 |
| SUVpeak‡ | 5.78 ± 3.17 | 7.93 ± 4.84 | 0.013 |
| SUVratio‡ | 4.83 ± 2.95 | 6.60 ± 4.18 | 0.010 |
Independent‐sample t and
Mann–Whitney U tests used for comparisons. FDG, fluorodeoxyglucose; SUV, standardized uptake value.
Multivariate logistic regression analysis
In the multivariate logistic regression analysis, SUVmax, SUVmean, SUVpeak, SUVratio, and smoking history were analyzed together. Smoking history (never smokers) was the the only independent predictor for the presence of EGFR mutation in lung adenocarcinoma (P = 0.010) (Table 3).
Table 3.
Multivariate logistic regression analysis of the significant clinicopathological characteristics and quantitative parameters based on FDG uptake measurements to predict EGFR mutation
| 95% CI for OR | P | |||
|---|---|---|---|---|
| Parameters | OR | Lower | Upper | |
| SUVmax | 1.457 | 0.595 | 3.571 | 0.410 |
| SUVmean | 0.440 | 0.155 | 2.247 | 0.440 |
| SUVpeak | 0.434 | 0.527 | 1.317 | 0.434 |
| SUVratio | 0.935 | 0.767 | 1.277 | 0.935 |
| Smoking history | 2.756 | 1.281 | 5.929 | 0.010 |
CI, confidence interval; FDG, fluorodeoxyglucose; OR, odds ratio; SUV, standardized uptake value.
Receiver operating characteristic curve analysis
The AUCs to identify EGFR mutation were 0.629, 0.632, 0.622, and 0.626 for SUVmax, SUVmean, SUVpeak, and SUVratio, respectively (Table 4). The cutoff value, sensitivity, specificity, positive LR (+LR), and negative LR (−LR) of each parameter are shown in Table 4. There were no significant differences in AUCs between SUVmax, SUVmean, SUVpeak, and SUVratio (SUVmax vs. SUVmean: P = 0.482; SUVmax vs. SUVpeak: P = 0.498; SUVmax vs. SUVratio: P = 0.883; SUVmean vs. SUVpeak: P = 0.352; SUVmean vs. SUVratio: P = 0.762; SUVpeak vs. SUVratio: P = 0.825) (Fig 1).
Table 4.
ROC analysis of the significant quantitative parameters to identify EGFR mutation
| 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Cutoff value | AUC | Lower | Upper | Sensitivity (%) | Specificity (%) | +LR | −LR |
| SUVmax | 11.19 | 0.629 | 0.535 | 0.723 | 41.5 | 82.4 | 1.41 | 0.42 |
| SUVmean | 6.06 | 0.632 | 0.538 | 0.726 | 52.3 | 71.6 | 1.50 | 0.54 |
| SUVpeak | 6.92 | 0.622 | 0.527 | 0.717 | 58.5 | 66.2 | 1.59 | 0.58 |
| SUVratio | 6.75 | 0.626 | 0.532 | 0.721 | 43.1 | 85.1 | 1.50 | 0.35 |
AUC, area under the curve; CI, confidence interval; LR, likelihood ratio; ROC, receiver operating characteristic; SUV, standardized uptake value.
Figure 1.
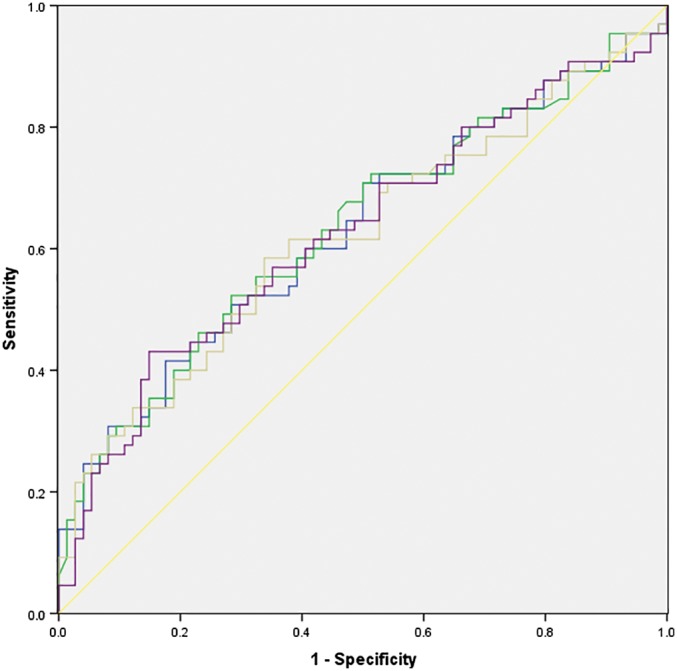
Receiver operating characteristic curve analysis and comparison of the significant quantitative parameters based on fluorodeoxyglucose uptake measurements to predict EGFR mutation. ( ) SUVmax, (
) SUVmax, ( ) SUVmean, (
) SUVmean, ( ) SUVpeak, (
) SUVpeak, ( ) SUVratio, and (
) SUVratio, and ( ) Reference line. SUV, standardized uptake value.
) Reference line. SUV, standardized uptake value.
Discussion
EGFR mutation is one of the most common druggable targets in NSCLC. The availability of effective EGFR‐TKIs in first‐line therapy requires the timely identification of suitable patients. Therefore, identifying factors to predict a positive EGFR mutation are clinically useful. In this study, we found that NSCLC patients with mutated‐EGFR had lower SUVmax, SUVmean, SUVpeak, and SUVratio measurements based on 18F‐FDG PET‐CT than NSCLC patients with wild‐type EGFR. These findings suggest that EGFR‐mutated lung adenocarcinomas could be biologically indolent with lower levels of glucose metabolism than EGFR‐wild tumors and these PET‐CT parameters could be potentially useful to discriminate the EGFR mutation status in NSCLC patients.
As one of the currently available noninvasive imaging methods, PET‐CT is widely used for lesion detection, lesion characterization, and clinical staging in patients with lung cancer. PET‐CT is based on the fact that the glucose metabolism of a tumor is partly reflected by FDG uptake. Previous studies have shown contradictory results for the correlation between EGFR mutation status and FDG uptake. Some data from previous studies revealed that a lower FDG avidity was an independent variate for predicting EGFR mutations, while other groups reported that no association existed between FDG uptake and EGFR status or that a higher SUVmax predicted EGFR mutation.6, 7, 8, 9, 10 In our study, NSCLC patients with EGFR mutations had lower FDG uptake measurements including SUVmax, SUVmean, SUVpeak, and SUVratio based on 18F‐FDG PET‐CT than NSCLC patients with wild‐type EGFR.
There are several possible reasons for these contradictory results. First, SUVmax, SUVmean, and SUVpeak are semi‐quantitative indexes that could vary with different PET scanners, fasting duration, level of plasma glucose, and ROI parameters. Given the limitations of these parameters, we chose another PET‐CT parameter, SUVratio, as an alternative variable to explore the relationship between PET‐CT and EGFR gene mutation status. Our results showed that SUVratio had a statistically significant predictive role for determining EGFR mutation status, which indicated that quantitative parameters based on 18F‐FDG PET‐CT could be regarded as predictors of EGFR mutation. Second, the difference between our study and previous reports is the homogeneity of the lesions. Because of the retrospective nature of our study, we selected lesions that were predominately solid and larger than 1 cm in order to minimize partial volume averaging effects in FDG‐PET interpretation. The pathological type of the lesions in our study was lung adenocarcinoma, not including squamous cell carcinoma. We suggest that these conditions resulted in a more reliable estimate of FDG uptake in lung cancer. Third, the possible mechanisms of lower FDG uptake and EGFR mutation in our study might be related to the following reasons. 18F‐FDG PET‐CT as a functional imaging modality is based on glucose metabolism. 18F‐FDG uptake in NSCLC patients correlates with the expression of GLUT1 in primary tumors and EGFR mutation decreases FDG uptake in NSCLC via the NOX4/reactive oxygen species/GLUT1 axis.13, 14 Although our results showed the statistically significant predictive roles of SUVmax, SUVmean, SUVpeak, and SUVratio for determining EGFR mutation status, the ROC curves showed no significant differences between their diagnostic efficiencies. Multivariate logistic regression analysis showed that being a never smoker was the only independent predictor of EGFR mutation. Moreover, the maximal AUC of these quantitative parameters was only 0.632. These results indicate that these FDG uptake measurements based on 18F‐FDG PET‐CT have only modest power to predict EGFR mutation in lung cancer. Furthermore, when compared with smoking history, they were not good or significant predictive factors.
Our study has some limitations. First, only a relatively small number of patients were evaluated for EGFR mutation analysis. Only 74 out of 139 patients had an EGFR mutation, which may be a potential selection bias. However, 53.2% of patients had an EGFR mutation, which was a relatively high incidence. A recent systematic review showed that EGFR mutation frequency in the Asia‐Pacific NSCLC/adenocarcinoma subgroup is 47.9%.15 Second, different driver gene mutations may result in distinct pathway activation and glycolytic features. Previous studies have reported that NSCLC patients with tumors harbouring K‐ras mutation or ALK rearrangement showed significantly higher 18F‐FDG uptake than wild‐type patients.9, 11 We did not take into consideration the roles of these drivers, which could cause bias in FDG uptake measurements in patients with EGFR‐wild to some extent.
In conclusion, our results indicate that EGFR‐mutated lung adenocarcinomas potentially have a lower level of glucose metabolism than wild‐type tumors. Quantitative parameters of FDG uptake measurements, such as SUVmax, SUVmean, SUVpeak, and SUVratio have modest power to predict EGFR status; however, when compared to smoking history, they are not good or significant predictive factors.
Acknowlegments
This work was supported by grants from the National Natural Science Foundation of China (81601377, 81501984, 81671771), the Tianjin Natural Science Fund (16JCZDJC35200, 17JCYBJC25100), The Science & Technology Development Fund of Tianjin Education Commission for Higher Education (2018KJ061, 2018KJ057), the Hainan Natural Science Fund (2018CXTD347), Tianjin Science and Technology Program Fund (18PTZWHZ00100) and Beijing‐Tianjin‐Hebei Basic Research Cooperation Project Fund (H2018206600).
Disclosure
No authors report any conflict of interest.
Contributor Information
Xubin Li, Email: lixb@bjmu.edu.cn.
Wengui Xu, Email: wenguixy@163.com.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2. Lee CK, Brown C, Gralla RJ et al Impact of EGFR inhibitor in non‐small cell lung cancer on progression‐free and overall survival: A meta‐analysis. J Natl Cancer Inst 2013; 105: 595–605. [DOI] [PubMed] [Google Scholar]
- 3. Hsu WH, Yang JC, Mok TS et al Overview of current systemic management of EGFR‐mutant NSCLC. Ann Oncol 2018; 29: i3–9. [DOI] [PubMed] [Google Scholar]
- 4. Stroobants S, Verschakelen J, Vansteenkiste J. Value of FDG‐PET in the management of non‐small cell lung cancer. Eur J Radiol 2003; 45: 49–59. [DOI] [PubMed] [Google Scholar]
- 5. Makinoshima H, Takita M, Matsumoto S et al Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR‐mutated lung adenocarcinoma. J Biol Chem 2014; 289: 20813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang CT, Yen RF, Cheng MF et al Correlation of F‐18 fluorodeoxyglucose‐positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol 2010; 27: 9–15. [DOI] [PubMed] [Google Scholar]
- 7. Mak RH, Digumarthy SR, Muzikansky A et al Role of 18F‐fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non‐small cell lung cancer. Oncologist 2011; 16: 319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ko KH, Hsu HH, Huang TW et al Value of 18F‐FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging 2014; 41: 1889–97. [DOI] [PubMed] [Google Scholar]
- 9. Caicedo C, Garcia‐Velloso MJ, Lozano MD et al Role of [18F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non‐small‐cell lung cancer. Eur J Nucl Med Mol Imaging 2014; 41: 2058–65. [DOI] [PubMed] [Google Scholar]
- 10. Lee SM, Bae SK, Jung SJ, Kim CK. FDG uptake in non‐small cell lung cancer is not an independent predictor of EGFR or KRAS mutation status: A retrospective analysis of 206 patients. Clin Nucl Med 2015; 40: 950–8. [DOI] [PubMed] [Google Scholar]
- 11. Lv Z, Fan J, Xu J et al Value of 18F‐FDG PET/CT for predicting EGFR mutations and positive ALK expression in patients with non‐small cell lung cancer: A retrospective analysis of 849 Chinese patients. Eur J Nucl Med Mol Imaging 2018; 45: 735–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagai Y, Miyazawa H, Huqun et al Genetic heterogeneity of the epidermal growth factor receptor in non‐small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid‐locked nucleic acid PCR clamp. Cancer Res 2005; 65: 7276–82. [DOI] [PubMed] [Google Scholar]
- 13. Higashi K, Ueda Y, Sakurai A et al Correlation of glut‐1 glucose transporter expression with [(18)F] FDG uptake in non‐small cell lung cancer. Eur J Nucl Med 2000; 27: 1778–85. [DOI] [PubMed] [Google Scholar]
- 14. Chen L, Zhou Y, Tang X et al EGFR mutation decreases FDG uptake in non‐small cell lung cancer via the NOX4/ROS/GLUT1 axis. Int J Oncol 2019; 54: 370–80. [DOI] [PubMed] [Google Scholar]
- 15. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non‐small‐cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015; 9: 2892–911. [PMC free article] [PubMed] [Google Scholar]


