Abstract
Background
It remains unclear why radiation clinically provides a synergistic effect when combined with immune checkpoint inhibitors such as nivolumab. The purpose of our study was to retrospectively evaluate whether the therapeutic efficacy of nivolumab is improved as a result of a history of radiotherapy (RT) in patients with previously treated advanced non‐small cell lung cancer (NSCLC).
Methods
From February 2016 to December 2017, 124 consecutive patients were administered nivolumab for pretreated advanced NSCLC. The patients were divided into RT and non‐RT groups.
Results
Sixty‐six (53%) of the 124 patients had been administered RT before the initiation of nivolumab, 52 (42%) received extracranial RT, and 40 (32%) were treated with thoracic RT. The median number of nivolumab cycles was 4 (range: 1–43). The overall response rate (ORR) and disease control rate (DCR) of nivolumab in all patients were 28.0% and 58.4%, respectively. The ORR (36.4%) was significantly higher in patients who had received previous RT than in patients who had not received any RT (19%). The therapeutic efficacy of nivolumab was particularly noteworthy in patients with non‐adenocarcinoma and squamous cell carcinoma histology administered extracranial RT, with ORRs of 48.3% and 52.6%, and DCRs of 87.1% and 84.2%, respectively.
Conclusion
Previous RT was an independent prognostic marker of favorable prognosis after nivolumab administration and improved the response rate to nivolumab treatment. Previous RT was clinically identified to have a synergistic effect with nivolumab treatment, increasing the response rate and improving the outcome of patients with advanced NSCLC.
Keywords: Nivolumab, NSCLC, PD‐1, radiotherapy, synergistic effect
Introduction
Nivolumab, a PD‐1 antibody, is an immune checkpoint inhibitor (ICI) that has been proven to be active in patients with several different tumor types. Nivolumab has been shown to have an overall response rate (ORR) of approximately 20% in patients with previously treated non‐small cell lung cancer (NSCLC).1, 2 However, nivolumab is not effective in more than 40% of NSCLC patients who experience disease progression, despite nivolumab treatment. To improve the efficacy of this promising immunotherapy, additional modalities such as chemotherapy, radiotherapy (RT), or other ICIs may also be administered to patients in whom ICIs have not been completely effective. Interestingly, RT stimulates a systemic immune response and causes the release of tumor‐related antigens.3 Recent preclinical studies have demonstrated a synergistic tumor response with RT and the blockade of PD‐1.4, 5, 6 It is possible that tumor‐specific immunity is induced by radiation. Although RT plays an important role in the local control and elimination of tumors, it also contributes to the induction of antitumor immune responses, and the immunosuppressive and immunostimulatory effects of RT.6 Radiation‐induced cell death causes a release of danger signals such as HMGB1, ATP, and HSP70, and the dendritic cells can stimulate activated CD8 T cells and tumor‐specific T cells.6 In addition to immune activation, RT induces transforming growth factor‐β (TGF‐β), an immunosuppressive cytokine. To reduce the immunosuppressive functions, a combination of RT and TGF‐β inhibitors has been identified as a valuable option in preclinical settings.6 A recent experimental study demonstrated that fractionated RT increases PD‐L1 surface expression on tumor cells, suggesting a key rationale for the combination of RT with ICIs.7
Of the 98 patients registered in the KEYNOTE‐001 phase I trial, Shaverdian et al. reported that the duration of progression‐free survival (PFS) with pembrolizumab was significantly longer in patients previously administered RT than in those not treated with RT.8 Fiorica et al. also reported that nivolumab treatment after hypofractionated RT improved the outcome in 35 patients with pretreated or metastatic NSCLC.9 These results suggest that previous RT clinically improves tumor response and immune reaction to ICIs, such as nivolumab or pembrolizumab. However, the synergistic effect of ICIs and previous RT was not fully elucidated in these studies. As the former study was a phase I trial, pembrolizumab was administered at different doses and treatment deliveries.8 The latter study was limited by a small sample of only 35 patients.9 Analysis of these studies shows that immunotherapy after previous RT prolongs survival;8, 9 however, neither the ORRs of ICIs after previous RT nor the populations that might benefit from ICI treatment were included. Little detailed clinical data of the effect of ICI administration after previous RT has been reported; therefore, we attempted to elucidate the potential synergistic antitumor effect of nivolumab after RT in patients with previously treated NSCLC.
Methods
Patient eligibility and data collection
The eligibility criteria for our retrospective analysis were: histologically or cytologically proven advanced NSCLC with stage III or IV disease or recurrence after surgical resection; age > 20 years; patients with disease progression after at least one prior cytotoxic chemotherapy treated with nivolumab; EGFR mutation‐positive patients administered EGFR‐tyrosine kinase inhibitors prior to any cytotoxic chemotherapy; and patients with available ORR data of nivolumab according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Patients were excluded if they had: a concomitant serious illness, such as myocardial infarction in the previous three months; uncontrolled angina pectoris, heart failure, uncontrolled diabetes mellitus, uncontrolled hypertension, interstitial pneumonia, or lung disease; an infection or other disease contraindicating chemotherapy; or were pregnant or breastfeeding. This study was approved by the institutional ethics committee of the Saitama Medical University International Medical Center.
Treatment and efficacy evaluation
Nivolumab was intravenously administered at 3 mg/kg every two weeks. A complete blood cell count, differential count, routine chemistry measurements, physical examination, and toxicity assessment were performed on a weekly basis. Acute toxicity was graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The tumor response was evaluated according to RECIST version 1.1.10
Statistical analysis
P < 0.05 indicated statistical significance. Fisher's exact tests were conducted to examine the association between the categorical variables. The Kaplan–Meier method was used to estimate survival as a function of time, and survival differences were analyzed by log‐rank tests. PFS was defined as the time from the initiation of nivolumab therapy to tumor recurrence or death from any cause, while overall survival (OS) was defined as the time from the initiation of nivolumab therapy to death from any cause. Statistical analyses were performed using GraphPad Prism 4 and JMP 8.0.
Results
Patient demographics
From February 2016 to December 2017, 152 patients with pretreated NSCLC were administered nivolumab. Twenty‐eight patients were excluded because of inadequate medical information or the absence of an evaluable target lesion. Thus, a total of 124 patients (n males = 93, n females = 31; median age: 69 years; range: 31–85 years) were eligible for analysis. Patient characteristics are listed in Table 1. A total of 99 patients had a smoking history. Clinical staging indicated that 27 patients had stage III disease, 77 had stage IV disease, and 20 patients developed recurrence after surgical resection. The patients were divided into RT and non‐RT groups.
Table 1.
Comparison of demographics in patients treated with or without RT before nivolumab
| Variables | All patients (n = 124) |
Patients administered any RT before Nivo (n = 66) | Patients not administered RT before Nivo (n = 58) | P |
|---|---|---|---|---|
| Age | ||||
| ≦ 69/> 69 | 65/59 | 36/30 | 29/29 | 0.71 |
| Gender | ||||
| Male/female | 93/31 | 51/15 | 42/16 | 0.54 |
| Smoking | ||||
| Yes/no | 99/25 | 52/14 | 47/11 | 0.82 |
| ECOG PS | ||||
| 0/1–3 | 59/65 | 31/35 | 18/40 | 0.09 |
| Stage | ||||
| III/IV | 27/97 | 20/46 | 7/51 | 0.71 |
| T factor | ||||
| T1–2/T3–4 | 68/56 | 35/31 | 33/25 | 0.58 |
| N factor | ||||
| N0/N1‐3 | 20/104 | 12/54 | 8/50 | 0.62 |
| Histology | ||||
| Adeno/non‐adeno | 65/59 | 31/35 | 34/24 | 0.21 |
| EGFR mutation status | ||||
| Mutant/wild | 14/104 | 10/51 | 4/53 | 0.15 |
| Nivo response | ||||
| CR or PR/SD or PD | 35/89 | 24/44 | 11/47 | 0.04 |
| White blood cells† | ||||
| High/low | 65 / 59 | 32/34 | 33/25 | 0.37 |
| Neutrophils1 | ||||
| High/low | 64 / 60 | 33/33 | 31/27 | 0.72 |
| Lymphocytes1 | ||||
| High/low | 62 / 62 | 26/40 | 36/22 | 0.01 |
Bold values indicates statistically significance.
Laboratory findings before nivolumab administration. IV, stage IV including recurrence after surgical resection; adeno, adenocarcinoma; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; Nivo, nivolumab; non‐adeno, non‐adenocarcinoma; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease; WBC, white blood cell.
The percentages of patients with an Eastern Cooperative Oncology Group performance score (PS) of 0–1 in the RT and non‐RT groups were 75% (50/66) and 82% (48/58), respectively, without significant difference. There were 65 patients with adenocarcinoma (AC), 38 with squamous cell carcinoma (SQC), and 21 with other histologies. EGFR mutation analysis was performed: 104 patients had wild‐type EGFR, 14 harbored mutant EGFR, and 6 patients had unknown EGFR status. Table 1 shows a comparison of the groups prior to nivolumab administration. The patient demographics in both groups were well balanced, except for the lymphocyte count.
Sixty‐six patients were administered any RT prior to nivolumab treatment. Of these 66 patients, 24 were treated with concurrent platinum‐based chemoradiotherapy (50–60 Gy), 16 with palliative thoracic RT (30–40 Gy), 14 with palliative bone RT (8–30 Gy), and 11 with cranial RT (30–50 Gy). In terms of systemic chemotherapy prior to nivolumab treatment, 118 patients were treated with platinum‐based regimens and 6 with non‐platinum regimens. In the 66 patients administered any previous RT, 52 were treated with extracranial RT and 40 with thoracic RT. Patients were subdivided into three groups for further analysis: any previous RT (n = 66), extracranial RT (n = 52), and thoracic RT (n = 40).
Palliative RT after nivolumab administration was administered to 25 of 66 patients (37.8%) who had undergone any previous RT (2 in thoracic sites, 13 in bone sites, 2 in lymph node metastases, and 8 in brain metastases) and 23 (of 58 patients 36.6%) without previous RT (4 in thoracic sites, 11 in bone sites, 2 in lymph node metastases and 6 in brain metastases), without statistical significance (37.8% vs. 36.6%; P = 0.85).
Treatment delivery and response rate
The median number of nivolumab cycles was 4 (range: 1–43). The ORR and disease control rate (DCR) of nivolumab were 28.0% (95% confidence interval [CI] 20.1–35.9%) and 58.4% (95% CI 49.8–67.0%), respectively. Furthermore, analysis of all patients according to the number of lymphocytes showed an ORR of nivolumab treatment of 32% (20/62) in patients with low lymphocytes and 27% (17/62) in patients with high lymphocytes (P = 0.69). The median duration of follow‐up after RT in the 66 patients administered any RT prior to nivolumab treatment was 314 days (range: 12–3768). We used this median value of 314 days as a cutoff, and found that the ORR and DCR in 35 patients with a follow‐up of < 314 days were 42% and 71%, and those in 31 patients with a follow‐up of > 314 days were 29% and 64%, respectively, demonstrating no significant difference between the two groups.
The ORR (36.4%, 95% CI 24.8–48.0%) in patients treated with previous RT was significantly higher than in patients without previous RT (19%, 95% CI 8.9–29.1%). The ORRs and DCRs of nivolumab in patients with or without previous RT are listed in Table 2. There was no statistically significant difference in the ORRs and DCRs among patients administered previous RT, extracranial RT, or thoracic RT. In the analysis according to histology, a statistically significant difference in the DCR, but not the ORR, was observed between AC and non‐AC histologies among patients with any previous RT, extracranial RT, and thoracic RT. No statistically significant differences in the ORRs and DCRs were observed between patients with stage III and IV. However, the ORR of patients with non‐AC histology and wild‐type EGFR seemed to be higher than in patients with AC histology, but the difference was not statistically significant. Among patients with SQC histology, the ORRs and DCRs were 43.4% and 78.2% in patients administered any previous RT (n = 23), 52.6% and 84.2% in patients administered extracranial RT (n = 19), and 52.6% and 89.4% in patients administered thoracic RT (n = 19), respectively.
Table 2.
Response of nivolumab in patients treated with or without previous RT
| Variables | CR | PR | SD | PD | ORR (%) | DCR (%) |
|---|---|---|---|---|---|---|
| Any previous RT (n = 66) | 1 | 23 | 21 | 21 | 36.4% | 68.2% |
| Adeno (n = 31) | 1 | 8 | 7 | 15 | 29.0% | 51.6% |
| Non‐adeno (n = 35) | 0 | 15 | 14 | 6 | 42.8% | 80.0% |
| EGFR wild type (n = 51) | 1 | 22 | 16 | 12 | 45.1% | 76.4% |
| EGFR mutant (n = 10) | 0 | 1 | 1 | 8 | 10.0% | 20.0% |
| Stage III (n = 20) | 0 | 8 | 6 | 6 | 40.0% | 70.0% |
| Stage IV (n = 37) | 0 | 12 | 11 | 14 | 32.4% | 62.2% |
| Extracranial RT (n = 52) | 1 | 19 | 17 | 15 | 38.4% | 71.2% |
| Adeno (n = 21) | 1 | 4 | 5 | 11 | 23.8% | 47.6% |
| Non‐adeno (n = 31) | 0 | 15 | 12 | 4 | 48.3% | 87.1% |
| EGFR wild type (n = 44) | 1 | 19 | 12 | 12 | 45.5% | 72.7% |
| EGFR mutant (n = 4) | 0 | 0 | 1 | 3 | 0.0% | 25.0% |
| Stage III (n = 19) | 0 | 8 | 5 | 6 | 42.1% | 68.4% |
| Stage IV (n = 24) | 0 | 8 | 8 | 8 | 33.3% | 66.7% |
| Thoracic RT (n = 40) | 0 | 17 | 14 | 9 | 42.5% | 77.5% |
| Adeno (n = 11) | 0 | 3 | 2 | 6 | 27.2% | 45.5% |
| Non‐adeno (n = 29) | 0 | 14 | 12 | 3 | 48.3% | 86.9% |
| EGFR wild type (n = 35) | 0 | 17 | 10 | 8 | 48.6% | 77.1% |
| EGFR mutant (n = 1) | 0 | 0 | 0 | 1 | 0.0% | 0.0% |
| Stage III (n = 19) | 0 | 8 | 6 | 5 | 42.1% | 73.7% |
| Stage IV (n n = 14) | 0 | 6 | 5 | 3 | 42.8% | 78.6% |
| No previous RT (n = 58) | 0 | 11 | 17 | 30 | 18.9% | 46.5% |
| Adeno (n = 34) | 0 | 6 | 10 | 18 | 17.6% | 47.1% |
| Non‐adeno (n = 24) | 0 | 5 | 7 | 12 | 20.8% | 50.0% |
| EGFR wild type (n = 53) | 0 | 11 | 14 | 28 | 20.7% | 47.2% |
| EGFR mutant (n = 4) | 0 | 0 | 2 | 2 | 0.0% | 50.0% |
| Stage III (n = 7) | 0 | 0 | 2 | 5 | 0.0% | 28.6% |
| Stage IV (n = 40) | 0 | 6 | 12 | 22 | 15.0% | 45.0% |
Adeno, adenocarcinoma; CR, complete response; DCR, disease control rate; non‐adeno, non‐adenocarcinoma; ORR, overall response rate; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease.
Survival analysis and toxicity
The median PFS and OS rates after nivolumab administration in all patients were 132 and 561 days, respectively. Of the 124 patients, 64 died and 101 experienced recurrence after initial nivolumab treatment. The median PFS of patients administered any previous RT (n = 66), extracranial RT (n = 52), thoracic RT (n = 40), and no previous RT (n = 58) were 204, 206, 233, and 79 days, respectively, and the median survival times were 562 days, not reached (NR), NR, and 524 days, respectively (Fig S1). Univariate and multivariate analyses were performed in all patients (Table 3). In univariate analysis, gender, smoking, histology, any previous RT, extracranial RT, and thoracic RT were identified as significant prognostic markers for PFS, while PS and neutrophil count were significant predictors for OS. In multivariate analysis, we included variables with P < 0.05 in univariate analysis. Multivariate analysis confirmed that any previous RT and smoking were independent prognostic factors for poor PFS, whereas PS was the only significant prognostic marker for OS (Table 3). Figure 1 shows the Kaplan–Meier survival curves according to any previous RT, extracranial RT, and thoracic RT.
Table 3.
Univariate and multivariate survival analyses
| Number of patients | PFS | OS | |||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||
| Variables | (n = 124) | Median PFS (P) | HR, 95% CI (P) | MST (M) (P) | HR, 95% CI (P) |
| Age ≦ 69/> 69 | 65/59 | 103/140 days (0.49) | ‐ | 709/524 days (0.68) | |
| Gender Male/female | 93/31 | 184/68 days (0.01) | 1.03(0.73–1.41) (0.85) | 709/445 days (0.27) | |
| Smoking Yes/no | 99/25 | 184/60 days (< 0.01) | 1.44(1.01–2.05) (0.04) | 709/284 days (0.24) | |
| ECOG PS 0/1–3 | 59/65 | 177/81 days (0.12) | — | 709/179 days (< 0.01) | 1.49(1.07–2.00) (0.01) |
| Stage III/IV | 27/97 | 139/176 days (0.89) | — | NR/528 days (0.40) | |
| LN metastasis Yes/no | 20/104 | 139/245 days (0.49) | — | 524/NR days (0.19) | |
| Histology Adeno/non‐adeno | 65/59 | 85/195 days (0.02) | 1.12(0.96–1.39) (0.29) | 528/709 days (0.38) | |
| Any RT Yes/no | 66/58 | 204/79 days (0.02) | 1.30(0.06–1.59) (0.01) | 562/524 days (0.48) | |
| Thoracic RT Yes/no | 40/58 | 233/79 days (< 0.01) | — | NR/528 days (0.41) | |
| Extracranial RT Yes/no | 52/58 | 206/79 days (0.01) | — | NR/528 days (0.29) | |
| White blood cells† High/low | 65/59 | 128/144 days (0.39) | — | 478/NR days (0.12) | |
| Neutrophils† High/low | 64/60 | 118/177 days (0.15) | — | 406/778 days (0.05) | 1.25(0.97–1.62) (0.07) |
| Lymphocytes† High/low | 62/62 | 180/129 days (0.35) | — | 778/409 days (0.14) | |
Laboratory findings before nivolumab administration. IV, stage IV including recurrence after surgical resection; CI, confidence interval; adeno, adenocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LN, lymph node; M, months; MST, median survival time; Nivo, nivolumab; non‐adeno, non‐adenocarcinoma; OS, overall survival; PFS, progression‐free survival; PR, partial response; RT, radiotherapy.
Figure 1.
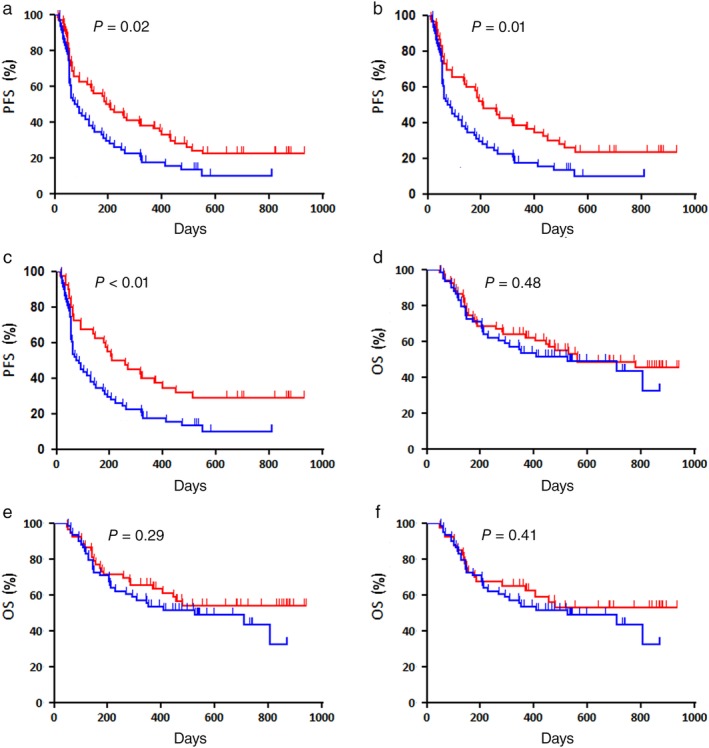
Kaplan–Meier survival curves of progression‐free survival (PFS) according to (a) any previous radiotherapy (RT) ( ) RT(+) (n = 66) and (
) RT(+) (n = 66) and ( ) RT(−) (n = 58), (b) extracranial RT (
) RT(−) (n = 58), (b) extracranial RT ( ) RT(+) (n = 52) and (
) RT(+) (n = 52) and ( ) RT(−) (n = 58), and (c) thoracic RT, (
) RT(−) (n = 58), and (c) thoracic RT, ( ) RT(+) (n = 40) and (
) RT(+) (n = 40) and ( ) RT(−) (n = 58). A statistically significant difference in PFS was observed between patients treated with and without RT. Kaplan–Meier survival curves of overall survival (OS) according to (d) any previous RT (
) RT(−) (n = 58). A statistically significant difference in PFS was observed between patients treated with and without RT. Kaplan–Meier survival curves of overall survival (OS) according to (d) any previous RT ( ) RT(+) (n = 66) and (
) RT(+) (n = 66) and ( ) RT(−) (n = 58), (e) extracranial RT (
) RT(−) (n = 58), (e) extracranial RT ( ) RT(+) (n = 52), and (
) RT(+) (n = 52), and ( ) RT(−) (n = 58) and (f) thoracic radiotherapy (
) RT(−) (n = 58) and (f) thoracic radiotherapy ( ) RT(+) (n = 40) and (
) RT(+) (n = 40) and ( ) RT(−) (n = 58). No statistically significant difference in OS was observed between the patients treated with and without RT.
) RT(−) (n = 58). No statistically significant difference in OS was observed between the patients treated with and without RT.
Figure 2 shows a forest plot of PFS and OS according to RT administration prior to nivolumab treatment for each variable. Compared to no RT, previous RT was significantly linked to favorable PFS in patients with wild‐type EGFR and stage IV disease, while extracranial RT and thoracic RT yielded significantly better PFS in patients with non‐AC histology and wild‐type EGFR, and better OS in patients with non‐AC histology (Figs 2, 3).
Figure 2.
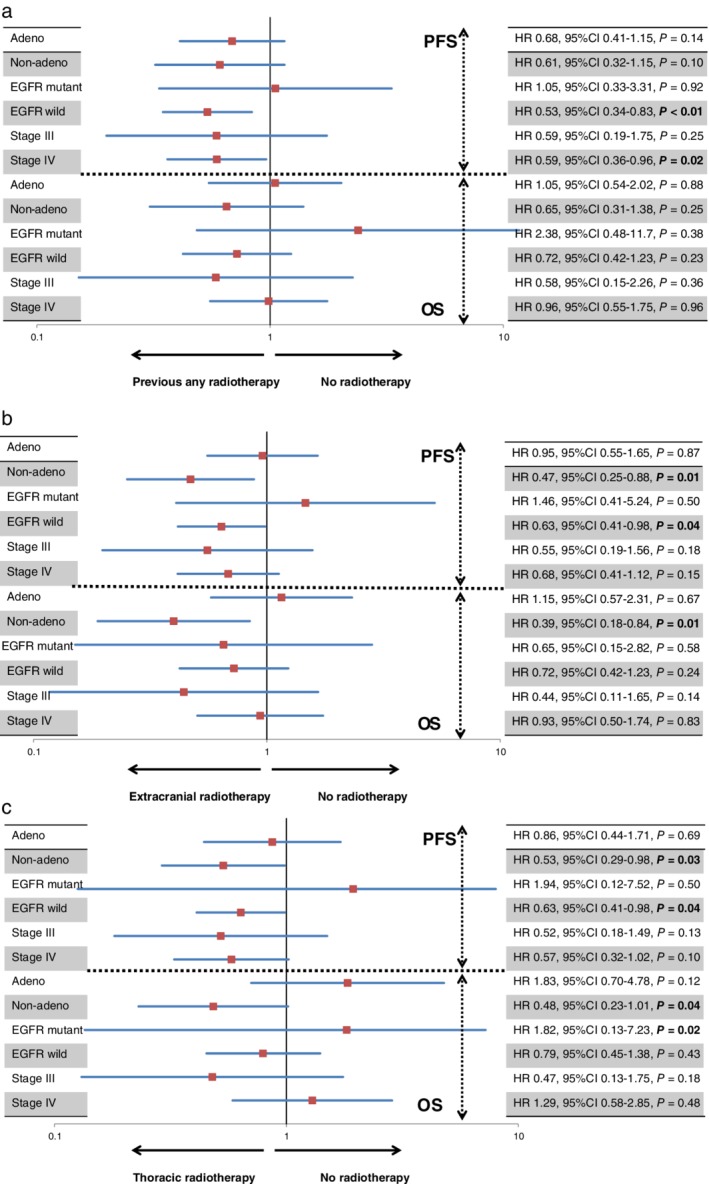
(a) Forest plots of progression‐free survival (PFS) and overall survival (OS) according to any previous radiotherapy (RT) before nivolumab administration for each variable. Patients with EGFR wild type or at stage IV administered any previous RT exhibited significantly better PFS than patients not administered RT. (b) Forest plots of PFS and OS according to extracranial RT before nivolumab administration for each variable. A statistically significant difference in PFS was observed in patients with EGFR wild type and non‐adenocarcinoma treated with and without extracranial RT. Moreover, patients with non‐adenocarcinoma administered extracranial RT yielded significantly favorable OS compared to those not administered extracranial RT. (c) Forest plots of PFS and OS according to thoracic RT before nivolumab administration for each variable. Patients with non‐adenocarcinoma administered thoracic RT yielded significantly favorable PFS and OS compared to those not administered extracranial RT. Thoracic RT yielded significantly better PFS in patients with EGFR wild type and poor OS in those with EGFR mutations.
Figure 3.
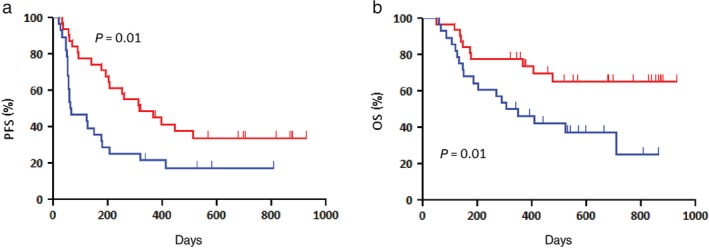
Patients (n = 59) with non‐adenocarcinoma administered extracranial radiotherapy (RT) exhibited significantly better (a) progression‐free survival (PFS) and (b) overall survival (OS) than those not administered extracranial RT ( ) RT(+) (n = 31) and (
) RT(+) (n = 31) and ( ) RT(−) (n = 28). The one and two‐year OS rates were 77% and 65%, respectively.
) RT(−) (n = 28). The one and two‐year OS rates were 77% and 65%, respectively.
In the analysis of pulmonary toxicities, 8 (20%) patients treated with previous thoracic RT experienced treatment‐related pulmonary toxicities compared to 12 (14%) patients not administered thoracic RT, without any statistical significance. Furthermore, no statistically significant difference in the incidence of grade 3 or higher pulmonary adverse events was observed between patients with or without a history of thoracic RT.
Discussion
This study was a retrospective evaluation of the efficacy of nivolumab treatment according to a history of previous RT in patients with previously treated NSCLC. We found that any previous RT was an independent prognostic marker of a favorable prognosis with nivolumab administration and could markedly improve the response rate and outcome of nivolumab treatment. The ORR of nivolumab was particularly improved in patients with non‐AC histology and wild‐type EGFR, with an increase of more than 40% if any previous RT was performed prior to nivolumab administration, whereas that of nivolumab in patients without previous RT was similar to that observed in previous phase III studies.1, 2 In addition, the frequency of low lymphocytes was significantly higher in patients administered previous RT; however, no statistically significant difference in the ORR of nivolumab was observed between patients with and without any previous RT. Therefore, we consider that the number of lymphocytes does not bias the efficacy of nivolumab, although it remains unclear why there is a trend of low lymphocytes in the RT group. Our detailed survival analysis also revealed that nivolumab increased PFS and OS in patients with non‐AC histology who were administered extracranial RT prior to nivolumab therapy. Of the 59 patients with non‐AC histology, SQC histology was noted in 38 (64.4%). There was no significant difference in OS between patients with and without previous RT in our study; however, any sequential treatment after nivolumab may have biased these results. In addition, the frequency of palliative RT administration after nivolumab treatment was not significantly different between patients with and without previous RT; thus, it did not affect the survival difference between the groups. We believe that any previous RT contributes to prolonged OS after the initiation of nivolumab. Based on these results, further investigation in prospective studies evaluating the efficacy of nivolumab following any previous RT is warranted. Extracranial RT seemed to increase the efficacy of nivolumab administration more than any RT, including for brain metastases, although the mechanism remains unclear.
Shaverdian et al. reported that any previous treatment with RT in patients with advanced NSCLC receiving pembrolizumab was associated with longer PFS and OS.9 They analyzed the clinical features of a subset of 97 patients administered pembrolizumab in the phase I KEYNOTE001 trial. Forty‐two (43%) of the 97 patients were administered any previous RT before the initiation of pembrolizumab, 38 (39%) were administered extracranial RT, and 24 (25%) were administered thoracic RT. The PFS (6.3 months) and OS (11.6 months) rates of patients who underwent extracranial RT were significantly longer than in patients who did not undergo extracranial RT (2.0 and 5.3 months, respectively). In their analysis according to the type of previous RT administered, extracranial RT seemed to improve prognosis after pembrolizumab compared to any RT. This phenomenon suggests that previous RT, except to the brain, strongly contributes to the synergistic effect of ICIs, which is similar to the results of our study. Fiorica et al. also confirmed the synergistic effect of RT on nivolumab against advanced NSCLC.8 Their study included 15 patients previously administered RT and 20 patients never administered RT, and PFS and OS after nivolumab treatment were compared. The outcome of patients administered RT prior to nivolumab treatment was markedly better than that of patients who were not. However, the relationship between the response rate of ICIs and previous RT remains unclear. To our knowledge, our study is the first to verify the improvement in the ORR of nivolumab after any previous RT. Nivolumab administration after RT increased the response rate nearly two‐fold (36.4% vs. 18.9%); a favorable trend was also observed in patients with non‐AC histology and wild‐type EGFR. Patients with SQC histology achieved an ORR of 52.6% and a DCR of 89.4% with nivolumab treatment. We found that previous RT yields a different synergistic effect in the response to nivolumab treatment according to histological type. The combined sequence of RT and nivolumab may be a promising treatment in patients with non‐AC histology, particularly SQC histology. Further study is warranted to elucidate the additive effect of radiation on the efficacy of nivolumab according to the histological type in advanced NSCLC.
Recently, Britschgi et al. reported the existence of abscopal effects induced by RT and nivolumab in patients with metastatic NSCLC, suggesting that this represented clinical determination of the synergistic effect of local RT and ICIs.11 An abscopal effect is a phenomenon wherein untreated tumor lesions regress after local treatment, such as RT. Theoretically, radiation‐triggered antitumor T cells are thought to kill tumor cells outside the irradiated tumor sites.12 However, this depends on many factors, such as whether tumor‐specific T cells are induced by RT and are effective for tumor control. In a preclinical study, Zhang et al. reported that the synergistic local and abscopal effects of hypofractionated RT and anti‐PD‐1 treatment are different in different tumor cell lines.13 Yuan et al. presented a case of lung SQC with systemic tumor regression by RT even after nivolumab had failed.14 Their result suggests that RT stimulated immune activation in nivolumab‐refractory circumstances and activated T cells killed tumor cells, even outside irradiated sites. Moreover, Meng et al. reported that cells in the immune microenvironment, such as CD4+, CD8+, and FOXP3+ tumor‐infiltrating lymphocytes, are likely different between non‐SQC and SQC patients.15 Although it is unknown whether the synergistic effect of RT and ICIs is stronger in SQC than in AC tumors, our results suggest that nivolumab has a stronger synergistic effect after previous RT in patients with SQC histology than those with AC histology.
Studies have evaluated the adverse events in patients treated with both ICIs and thoracic RT.8, 9, 16, 17 No statistically significant difference in the frequency of grade 3 or higher pulmonary toxicities was observed in patients with or without a history of previous thoracic RT prior to ICI treatment. Our results are consistent with these findings. Therefore, RT prior to nivolumab administration is acceptable in terms of safety.
Our study has several limitations. First, as this was a retrospective study with a small sample, bias may be present in our results. Second, the patients in our study received different total doses and schedules of RT according to the stage or extent of their tumor, therefore we could not confirm whether the efficacy of nivolumab varies according to the total RT dose. However, an optimal trend between the presence of previous RT and the efficacy of nivolumab was identified. Finally, it remains unclear why previous RT enhances the response rate of nivolumab. Although many experimental studies have explored the relationship between radiation and immune reaction, little is known about the clinical significance of RT as a sensitizer to immunotherapy. Our investigation verified that previous RT enhances the response rate of nivolumab and contributes to the prolongation of disease‐free survival.
In the randomized phase III PACIFIC study, the anti‐PD‐L1 antibody durvalumab significantly improved the survival duration of patients with locally advanced NSCLC administered platinum‐based concurrent chemoradiotherapy.18 Therefore, the prognostic significance of administering an anti‐PD‐L1 antibody following thoracic radiation has been established in patients with locally advanced disease. Currently, there are planned or ongoing prospective clinical studies investigating RT combined with ICIs in patients with NSCLC.17
In conclusion, RT prior to nivolumab therapy influences the efficacy of nivolumab and is intricately linked with favorable prognosis after immunotherapy. Extracranial RT has been clinically identified as a better modality to improve the efficacy of nivolumab compared to any other RT, including for brain metastases. Further investigation is required to establish the promising sequential strategy of nivolumab followed by RT.
Disclosure
OY, AM, KK, and HK received research grants and speaker honorarium from Ono Pharmaceutical Company and Bristol‐Myers Company.
All remaining authors report no conflict of interest.
Supporting information
Figure S1. The median progression‐free survival rates of patients administered any previous radiotherapy (RT, n = 66), extracranial RT (n = 52), thoracic RT (n = 40), and no previous RT (n = 58) were 204, 206, 233, and 79 days, and the median survival times were 562, not reached (NR), NR, and 524 days, respectively.
References
- 1. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz‐Ares L, Hom L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy. Nat Rev Clin Oncol 2017; 14: 365–79. [DOI] [PubMed] [Google Scholar]
- 4. Dovedi S, Adlard AL, Lipowska‐Bhalla G et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD‐L1 blockade. Cancer Res 2014; 74: 5458–68. [DOI] [PubMed] [Google Scholar]
- 5. Deng L, Liang H, Burnette B et al. Irradiation and anti‐PD‐L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124: 687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rückert M, Deloch L, Fietkau R, Frey B, Hecht M, Gaipl US. Immune modulatory effects of radiotherapy as basis for well‐reasoned radioimmunotherapies. Strahlenther Onkol 2018; 194: 509–19. [DOI] [PubMed] [Google Scholar]
- 7. Derer A, Spiljar M, Bäumler M et al. Chemoradiation increases PD‐L1 expression in certain melanoma and glioblastoma cells. Front Immunol 2016; 7: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaverdian N, Lisberg AE, Bornazyan K et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non‐small‐cell lung cancer: A secondary analysis of the KEYNOTE‐001 phase 1 trial. Lancet Oncol 2017; 18: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fiorica F, Belluomini L, Stefanelli A et al. Immune check inhibitor nivolumab and radiotherapy in pretreated lung cancer patients: Efficacy and safety of combination. Am J Clin Oncol 2018; doi: 10.1097/COC.0000000000000428 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Eisenhauer E, Therasse P, Bogaerts J et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 11. Britschgi C, Riesterer O, Burger IA, Guckenberger M, Curioni‐Fontecedro A. Report of an abscopal effect induced by stereotactic body radiotherapy and nivolumab in a patient with metastatic non‐small cell lung cancer. Radiat Oncol 2018; 13: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhalla N, Brooker R, Brada M. Combining immunotherapy and radiotherapy in lung cancer. J Thorac Dis 2018; 10 (Suppl 13): S1447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang X, Niedermann G. Abscopal effects with hypofractionated schedules extending into the effector phase of the tumor‐specific T‐cell response. Int J Radiat Oncol Biol Phys 2018; 101: 63–73. [DOI] [PubMed] [Google Scholar]
- 14. Yuan Z, Fromm A, Ahmed KA et al. Radiotherapy rescue of a nivolumab‐refractory immune response in a patient with PD‐L1‐negative metastatic squamous cell carcinoma of the lung. J Thorac Oncol 2017; 12: e135–6. [DOI] [PubMed] [Google Scholar]
- 15. Meng X, Gao Y, Yang L et al. Immune microenvironment differences between squamous and non‐squamous non‐small‐cell lung cancer and their influence on the prognosis. Clin Lung Cancer 2019; 20: 48–58. [DOI] [PubMed] [Google Scholar]
- 16. von Reibnitz D, Chaft JE, Wu AJ et al. Safety of combining thoracic radiation therapy with concurrent versus sequential immune checkpoint inhibition. Adv Radiat Oncol 2018; 3: 391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ko EC, Raben D, Formenti SC. The integration of radiotherapy with immunotherapy for the treatment of non‐small cell lung cancer. Clin Cancer Res 2018; 24: 5792–806. [DOI] [PubMed] [Google Scholar]
- 18. Antonia SJ, Villegas A, Daniel D et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017; 377: 1919–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The median progression‐free survival rates of patients administered any previous radiotherapy (RT, n = 66), extracranial RT (n = 52), thoracic RT (n = 40), and no previous RT (n = 58) were 204, 206, 233, and 79 days, and the median survival times were 562, not reached (NR), NR, and 524 days, respectively.


