Abstract
Background
It is unclear whether the chemotherapy response improves after exposure to immunotherapy. Antiangiogenic agents have been shown to stimulate the immune system and cause synergistic effects that stimulate tumor shrinkage. We conducted a retrospective study to evaluate improvement of the efficacy of ramucirumab plus docetaxel after the failure of nivolumab as a PD‐1 inhibitor.
Methods
From February 2016 to December 2017, 152 patients with non‐small cell lung cancer (NSCLC) administered nivolumab in our institution were identified. We reviewed the records of 20 NSCLC patients administered ramucirumab plus docetaxel after nivolumab failure. The overall response rate (ORR), progression‐free survival (PFS), and overall survival (OS) were investigated. Pegylated granulocyte colony‐stimulating factor was prophylactically administered to 18 patients (90%) after the administration of ramucirumab plus docetaxel.
Results
The median age of the patients was 70 (range: 55–77) years. Twelve patients were male and eight were female. The histology was adenocarcinoma in 16 patients, squamous cell carcinoma in three, and other in one. The ORR of ramucirumab plus docetaxel was 60%, and the PFS and OS were 169 and 343 days, respectively. Among the 20 patients, 12 achieved a partial response, giving an ORR of 60.0%. Six patients had stable disease and two had progressive disease. The disease control rate was 90%. Gastrointestinal adverse events were frequently observed in 19 patients.
Conclusions
Ramucirumab plus docetaxel achieved a higher response rate when administered immediately after nivolumab failure compared to regimens without prior nivolumab administration.
Keywords: Chemotherapy, docetaxel, increased response, nivolumab, ramucirumab
Introduction
Lung cancer is a major neoplasm with a dismal prognosis and a high death rate worldwide. Unfortunately, systemic chemotherapy yields a poor response against previously treated non‐small cell lung cancer (NSCLC). Recently, immunotherapy, such as immune checkpoint inhibitors (ICIs), has been developed for patients with advanced NSCLC. Approximately 16% of patients administered nivolumab as anti‐PD‐1 monotherapy have achieved survival of more than five years.1 Thus, determining an ICI treatment sequence to prolong the survival of cancer patients is a critical issue.
The best chemotherapeutic regimens to apply after ICI failure have not yet been elucidated. We usually administer cytotoxic agents, such as docetaxel, S‐1, pemetrexed, and ramucirumab plus docetaxel. Recently, Schvartman et al. reported that the response rates to single‐agent chemotherapy after exposure to ICIs were higher in 28 patients with advanced NSCLC compared to those in historical controls.2 In their study, the overall response rate (ORR) of single agents after ICIs was 39%. Although single‐agent chemotherapy consists of docetaxel, mitomycin, gemcitabine, and pemetrexed, half of the 28 patients in the study received docetaxel alone and achieved an ORR of 43%. Park et al. also reported that ICIs could improve the ORR of salvage chemotherapy administered after immunotherapy in patients with NSCLC, and 39 (53.4%) of 73 patients achieved the ORR.3 These phenomena suggest a possible immunotherapy‐induced chemo‐sensitization effect, although the detailed mechanism remains unknown.
Ramucirumab was developed as a human immunoglobulin G1 monoclonal antibody that targets the vascular endothelial growth factor receptor 2 (VEGFR2) extracellular domain. A phase III trial (REVEL study) reported that the combination of ramucirumab plus docetaxel achieved a significantly better prognosis than docetaxel monotherapy.4 Ramucirumab is indeed active, achieving a response rate of approximately 28.9% when combined with docetaxel in Japanese patients.5 Nowadays, ICIs, docetaxel, and docetaxel plus ramucirumab are recommended as optimal treatment in patients with previously treated NSCLC. However, whether ramucirumab plus docetaxel should be considered before the administration of ICIs and after ICI failure is unknown.
A recent basic study showed that simultaneous treatment of a PD‐1 inhibitor and anti‐VEGFR2 antibody synergistically inhibits tumor growth in vivo.6 Allen et al. also showed that anti‐PD‐L1 therapy can sensitize tumors to antiangiogenic treatment and prolong its efficacy, and antiangiogenic therapy can improve the efficacy of anti‐PD‐L1 antibodies in preclinical models.7 The immunotherapy‐induced chemo‐sensitization effect may be superior in the combination of a single agent plus anti‐VEGFR2 antibody than in a single agent alone. Although several reports have shown the efficacy of single‐agent chemotherapy after PD‐1 or PD‐L1 antibody failure, the efficacy of ramucirumab plus docetaxel in patients with advanced NSCLC remains unknown.
Based on this background, we retrospectively evaluated the clinical features of ramucirumab plus docetaxel as a sequential treatment after nivolumab failure in patients with previously treated NSCLC.
Methods
Patient eligibility and data collection
The inclusion criteria were: histologically or cytologically proven NSCLC, an Eastern Cooperative Oncology Group performance status score of 0–2, age ≥ 20 years, life expectancy of ≥ 3 months, exhibited disease progression after nivolumab treatment, administered first‐line platinum‐based chemotherapy, administered EGFR‐tyrosine kinase inhibitors (TKIs) prior to platinum combination chemotherapy for an EGFR mutation, administered ramucirumab plus docetaxel after nivolumab failure, and efficacy data of ramucirumab plus docetaxel was available. Patients were excluded if they had any of the following: a concomitant serious illness such as myocardial infarction in the previous three months, uncontrolled angina pectoris, heart failure, uncontrolled diabetes mellitus, uncontrolled hypertension, interstitial pneumonia, or lung disease; infection or other diseases contraindicating chemotherapy; pregnancy; or breast‐feeding. The institutional ethics committee of the Saitama Medical University International Medical Center approved this study. The requirement for written informed consent was waived because of the retrospective nature of the study.
Efficacy evaluation
Prior to treatment patients were evaluated with a complete blood cell count, a differential count, routine chemistry measurements, chest radiography, chest computed tomography (CT), abdominal CT, whole‐brain magnetic resonance imaging or CT, and isotope bone scintigraphy. Complete blood cell counts, differential counts, routine chemistry measurements, physical examination, and toxicity assessment were evaluated weekly. Acute toxicities were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1.8 Responses based on target (and non‐target) lesions were defined as follows: complete response (CR), disappearance of all target (and non‐target) lesions; partial response (PR), ≥ 30% reduction in size (or disappearance of ≥ 1 non‐target lesions); stable disease (SD), < 30% decrease or < 20% increase in size (or the persistence of ≥ 1 non‐target lesions); and progressive disease (PD), > 20% increase in size (or the appearance of new non‐target lesions and/or progression of existing non‐target lesions). The ORR was defined as the best response recorded from the initiation of treatment until disease progression or recurrence, confirmed by repeat assessments performed no less than four weeks after the criteria for response were first met. Survival was recorded from the first day of ramucirumab plus docetaxel treatment to the date of death or last follow‐up, and survival curves were calculated according to the Kaplan–Meier method. Overall survival (OS) was determined as the interval from the first day of chemotherapy to death from any cause. Progression‐free survival (PFS) was defined as the interval from the first day of chemotherapy to the first sign of disease progression or death.
Results
Patient demographics
From February 2016 to December 2017, 152 NSCLC patients with NSCLC were treated with nivolumab at our institute. Forty patients were administered cytotoxic chemotherapy after nivolumab failure. Efficacy data was not available for three patients. Nine patients were treated with cytotoxic agents other than docetaxel, and eight patients received docetaxel single‐agent chemotherapy. Twenty patients were eligible for the study (Fig 1). The patient demographics are listed in Table 1. The median age was 70 (range: 55–77) years; 12 patients were male and eight were female; and 12 patients (60%) had a history of smoking. The histology was: adenocarcinoma (16 patients), squamous cell carcinoma (3 patients), and other (1 patient). Sixteen patients were administered ramucirumab plus docetaxel as third‐line chemotherapy, three patients as fourth‐line chemotherapy, and one patient as fifth‐line chemotherapy. Seven patients were administered taxane‐containing regimens and 13 were administered regimens including pemetrexed as platinum‐based chemotherapy. Pegylated‐granulocyte‐colony stimulating factor (PEG‐G‐CSF) was prophylactically administered in 18 patients (90%) within 72 hours after the administration of ramucirumab plus docetaxel.
Figure 1.
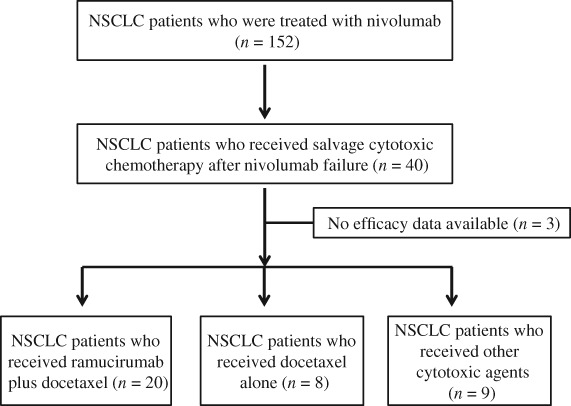
CONSORT flow diagram of this study. NSCLC, non‐small cell lung cancer
Table 1.
Patient characteristics
| Characteristics | N = 20 |
|---|---|
| Age | |
| Median years (range) | 70 years (55–77 years) |
| Gender | |
| Male | 12 |
| Female | 8 |
| ECOG PS | |
| 0 | 9 |
| 1 | 9 |
| 2 | 2 |
| Smoking history | |
| Yes | 12 |
| No | 8 |
| Histology | |
| ADC | 16 |
| SCC | 3 |
| Other | 1 |
| Clinical staging | |
| III | 3 |
| IV | 12 |
| Recurrence after surgery | 5 |
| EGFR mutation status | |
| Wild type | 17 |
| Mutant type | 3 |
| Number of treatment lines of prior nivolumab | |
| 1 | 13 |
| 2 | 3 |
| 3 | 1 |
| Number of treatment lines between nivolumab and docetaxel plus ramucirumab | |
| 0 | 17 |
| 1 | 3 |
| Duration of nivolumab treatment | |
| Median days (range) | 68 days (29–554) |
| Median number of docetaxel plus ramucirumab cycles (range) | 4 cycles (1–11) |
| †Prophylactic administration of G‐CSF† | |
| Yes | 18 |
| No | 2 |
Granulocyte‐colony stimulating factor (G‐CSF) was administered within 72 hours of docetaxel plus ramucirumab. ADC, adenocarcinoma; ECOG, Eastern Cooperative Oncology Group; G‐CSF, granulocyte‐colony stimulating factor; PS, performance status; SCC, squamous cell carcinoma.
Efficacy and survival analysis
All patients were evaluable for therapeutic response (Table 2). Among the 20 patients, 12 achieved PR (ORR 60.0%, 95% confidence interval 38.5–81.4%), 6 achieved SD, and 2 achieved PD. The disease control rate (DCR) was 90% (95% confidence interval 76.9–103%). A median of 5 patients in PR, 4 in SD, and 3 in PD were administered ramucirumab plus docetaxel after nivolumab failure. Figure 2 shows the maximum tumor reduction following treatment with ramucirumab plus docetaxel. Tumor shrinkage was observed in almost all patients. On the other hand, an ORR of 10% and a DCR of 30% were observed in all patients administered nivolumab. The median interval between the final administration of nivolumab and the initiation of docetaxel plus ramucirumab was 34 days (range: 25–168): 30 days (range: 15–161) in patients with PR, 102 days (range: 21–474) in patients with SD, and 42 days (range: 21–63) in patients with PD.
Table 2.
Objective response rate (n = 20)
| Efficacy | Patients (n) | Percentage |
|---|---|---|
| CR | 0 | 0% |
| PR | 12 | 60% |
| SD | 6 | 30% |
| PD | 2 | 10% |
| ORR (95% CI) | 60% (38.5–81.4%) | |
| DCR (95% CI) | 90% (76.9–103%) | |
CI, confidence interval; CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 2.
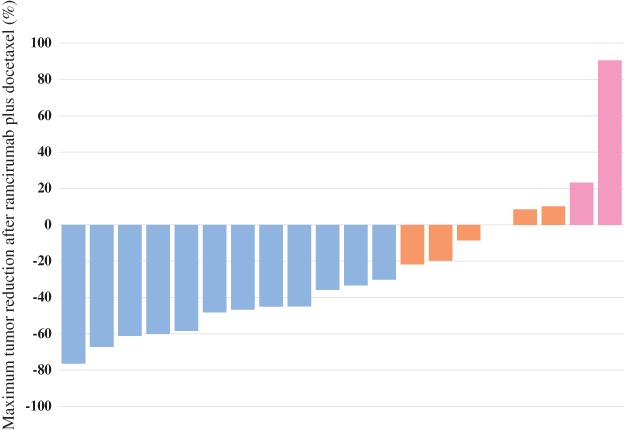
Waterfall plot showing maximum tumor reduction after the administration of ramucirumab plus docetaxel. Blue denotes a partial response (PR); orange, stable disease (SD); and pink, progressive disease (PD).
The median PFS and OS rates of ramucirumab plus docetaxel after nivolumab failure were 169 days and 343 days, respectively. The median duration of nivolumab therapy was 68 days (range: 29–554).
Figure 3 shows the treatment durations of nivolumab and ramucirumab plus docetaxel. The treatment duration of nivolumab was < 90 days in 9 (75%) patients with PR, 3 (50%) patients with SD, and 2 (100%) patients with PD administered ramucirumab plus docetaxel.
Figure 3.
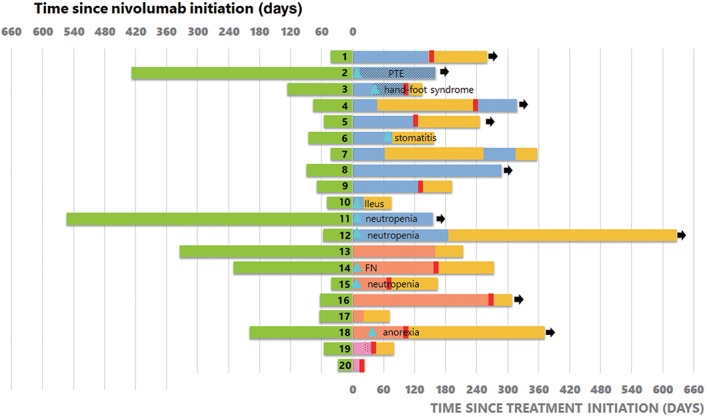
Treatment duration of nivolumab and ramucirumab (RAM) plus docetaxel (DTX) in all patients. Nine out of 20 patients experienced grade 3 adverse events. The treatment duration of nivolumab was < 90 days in 9 (75%) patients with a partial response (PR), 3 (50%) patients with stable disease (SD) and 2 (100%) patients with progressive disease (PD) on RAM + DTX. FN, febrile neutropenia; OS, overall survival; PTE, pulmonary thromboembolism.  , Time receiving Nivolumab;
, Time receiving Nivolumab;  , Time receiving DTX+RAM (PR Group);
, Time receiving DTX+RAM (PR Group);  , Time receiving DTX+RAM (SD Group);
, Time receiving DTX+RAM (SD Group);  , Time receiving DTX+RAM (PD Group) (dots DTX only);
, Time receiving DTX+RAM (PD Group) (dots DTX only);  , OS not receiving DTX+RAM;
, OS not receiving DTX+RAM;  , Progressive disease;
, Progressive disease;  , Adverse event, ≧Grade3;
, Adverse event, ≧Grade3;  , Alive as of database lock.
, Alive as of database lock.
Adverse events
The adverse events are listed in Table 3. Nineteen patients (95%) experienced any grade adverse events after the administration of ramucirumab plus docetaxel. The dose of docetaxel was reduced in seven patients because of toxicity. Two patients discontinued ramucirumab plus docetaxel because of stomatitis and ileus, while treatment was revised to docetaxel alone in three patients because of grade 3 pulmonary thromboembolism, grade 3 hand–foot syndrome, and grade 2 pulmonary hemorrhage (Fig 3). Gastric intestinal adverse events were frequently observed in 19 patients. In particular, one patient experienced a grade 3 intestinal obstruction on day 8 and required treatment in intensive care. This patient was not able to receive the combination chemotherapy because of a poor performance status score. No pulmonary toxicities, such as interstitial pneumonitis, or treatment‐related deaths were observed. One patient who experienced grade 3 febrile neutropenia was not administered prophylactic PEG‐G‐CSF (Fig 3).
Table 3.
Hematological and non‐hematological adverse events
| N = 20 (%) | ||
|---|---|---|
| Variables | All grade | ≧ Grade 3 |
| Neutropenia | 3 (15%) | 3 (15%) |
| Febrile neutropenia | 1 (5%) | 1 (5%) |
| Thrombocytopenia | 6 (30%) | 0 (0%) |
| Epistaxis | 7 (35%) | 0 (0%) |
| Pulmonary hemorrhage | 1 (5%) | 0 (0%) |
| Pulmonary thromboembolism | 1 (5%) | 1 (5%) |
| Fatigue | 7 (35%) | 0 (0%) |
| Stomatitis | 4 (20%) | 1 (5%) |
| Anorexia | 13 (65%) | 1 (5%) |
| Dysgeusia | 3 (15%) | 0 (0%) |
| Peripheral edema | 5 (25%) | 0 (0%) |
| Intestinal obstruction | 1 (5%) | 1 (5%) |
| Diarrhea | 3 (15%) | 0 (0%) |
| Constipation | 3 (15%) | 0 (0%) |
| Hand‐foot syndrome | 2 (10%) | 1 (5%) |
| Sensory neuropathy | 2 (10%) | 0 (0%) |
| Skin rash | 3 (15%) | 0 (0%) |
| Alopecia | 1 (5%) | 0 (0%) |
Discussion
To the best of our knowledge, this is the first retrospective study to assess the response rate of ramucirumab plus docetaxel after nivolumab failure in patients with previously treated NSCLC. We found a high ORR of 60% for this combination regimen. Compared to 23% in the REVEL study and 28.9% in the JVCG study, our ORR is impressive, similar to that of molecular targeted agents such as EGFR‐TKIs. However, the frequency of adverse events observed in our sample was slightly different from those reported previously.4,5 Gastrointestinal toxicities such as ileus, stomatitis, and intestinal obstruction were frequently observed. Although this phenomenon may be associated with the effect of prior nivolumab, we do not have a sufficient explanation regarding the relationship between gastrointestinal toxicity and ramucirumab plus docetaxel. Moreover, the efficacy of ramucirumab plus docetaxel was not closely linked to that of prior nivolumab. The prior administration of nivolumab may contribute to the improved response of ramucirumab plus docetaxel, regardless of the treatment duration of nivolumab.
Several retrospective studies have evaluated the efficacy of salvage chemotherapy following exposure to PD‐1/PD‐L1 inhibitors and reported an improved antitumor effect of salvage chemotherapy after ICI failure, achieving ORRs ranging from 25 to 46.9%.2, 3, 9, 10 Park et al. reported that gemcitabine‐based salvage chemotherapy yielded an ORR of 63.6% (14/22 patients), and 12 of the 14 patients with PR were administered gemcitabine/platinum doublet chemotherapy.3 They reported that the ORR of salvage chemotherapy after immunotherapy failure was significantly higher than that of the final chemotherapy administered before immunotherapy (53.4% vs. 34.9%; P = 0.03). In the English literature, cytotoxic agents such as gemcitabine, pemetrexed, docetaxel, mitomycin, vinorelbine, nab‐paclitaxel, and S‐1 have been identified as salvage chemotherapy after ICI failure2, 3, 11 however, no reports on combination chemotherapies including antiangiogenic agents have been published. Our data indicate that the combination of docetaxel plus ramucirumab as a VEGFR2 inhibitor may contribute to the improved response rate.4,5 Previous studies suggest that immunotherapy may introduce tumor synergistic effects to subsequent cytotoxic therapy; our results are consistent with this finding.2, 3, 9, 10, 11 Moreover, it has been reported that therapeutic level of anti‐PD‐1 is maintained when chemotherapy is administered because of the long half‐life of the PD‐1 antibody.12 A recent trial indicated that nivolumab as a PD‐1 antibody continues to bind to the PD‐L1 for approximately two months.13 Likewise, dramatic tumor shrinkage with ramucirumab plus paclitaxel after anti‐PD‐1 antibody failure was observed in two cases of metastatic gastroesophageal adenocarcinoma.14 This case report suggests that the administration of PD‐1 blockade followed by chemotherapy could enhance the tumor response to ramucirumab plus taxane in advanced human neoplasms, such as gastrointestinal cancer. Therefore, immunotherapy followed by cytotoxic therapy may be a promising chemotherapeutic sequence compared to chemotherapy administered before immunotherapy. A prospective study conducted in multicenter institutions is warranted to confirm these findings.
There have been recent discussions regarding the mechanism by which cytotoxic chemotherapy positively improves immunomodulatory effects.15, 16 The immunomodulatory effects of chemotherapy, as a possible mechanism, include the elimination of myeloid‐derived suppressor cells (MDSCs) and T‐regulatory cells (Tregs), augmentation of an antigen‐specific immune response, enhancement of antigen presentation and processing, and upregulation of death receptors in tumor cells.2, 3, 15, 16 The synergistic combination of antiangiogenesis and immunotherapy has also been discussed at the preclinical level.17 Angiogenic agents stimulate the immune system; conversely, immunotherapy is suggested to be antiangiogenic. VEGF plays a crucial role in mediating the immunosuppressive microenvironment. In particular, VEGFR2 inhibition is reported to significantly increase cluster of differentiation (CD)4‐positive and CD8‐positive T cell infiltration and reduce the number of Tregs and MDSCs within tumor tissues in mice.17, 18, 19 A recent experimental study showed that simultaneous inhibition of PD‐1 and VEGFR2 significantly suppressed tumor growth compared to monotherapy.6 Given these findings it is not surprising that chemotherapy confers a synergistic effect on immunotherapy and that antiangiogenic agents enhance the immune response. A recent clinical study showed that pembrolizumab plus chemotherapy (carboplatin and pemetrexed) significantly improves efficacy compared to chemotherapy alone,20 suggesting a synergistic effect between the cytotoxic agent and PD‐1 inhibitor.
The current study has several limitations. First, our study had a small sample size that may be subject to patient selection bias. As 90% of patients had a performance status score of 0 or 1, we included patients with a favorable general condition. It is likely that patients eligible for salvage combination chemotherapy after ICI failure had a good general condition and thus a better expected outcome. Second, our study followed a retrospective approach and included a heterogeneous population; however, no prospective studies evaluating the efficacy of salvage chemotherapy following exposure to PD‐1/PD‐L1 inhibitors have been conducted. Further study is warranted to investigate whether the response of salvage chemotherapy after ICI failure can improve compared to that in historical controls using regimens including antiangiogenic agents if possible. Finally, because of the limited sample, it remains unclear whether our regimen after nivolumab failure can benefit survival rates. Considering the results of previous studies, salvage chemotherapy after ICI failure does not seem to prolong the survival of patients with advanced NSCLC. It is necessary to select chemotherapeutic agents that are easily affected by immunotherapy to improve outcomes.
In conclusion, the ORRs in patients with NSCLC administered ramucirumab plus docetaxel after nivolumab failure were higher compared to the rates described in previous clinical studies. Gastrointestinal adverse events were frequently observed but no typical toxicities regarding the combination of ramucirumab plus docetaxel. If the combination of platinum‐based chemotherapy plus ICIs is administered as first‐line treatment in patients with advanced NSCLC, ramucirumab plus docetaxel may be suitable as an optimal treatment sequence. Further investigation in a large prospective trial is required to confirm our results.
Disclosure
AM, KK and HK received research grants and a speaker honorarium from Eli Lilly Company.
None of the remaining authors report any conflict of interest.
References
- 1. Gettinger S, Horn L, Jackman D et al. Five‐year follow‐up of nivolumab in previously treated advanced non‐small‐cell lung cancer: Results from the CA209‐003 study. J Clin Oncol 2018; 36: 1675–84. [DOI] [PubMed] [Google Scholar]
- 2. Schvartsman G, Peng SA, Bis G et al. Response rates to single‐agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non‐small cell lung cancer. Lung Cancer 2017; 112: 90–5. [DOI] [PubMed] [Google Scholar]
- 3. Park SE, Lee SH, Ahn JS et al. Increased response rates to salvage chemotherapy administered after PD‐1/PD‐L1 inhibitors in patients with non‐small cell lung cancer. J Thorac Oncol 2018; 13: 106–11. [DOI] [PubMed] [Google Scholar]
- 4. Garon EB, Ciuleanu TE, Arrieta O et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): A multicentre, double‐blind, randomised phase 3 trial. Lancet 2014; 384: 665–73. [DOI] [PubMed] [Google Scholar]
- 5. Yoh K, Hosomi Y, Kasahara K et al. Randomized, double‐blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non‐small cell lung cancer after disease progression on platinum‐based therapy. Lung Cancer 2016; 99: 186–93. [DOI] [PubMed] [Google Scholar]
- 6. Yasuda S, Sho M, Yamamoto I et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti‐tumour effect in vivo. Clin Exp Immunol 2013; 172: 500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen E, Jabouille A, Rivera LB et al. Combined antiangiogenic and anti‐PD‐L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017; 9: eaak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisenhauer E, Therasse P, Bogaerts J et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 9. Leger PD, Rothschild S, Castellans E et al. Response to salvage chemotherapy following exposure to immune checkpoint inhibitors in patients with non‐small cell lung cancer. 2017 ASCO Annual Meeting Proceedings. J Clin Oncol 2017; 35: Abstract 9084. [Google Scholar]
- 10. Grigg C, Reuland BD, Sacher AG et al. Clinical outcome of patients with non‐small cell lung cancer (NSCLC) receiving chemotherapy after immune checkpoint blockade. 2017 ASCO Annual Meeting Proceedings. J Clin Oncol 2017; 35: Abstract 9082. [Google Scholar]
- 11. Ogawara D, Soda H, Iwasaki K et al. Remarkable response of nivolumab‐refractory lung cancer to salvage chemotherapy. Thorac Cancer 2018; 9: 175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patnaik A, Kang SP, Rasco D et al. Phase I study of pembrolizumab (MK‐3475;anti‐PD‐1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015; 21: 4286–93. [DOI] [PubMed] [Google Scholar]
- 13. Brahmer JR, Drake CG, Wollner I et al. Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chakrabarti S, Dong H, Paripati HR et al. First report of dramatic tumor responses with ramucirumab and paclitaxel after progression on pembrolizumab in two cases of metastatic gastroesophageal adenocarcinoma. Oncologist 2018; 23: 840–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramakrishnan R, Gabrilovich DI. Novel mechanism of synergic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother 2013; 62: 405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol Res 2015; 3: 436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manegold C, Dingemans AM, Gray JE et al. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol 2016; 12: 194–207. [DOI] [PubMed] [Google Scholar]
- 18. Garber K. Promising early results for immunotherapy‐antiangiogenesis combination. J Natl Cancer Inst 2014; 106: pii: dju392. [DOI] [PubMed] [Google Scholar]
- 19. Kwilas AR, Ardiani A, Donahue RN et al. Dual effects of a targeted small‐molecule inhibitor (cabozantinib) on immune‐mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med 2014; 12: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langer CJ, Gadgeel SM, Borghaei H et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: A randomized, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet 2016; 17: 1497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]


