Abstract
Background
The aim of this study was to evaluate the usefulness of the presence of malignant pleural effusion (MPE) as a negative predictor of anti‐PD‐1 antibody efficacy.
Methods
A retrospective review of patients with advanced or recurrent non‐small cell lung cancer treated with an anti‐PD‐1 antibody between December 2015 and March 2018 at the National Cancer Center Hospital, Japan, was conducted. Progression‐free survival (PFS) and overall survival (OS) were compared between patients with and without MPE. Additional survival analysis according to PD‐L1 expression status was conducted. Univariate and multivariate analyses were performed.
Results
A total of 252 patients were identified before the commencement of anti‐PD‐1 antibody treatment: 33 with MPE and 219 without MPE. PFS and OS were significantly shorter in patients with MPE than in patients without MPE (median PFS 3.0 vs. 5.8 months, hazard ratio [HR] 1.7, P = 0.014; median OS 7.9 vs. 15.8 months, HR 2.1, P = 0.001). In patients with PD‐L1 expression in ≥ 1% of their tumor cells, the PFS of patients with MPE was significantly shorter than of patients without MPE (median PFS 3.1 vs. 6.5 months, HR 2.0, 95% confidence interval 1.0–3.5; P = 0.021). The presence of MPE was independently associated with a shorter PFS and OS in multivariate analysis.
Conclusion
The presence of MPE in patients administered an anti‐PD‐1 antibody is associated with shorter PFS and OS, regardless of the presence of PD‐L1 expression ≥ 1% of tumor cells.
Keywords: Anti‐PD‐1 antibody, malignant pleural effusion, nivolumab, non‐small cell lung cancer, pembrolizumab
Introduction
PD‐1/PD‐L1 checkpoint inhibitors, such as nivolumab, pembrolizumab, and atezolizumab, exhibit definite antitumor activity for the treatment of advanced non‐small cell lung cancer (NSCLC) and enable longer progression‐free survival (PFS) and overall survival (OS) periods than cytotoxic drugs. These agents are now approved and have become standard treatment options for patients with NSCLC. PD‐L1 expression in tumor cells is a predictor of the efficacy of PD‐1/PD‐L1 checkpoint inhibitors and is used for making treatment strategy decisions. In patients with advanced NSCLC and positive PD‐L1 expression in ≥ 50% of their tumor cells, pembrolizumab has become the standard of care for first‐line treatment.1 However, the response rate remains approximately 45%, and approximately 30% of patients progress during the first three months of pembrolizumab treatment. PD‐L1 expression alone is not sufficient as a definitive predictor, thus there remains a need to identify other predictors or patient characteristics capable of predicting the response of patients to PD‐1/PD‐L1 checkpoint inhibitors.
Malignant pleural effusion (MPE) occurs in approximately 15% of lung cancer patients.2 MPE is managed using several treatment options, including pleural drainage, pleurodesis, and pleuroperitoneal shunts. The presence of MPE is known to be an independent predictor of poor survival in patients with advanced NSCLC.3 For most patients with a good performance status whose lungs re‐expand after pleural drainage, systemic chemotherapy is recommended, as it is for patients without MPE. Some chemotherapeutic regimens containing an anti‐vascular endothelial growth factor (VEGF) antibody, such as bevacizumab, have shown promising efficacy and a high MPE control rate.4, 5, 6, 7 This tendency has also been observed with molecular target drugs, which have a higher efficacy than cytotoxic agents. Recent studies examining EGFR‐mutated NSCLC patients administered EGFR‐tyrosine kinase inhibitors (TKIs) have shown that the presence of MPE at baseline is associated with shorter PFS and OS.8, 9 These findings suggest that the presence of MPE might be a strong negative predictor of the efficacy of some agents.
Several studies have shown that the efficacy of PD‐1/PD‐L1 checkpoint inhibitors is correlated with several patient characteristics. EGFR mutation, a never‐smoker status, and a poor performance status (PS) were all correlated with a lower efficacy of PD‐1/PD‐L1 checkpoint inhibitors, compared to EGFR wild‐type, smoker status, and a good PS, respectively.10, 11, 12, 13, 14, 15 A previous study also reported that the presence of liver or lung metastases was a negative predictor of anti‐PD‐1 efficacy in patients with advanced NSCLC. However, no previous reports have compared the efficacy of anti‐PD‐1 antibodies between NSCLC patients with and without MPE. Thus, we retrospectively investigated the efficacy of anti‐PD‐1 antibodies in advanced NSCLC patients with or without MPE.
Methods
Patients
We retrospectively reviewed the medical records of patients with advanced or recurrent NSCLC who received nivolumab or pembrolizumab as first, second, or third‐line treatment between 1 December 2015 and 31 March 2018 at the National Cancer Center Hospital, Japan. The end of the follow‐up period was 31 July 2018.
Patients with positive pleural fluid cytology results, pleural effusion requiring drainage, or presenting with multiple pleural nodules and nodular pleural thickening with pleural effusion on a computed tomography (CT) scan were diagnosed as having MPE. We diagnosed the presence of MPE before commencing anti‐PD‐1 antibody treatment.
Tumor response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 using CT images. We did not consider an increase in pleural effusion as a progressive event.
PFS was defined as the interval between the first dose of anti‐PD‐1 antibody treatment and the date of clinical or radiographic disease progression or death from any cause; in the absence of confirmation of disease progression or death, data were censored at the last date the patient was known to be alive. OS was defined as the interval between the first dose of anti‐PD‐1 antibody treatment and the date of death from any cause; in the absence of confirmation of death, data were censored at the last date the patient was known to be alive.
PD‐L1 expression in the tumor cells of patients with NSCLC was analyzed using the commercially available PD‐L1 immunohistochemistry 22C3 pharmDx assay (Dako; Agilent Technologies, Santa Clara, CA, USA).16 Positive PD‐L1 expression in ≥ 1% of all tumor cells was classified as a positive result, while positive PD‐L1 expression in ≥ 50% was classified as strongly positive, consistent with the methodology used in other studies involving anti‐PD‐1 antibodies (Fig 1).1, 17, 18
Figure 1.
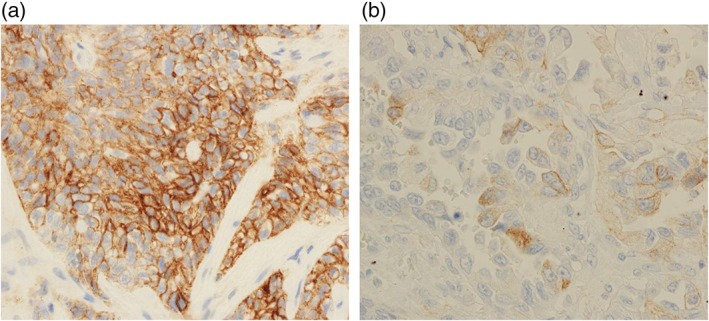
Immunohistochemical analysis of PD‐L1 expression in (a) strongly positive (≥ 50%) and (b) positive (≥ 1%) tumor cells.
Statistical analysis
Baseline characteristics were compared between patients with and without MPE using the Fisher's exact test for categorical variables. PFS and OS curves were estimated using the Kaplan–Meier method, and differences according to the absence or presence of MPE were evaluated using a log‐rank test. Univariate and multivariate analyses were performed using Cox proportional hazard regression models for performance status, smoking status, EGFR mutational status, PD‐L1 expression status, treatment line, and the presence of MPE. The covariates other than MPE were adopted based on the results of recent trials suggesting that they might affect the efficacy of PD‐1/PD‐L1 checkpoint inhibitors.1, 11, 13, 14, 15, 17, 19, 20 All P values were based on a one‐sided hypothesis, and values < 0.05 were considered statistically significant. All statistical analyses were performed using JMP Pro version 13.0.0 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 252 patients with advanced or recurrent NSCLC administered nivolumab or pembrolizumab were identified. The patient characteristics are summarized in Table 1. Twelve percent of the patients had an Eastern Cooperative Oncology Group PS of ≥ 2, 19% were never‐smokers, 7.9% had EGFR mutations, 13% had a PD‐L1 negative status, and 84% received an anti‐PD‐1 antibody as second or third‐line treatment. Of the 252 patients, 33 patients had MPE (cytologically confirmed malignant cells, n = 20; pleural effusion drainage, n = 26; presence of pleural lesions, n = 6). The baseline characteristics were not significantly different between patients with and without MPE. The median follow‐up period was 7.4 (range: 0.4–27.5) months.
Table 1.
Baseline characteristics (n = 252)
| Malignant pleural effusion | P | |||
|---|---|---|---|---|
| Characteristics | All, N (%) | No, N (%) | Yes, N (%) | |
| Patients | 252 | 219 | 33 | |
| Age | ||||
| Median (range) | 61 (30–83) | 63 (33–83) | 61 (30–79) | |
| ≥ 75 years | 28 (11) | 25 (11) | 3 (9.1) | 0.48 |
| Gender | 0.49 | |||
| Male | 172 (68) | 150 (68) | 22 (66) | |
| ECOG PS | 0.094 | |||
| 0–1 | 221 (88) | 195 (89) | 26 (79) | |
| ≥ 2 | 31 (12) | 24 (11) | 7 (21) | |
| Smoking status | 0.14 | |||
| Never‐smoker | 47 (19) | 38 (17) | 9 (27) | |
| Current or former smoker | 200 (79) | 176 (80) | 24 (73) | |
| Histology | 0.078 | |||
| Adenocarcinoma | 162 (64) | 135 (62) | 27 (82) | |
| Squamous | 58 (23) | 54 (25) | 4 (12) | |
| Other | 32 (13) | 30 (14) | 2 (6) | |
| Clinical stage | 0.35 | |||
| III/IV | 186 (74) | 163 (74) | 23 (70) | |
| Recurrence | 66 (26) | 56 (26) | 10 (30) | |
| EGFR mutated | 20 (7.9) | 17 (7.8) | 3 (9.1) | 0.61 |
| PD‐L1 22C3 status | 0.33 | |||
| < 1% | 33 (13) | 27 (12) | 6 (18) | |
| ≥ 1% | 132 (52) | 114 (52) | 18 (55) | |
| Brain metastasis | 55 (22) | 50 (23) | 5 (15) | 0.23 |
| Treatment line | 0.062 | |||
| 1 | 41 (16) | 32 (15) | 9 (27) | |
| 2/3 | 211 (84) | 187 (85) | 24 (73) | |
| Anti‐PD‐1 antibody | 0.34 | |||
| Nivolumab | 179 (71) | 157 (72) | 22 (67) | |
| Pembrolizumab | 73 (29) | 62 (28) | 11 (33) | |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Efficacy
The overall response rate (ORR) was similar in patients with and without MPE (ORR 24% vs. 26%; P = 0.49) (Table 2). Although there was no statistical significance, the disease control rate (DCR) in the patients with MPE was lower than in patients without MPE (DCR 58% vs. 42%; P = 0.068). None of the patients with MPE showed an increase in MPE before a progressive event. The subsequent treatment rate after disease progression among patients administered an anti‐PD‐1 antibody was similar between patients with and without MPE (57% vs. 56%; P = 1.00). The PFS and OS of patients with MPE were significantly shorter than in patients without MPE (median PFS 3.0 vs. 5.8 months, hazard ratio [HR] 1.7, 95% confidence interval [CI] 1.1–2.5, P = 0.014; median OS 7.9 vs. 15.8 months, HR 2.1; 95% CI 1.3–3.3, P = 0.001) (Fig 2).
Table 2.
Response to anti‐PD‐1 antibody
| Malignant pleural effusion | P | ||
|---|---|---|---|
| Response | No (n = 219) | Yes (n = 33) | |
| Best overall response, N (%) | |||
| Complete response | 1 (0.5) | 0 (0) | |
| Partial response | 57 (26) | 8 (24) | |
| Stable disease | 69 (32) | 6 (18) | |
| Progressive disease | 84 (38) | 17 (52) | |
| Not evaluable | 8 (3.7) | 2 (6) | |
| Objective response, N (%, 95% CI) | 58 (26, 21–33) | 8 (24, 13–41) | 0.49 |
| Disease control, N (%, 95% CI) | 127 (58, 51–64) | 14 (42, 27–59) | 0.068 |
CI, confidence interval.
Figure 2.
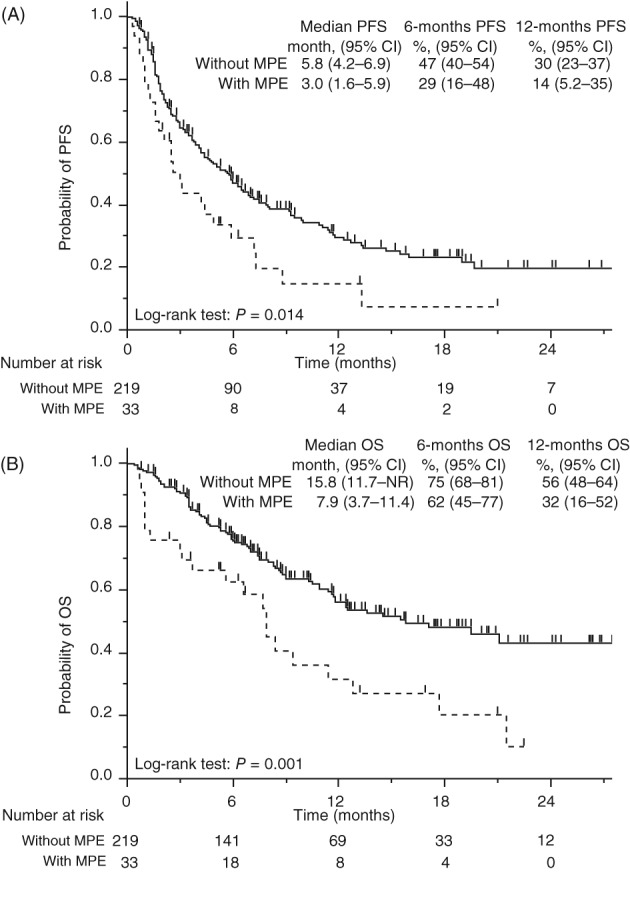
Kaplan–Meier curves of (a) progression‐free survival (PFS) and (b) overall survival (OS) of all patients. CI, confidence interval; MPE, malignant pleural effusion; NR, not reached. ( ) Without MPE, and (
) Without MPE, and ( ) With MPE.
) With MPE.
Additional survival analysis of all patients grouped according to PD‐L1 expression was performed. In the group with strongly positive PD‐L1 expression, the PFS of the patients with MPE was shorter but not significantly different from that of patients without MPE (median PFS 5.9 vs. 8.1 months, HR 1.8, 95% CI 0.75–3.9; P = 0.14) (Fig 3a). In the PD‐L1 expression‐positive group, the PFS of patients without MPE was significantly shorter than of patients with MPE (median PFS 3.1 vs. 6.5 months, HR 2.0. 95% CI 1.0–3.5; P = 0.021) (Fig 3b).
Figure 3.
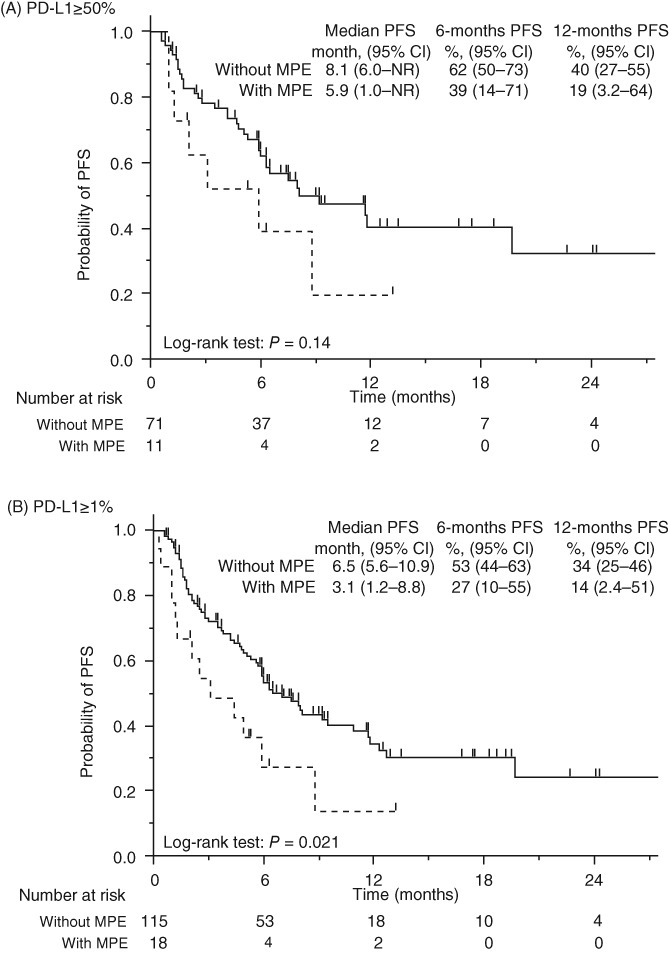
Kaplan–Meier curves of progression‐free survival (PFS) in patients with PD‐L1 expression in (a) ≥ 50% of tumor cells and (b) ≥ 1% of tumor cells. MPE, malignant pleural effusion; NR, not reached. ( ) Without MPE, and (
) Without MPE, and ( ) With MPE.
) With MPE.
Multivariate analysis showed that the presence of MPE was significantly and independently associated with shorter PFS (HR 2.0, 95% CI 1.1–3.5; P = 0.019) and OS (HR 2.4, 95% CI 1.2–4.6; P = 0.011) (Table 3). As is well known, first‐line treatment was significantly associated with longer PFS (HR 0.55, 95% CI 0.28–0.99; P = 0.046). In addition, a good PS was independently associated with longer OS (HR 0.38, 95% CI 0.19–0.83; P = 0.018).
Table 3.
Cox proportional hazard regression analyses
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P |
| PFS | ||||
| Malignant pleural effusion (+/−) | 1.7 (1.1–2.5) | 0.023 | 2.0 (1.1–3.5) | 0.019 |
| ECOG PS (2–3/0–1) | 2.1 (1.3–3.1) | 0.003 | 1.5 (0.74–2.7) | 0.24 |
| Smoking status (never/current or former) | 1.9 (1.3–2.7) | 0.002 | 1.5 (0.80–2.7) | 0.2 |
| EGFR mutated (+/−) | 1.8 (1.0–2.9) | 0.043 | 0.80 (0.29–1.9) | 0.63 |
| PD‐L1 status (≥ 1%/< 1%) | 0.59 (0.38–0.94) | 0.026 | 0.61 (0.36–1.1) | 0.085 |
| Treatment line (1/2–3) | 0.61 (0.37–0.96) | 0.03 | 0.55 (0.28–0.99) | 0.046 |
| OS | ||||
| Malignant pleural effusion (+/−) | 2.1 (1.3–3.3) | 0.003 | 2.4 (1.2–4.6) | 0.011 |
| ECOG PS (2–3/0–1) | 3.1 (2.1–5.4) | <0.001 | 2.7 (1.2–5.3) | 0.018 |
| Smoking status (never/current or former) | 1.1 (0.70–1.8) | 0.61 | 0.95 (0.45–1.8) | 0.87 |
| EGFR mutated (+/−) | 1.1 (0.49–2.0) | 0.88 | 0.15 (0.008–0.73) | 0.013 |
| PD‐L1 status (≥1%/<1%) | 0.53 (0.32–0.90) | 0.021 | 0.55 (0.29–1.1) | 0.075 |
| Treatment line (1/2–3) | 0.95 (0.52–1.6) | 0.85 | 0.86 (0.38–1.8) | 0.7 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PFS, progression free survival.
Discussion
In this retrospective study we showed that in patients who received anti‐PD‐1 antibodies, those with MPE had significantly shorter PFS and OS than those without MPE, and the presence of MPE was an independent negative predictor affecting PFS and OS, regardless of the presence of positive PD‐L1 expression.
Pleural effusion is often found during the clinical course of advanced lung cancer. MPE results from increased fluid production as a result of increased capillary permeability secondary to tumor implantation on the pleural surface or impaired fluid resorption caused by tumor invasion into the pleuromediastinal lymphatic system. The presence of MPE is reportedly an independent predictor of poor survival in patients with advanced NSCLC.2, 3 Several studies have shown that VEGF plays a pivotal role in the pathogenesis of MPE and that the level of VEGF in plasma is relatively high in patients with MPE.21, 22, 23 VEGF promotes the development of MPE by increasing vascular permeability and by promoting angiogenesis.24 VEGF is also known as an important regulatory factor that activates host vascular endothelial cells and promotes malignant proliferation, increasing oxygen and nutrients for tumor metastasis.25 On the other hand, VEGF has recently been identified as a key factor in tumor‐induced immunosuppression. In a mouse model, infusion of VEGF promoted the production of granulocyte‐differentiation antigen‐1‐positive myeloid‐derived suppressor cells (MDSCs) and blocked dendritic cell development.26 MDSCs cause the development of other immunosuppressive cells, such as regulatory T cells.27, 28 VEGF also decreased the cytotoxic activity of T cells expressing VEGF receptor 2, which was recovered by an anti‐VEGF antibody.29 Our results may be biologically plausible because VEGF may lead to the development of MPE and the ineffectiveness of the anti‐PD‐1 antibody.
Several trials have shown that PD‐L1 expression is a predictor of the efficacy of PD‐1/PD‐L1 checkpoint inhibitors. Pembrolizumab is highly effective in NSCLC patients with positive PD‐L1 expression in ≥ 50% of their tumor cells.1 PD‐L1 expression is also associated with the efficacy of other PD‐1/PD‐L1 checkpoint inhibitors13, 20 However, even if PD‐L1 expression is strongly positive, some patients exhibit disease progression immediately after receiving PD‐1/PD‐L1 checkpoint inhibitors. Therefore, additional predictors and patient characteristics are needed for optimal patient selection to achieve a good outcome and to avoid initial tolerance. Several subgroup analyses and retrospective studies have revealed that a poor PS, never‐smoker status, and EGFR mutations are negative predictors of the efficacy of PD‐1/PD‐L1 checkpoint inhibitors. Our results suggest that the presence of MPE could also be a strong negative predictor. Despite having a high PD‐L1 expression level, patients with MPE tended to have shorter PFS than those without MPE. Moreover, in multivariate analysis, the presence of MPE showed stronger associations with poor PFS than a poor PS, never smoking, EGFR mutations, or negative PD‐L1 expression.
In contrast, Tamiya et al. previously reported that liver or lung metastases, but not MPE, were predictors of efficacy in NSCLC patients administered anti‐PD‐1 antibodies.19 Moreover, the proportion of patients diagnosed with MPE in their sample was higher than in ours (44% vs. 13%). Some studies have reported that the incidence of MPE in lung cancer patients is approximately 15%,2, 30, 31 which is similar to our result. The discrepancy in results between the study by Tamiya et al. and ours, in which patients with MPE had shorter PFS than those without MPE, is most likely the result of differences in the definition of MPE. Cancer‐related pleural effusion can be classified as MPE or paramalignant pleural effusion. Paramalignant pleural effusion is associated with bronchial obstruction and distal pneumonitis, prior mediastinal irradiation, and hypoalbuminemia.32 These facts may suggest that Tamiya et al. included patients with paramalignant pleural effusions who would not have been diagnosed as such in our study.
Our study had several limitations. First, this study was conducted based on a retrospective review of medical records. Most of the patients had cytologically confirmed MPE, but pleural fluid cytology results were not available for some of the patients in the sample. Therefore, we classified patients with pleural effusion requiring drainage or those who presented with pleural nodules and nodular pleural thickening as having MPE, regardless of the cytology results. Second, consensus criteria for assessing the response of patients with MPE have not yet been established. We used RECIST version 1.1 and did not count an increase in pleural effusion as a progression event. Therefore, the reported PFS of patients with MPE in this trial might have been longer than the actual PFS. Finally, we did not examine the correlation between VEGF level and the efficacy of PD‐1/PD‐L1 checkpoint inhibitors. A recent study has shown that patients receiving a drug combination that included bevacizumab had fewer MDSCs, and larger numbers of type 1 helper T cells and cytotoxic T lymphocytes after treatment compared to patients who did not receive the drug combination.33 These efficacies are thought be a result of the reduction in MDSCs arising from treatment with bevacizumab, possibly leading to the recovery of effective antitumor immunity. Clinical trials of drug combinations including a PD‐1/PD‐L1 checkpoint inhibitor and agents targeting VEGF/VEGFR are underway for several kinds of cancer, including NSCLC. These future studies may help to clarify the mechanisms behind this phenomenon.
In conclusion, this study showed that the presence of MPE is a negative predictor of anti‐PD‐1 antibody efficacy in patients with advanced NSCLC. Therefore, future studies evaluating new treatment strategies for MPE in patients receiving anti‐PD‐1 antibody are required.
Disclosure
YM has served on speaker's bureaus for Cook Medical, Olympus, and AstraZeneca; and received research funding from Hitachi, Hitachi High‐Technologies, and Boston Scientific.
YG has had consulting/advisory roles for Eli Lilly, Chugai, Taiho Pharmaceutical, Boehringer Ingelheim, Pfizer, and Novartis; served on speaker's bureaus for AstraZeneca, Eli Lilly, Chugai, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, Bristol‐Myers Squibb, Pfizer, MSD, Shionogi Pharma, and Novartis; and received research funding from AbbVie, Eli Lilly, Taiho Pharmaceutical, Bristol‐Myers Squibb, and Ono Pharmaceutical.
SK has received research funding from AstraZeneca, Ono Pharmaceutical, and AbbVie; and received honoraria from AstraZeneca, Ono Pharmaceutical, Bristol‐Myers Squibb, and Chugai.
HH has received research funding from MSD, Bristol‐Myers Squibb, Ono Pharmaceutical, Merck Serono, Novartis, Astellas, Chugai, Taiho Pharmaceutical, and Genomic Health; and received honoraria from Eli Lilly.
YF has received research funding from AbbVie, AstraZeneca, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Incyte, Merck Serono, MSD, Novartis, and Bristol‐Myers Squibb; and served on speaker's bureaus from AstraZeneca, MSD, Taiho Pharmaceutical, Bristol‐Myers Squibb, and Ono Pharmaceutical.
NY has had consulting/advisory roles for Eisai, Takeda, Otsuka, and Boehringer Ingelheim; received research funding from Eli Lilly, Quintiles, Astellas, Chugai, Eisai, Taiho Pharmaceutical, Bristol‐Myers Squibb, Pfizer, Novartis, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Kyowa Hakko Kirin, Takeda, and Ono Pharmaceutical; and served on speaker's bureaus from Bristol‐Myers Squibb, Pfizer, AstraZeneca, Eli Lilly, Ono Pharmaceutical, and Chugai.
YO has received research funding from AstraZeneca, Chugai, Eli Lilly, Taiho Pharmaceutical, Pfizer, MSD, Novartis, Kyorin, Dainippon‐Sumitomo, and Ignyta; and received honoraria from AstraZeneca, Chugai, Eli Lilly, Taiho Pharmaceutical, Pfizer, MSD, Novartis, Daiichi Sankyo, Boehringer Ingelheim, and Bayer.
None of the remaining authors report any conflict of interest.
References
- 1. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 2. Naito T, Satoh H, Ishikawa H et al Pleural effusion as a significant prognostic factor in non‐small cell lung cancer. Anticancer Res 1997; 17: 4743–6. [PubMed] [Google Scholar]
- 3. Sugiura S, Ando Y, Minami H, Ando M, Sakai S, Shimokata K. Prognostic value of pleural effusion in patients with non‐small cell lung cancer. Clin Cancer Res 1997; 3: 47–50. [PubMed] [Google Scholar]
- 4. Masago K, Fujimoto D, Fujita S et al Response to bevacizumab combination chemotherapy of malignant pleural effusions associated with non‐squamous non‐small‐cell lung cancer. Mol Clin Oncol 2015; 3: 415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Usui K, Sugawara S, Nishitsuji M et al A phase II study of bevacizumab with carboplatin‐pemetrexed in non‐squamous non‐small cell lung carcinoma patients with malignant pleural effusions: North East Japan study group trial NEJ013A. Lung Cancer 2016; 99: 131–6. [DOI] [PubMed] [Google Scholar]
- 6. Kitamura K, Kubota K, Ando M et al Bevacizumab plus chemotherapy for advanced non‐squamous non‐small‐cell lung cancer with malignant pleural effusion. Cancer Chemother Pharmacol 2013; 71: 457–61. [DOI] [PubMed] [Google Scholar]
- 7. Fujita A, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy in patients with malignant pleural effusions from non‐small cell lung cancer: Cisplatin, ifosfamide, and irinotecan with recombinant human granulocyte colony‐stimulating factor support. Chest 2001; 119: 340–3. [DOI] [PubMed] [Google Scholar]
- 8. Atagi S, Goto K, Seto T et al Erlotinib for Japanese patients with activating EGFR mutation‐positive non‐small‐cell lung cancer: Combined analyses from two phase II studies. Future Oncol 2016; 12: 2117–26. [DOI] [PubMed] [Google Scholar]
- 9. Masuhiro K, Shiroyama T, Suzuki H et al Impact of pleural effusion on outcomes of patients receiving osimertinib for NSCLC harboring EGFR T790M. Anticancer Res 2018; 38: 3567–71. [DOI] [PubMed] [Google Scholar]
- 10. Lee CK, Man J, Lord S et al Checkpoint inhibitors in metastatic EGFR‐mutated non‐small cell lung cancer: A meta‐analysis. J Thorac Oncol 2017; 12: 403–7. [DOI] [PubMed] [Google Scholar]
- 11. Lisberg A, Cummings A, Goldman JW et al A phase II study of pembrolizumab in EGFR‐mutant, PD‐L1+, tyrosine kinase inhibitor (TKI) naïve patients with advanced NSCLC. Journal of Thoracic Oncology 2018; 13: 1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gettinger S, Politi K. PD‐1 axis inhibitors in EGFR‐ and ALK‐driven lung cancer: Lost cause? Clin Cancer Res 2016; 22: 4539–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin SY, Yang CY, Liao BC et al Tumor PD‐L1 expression and clinical outcomes in advanced‐stage non‐small cell lung cancer patients treated with nivolumab or pembrolizumab: Real‐world data in Taiwan. J Cancer 2018; 9: 1813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mezquita L, Auclin E, Ferrara R et al Association of the Lung Immune Prognostic Index with immune checkpoint inhibitor outcomes in patients with advanced non‐small cell lung cancer. JAMA Oncol 2018; 4: 351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roach C, Zhang N, Corigliano E et al Development of a companion diagnostic PD‐L1 immunohistochemistry assay for pembrolizumab therapy in non‐small‐cell lung cancer. Appl Immunohistochem Mol Morphol 2016; 24: 392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hui R, Garon EB, Goldman JW et al Pembrolizumab as first‐line therapy for patients with PD‐L1‐positive advanced non‐small cell lung cancer: A phase 1 trial. Ann Oncol 2017; 28: 874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 19. Tamiya M, Tamiya A, Inoue T et al Metastatic site as a predictor of nivolumab efficacy in patients with advanced non‐small cell lung cancer: A retrospective multicenter trial. PLoS One 2018; 13: e0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fafliora E, Hatzoglou C, Gourgoulianis KI, Zarogiannis SG. Systematic review and meta‐analysis of vascular endothelial growth factor as a biomarker for malignant pleural effusions. Physiol Rep 2016; 4: e12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ribeiro SC, Vargas FS, Antonangelo L et al Monoclonal anti‐vascular endothelial growth factor antibody reduces fluid volume in an experimental model of inflammatory pleural effusion. Respirology 2009; 14: 1188–93. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Mathy NW, Lu H. The role of VEGF in the diagnosis and treatment of malignant pleural effusion in patients with nonsmall cell lung cancer (review). Mol Med Rep 2018; 17: 8019–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep 2013; 15: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harmey JH, Bouchier‐Hayes D. Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: Implications for anti‐angiogenic therapy. Bioessays 2002; 24: 280–3. [DOI] [PubMed] [Google Scholar]
- 26. Gabrilovich D, Ishida T, Oyama T et al Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998; 92: 4150–66. [PubMed] [Google Scholar]
- 27. Huang B, Pan PY, Li Q et al Gr‐1 + CD115+ immature myeloid suppressor cells mediate the development of tumor‐induced T regulatory cells and T‐cell anergy in tumor‐bearing host. Cancer Res 2006; 66: 1123–31. [DOI] [PubMed] [Google Scholar]
- 28. Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid‐derived suppressor cells promote cross‐tolerance in B‐cell lymphoma by expanding regulatory T cells. Cancer Res 2008; 68: 5439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziogas AC, Gavalas NG, Tsiatas M et al VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor type 2. Int J Cancer 2012; 130: 857–64. [DOI] [PubMed] [Google Scholar]
- 30. Cohen S, Hossain SA. Primary carcinoma of the lung. A review of 417 histologically proved cases. Dis Chest 1966; 49: 67–74. [DOI] [PubMed] [Google Scholar]
- 31. Emerson GL, Emerson MS, Sherwood CE. The natural history of carcinoma of the lung. J Thorac Surg 1959; 37: 291–304. [PubMed] [Google Scholar]
- 32. Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10: 1907–13. [DOI] [PubMed] [Google Scholar]
- 33. Feng PH, Chen KY, Huang YC et al Bevacizumab reduces S100A9‐positive MDSCs linked to intracranial control in patients with EGFR‐mutant lung adenocarcinoma. J Thorac Oncol 2018; 13: 958–67. [DOI] [PubMed] [Google Scholar]


