Abstract
Background
Non‐small cell lung cancer (NSCLC) is the predominant type of lung cancer, and most clinically curable patients are diagnosed with locally advanced disease. Although the efficacy of standard platinum‐based chemotherapy doublets is relatively limited. The effect of immune checkpoint inhibitors (ICIs) remains controversial, and its role in the first‐line treatment of advanced NSCLC is obscure. Thus, we carried out a systematic review and meta‐analysis to compare the efficacy and safety of ICIs for advanced NSCLC.
Methods
The PubMed, Cochrane Central Register Trial, and American Society of Clinical Oncology databases were searched from inception to 30 April 2018. We searched for randomized controlled trials comparing single‐agent programmed cell death protein 1/programmed death‐ligand 1 inhibitors (nivolumab, pembrolizumab, or atezolizumab) or cytotoxic T‐lymphocyte‐associated antigen 4 inhibitor (ipilimumab) with chemotherapy in NSCLC patients. Progression‐free survival, overall survival, objective response rate, and adverse events were pooled for meta‐analysis by Review Manager (RevMan version 5.3) software.
Results
After exclusion of ineligible studies, 12 eligible randomized controlled trials were included. Data showed that ICIs significantly improved progression‐free survival (HR 0.66, 95% CI 0.57–0.77, P < 0.00001), overall survival (HR 0.77, 95% CI 0.64–0.91, P = 0.003), and but not objective response rate (RR 1.97, 95% CI 1.25–3.13, P = 0.004) in all unselected NSCLC populations. However, they failed to increase the OS of programmed death‐ligand 1 = 1–49% subgroup (HR 0.78, 95% CI 0.51–1.19, P = 0.25) and PFS of programmed death‐ligand 1<1% subgroup (HR 0.85; 95%CI 0.70 to 1.03, P=0.09) in ICIs+chemotherapy over chemotherapy. Meanwhile, OS of programmed death‐ligand =1‐49% subgroup (HR 0.92; 95%CI 0.77 to 1.10, P=0.36) and PFS of programmed death‐ligand 1≥50% subgroup (HR 0.76; 95%CI 0.52 to 1.11, P=0.15) showed no significant differences in ICIs over chemotherapy. Furthermore, fewer adverse events were observed in the ICIs groups than control groups.
Conclusion
ICIs are overall better tolerated than chemotherapy. Our results provide further evidence supporting the favorable risk/benefit ratio for ICIs.
Keywords: Chemotherapy, immune checkpoint inhibitor, meta‐analysis, non‐small cell lung cancer, programmed cell death protein 1/programmed death‐ligand 1
Introduction
Lung cancer has far‐reaching medical, psychosocial, and economic impacts, and is a burden on society. Worldwide, lung cancer is a major cause of death from malignant tumors, accounting for approximately 20% of all cancer‐related mortality.1 Advanced non‐small‐cell lung carcinoma (NSCLC) constituted 85% of all primary lung cancers and presented with advanced, unresectable disease at the time of diagnosis, and a <15% five‐year survival rate.2, 3 A great number of patients with NSCLC also receive palliative systemic care. The effectiveness of current standard first‐line treatment (i.e. platinum‐based chemotherapy doublets) seems to have reached a “plateau”, which has been shown to yield objective responses with a median overall survival (OS) of 8–10 months in approximately 30–40% of patients, and in particular, the role of chemotherapy has frequently been denigrated as toxic and ineffective.4 Over the past few decades, doctors have investigated new tactics for treating NSCLC, but still, the median OS with chemotherapy has not surpassed 15 months.5
One of the important features of carcinoma is avoiding immune surveillance.6 Cancer cells always make use of the programmed cell death protein 1/programmed death‐ligand 1/2 (PD‐1–PD‐L1/2) pathway to escape from immune‐cell attack. The development of therapies to enhance tumor immunity has turned into an important target for cancer treatment strategies.7 Recently, immune checkpoint inhibitors (ICIs), including the B7/CD28 receptor superfamily, have become increasingly important targets for the pharmacological blockade, and have emerged as promising therapeutic agents in NSCLC.8 Cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4) and the PD‐1 pathway have been the best characterized and most therapeutically relevant immune checkpoints, CTLA‐4 and PD‐1 pathway inhibitors have entered routine clinical use because the results from recent randomized controlled trials (RCTs) showed significant antitumor activity across a range of solid tumors.9, 10 These receptors play significant roles in regulating the immune response against malignancy.
Ipilimumab, a CTLA antagonist, is a fully humanized monoclonal antibody that blocks the interaction of CTLA‐4, a negative regulator of T‐cell activation, with its ligands (CD80/CD86), thereby allowing augmented antitumor T‐cell activation and proliferation, leading to tumor infiltration by T cells and tumor regression. More recently, however, combination ipilimumab with chemotherapy has been considered to be a reasonable therapy for NSCLC patients, for the reason that preclinical studies have shown that chemotherapy can lead to the release of tumor‐specific antigens, initiating T‐cell activation and sensitizing tumor cells to T cell‐mediated killing, and cooperating with anti‐CTLA‐4 antibody therapy.11, 12
PD‐L1 is an immune checkpoint protein that is expressed on tumor cells or tumor‐infiltrating immune cells. The binding of PD‐L1 with PD‐1 receptors on activated T cells induces tumor immune escape by downregulating antitumoral T‐cell function.13, 14 Monoclonal antibodies targeting the PD‐1 molecule and its ligand, PD‐L1, inhibit immune checkpoint receptors and can disrupt normal mechanisms of immune tolerance, resulting in increased immune activation in normal tissue.15
Although ICIs showed impressive clinical activity with high response rates and durable tumor remission in the treatment of NSCLC (nivolumab, pembrolizumab, and atezolizumab blocking the PD‐1/PD‐L1 pathway are approved by the US Food and Drug Administration for the treatment of patients with advanced NSCLC), some questions regarding lung cancer immunotherapy with these agents remain unclear, and there is a great need to identify candidates who are most likely to respond to ICIs. In addition, there is a lack of data comparing agents with one another. Many studies showed the correlation between the efficacy of ICIs and PD‐L1 expression on tumor cells and/or tumor infiltrating immune cells.16, 17, 18 Pembrolizumab was recently approved by the US Food and Drug Administration for treatment of patients with NSCLC in the frontline setting when the tumor PD‐L1 expression is >50%. Patients with PD‐L1‐negative NSCLC could also benefit from ICIs,19 nevertheless, the predictive value of PD‐L1 expression is still controversial.20, 21 Mutational load might be another possible marker of response to ICIs in NSCLC.22, 23 Thus, the complexity of tumor‐immune interactions requires other biomarkers in addition to or beyond PD‐L1.
In the present article, we present a network meta‐analysis comparing the relative efficacy and safety of ICIs for first‐line treatment of advanced NSCLC in naive patients. Furthermore, a meta‐analysis should provide a better understanding regarding biomarkers and indirectly compare each immunotherapy agent.
Methods
Searching strategy
We carried out a comprehensive systematic retrieval for potential articles in the PubMed database and Cochrane Central Register Trial from inception to 30 April 2018. Furthermore, the whole abstracts and virtual meeting presentations from proceedings of the American Society of Clinical Oncology 2018 Congress were searched manually. We also looked into all the references of identified relevant articles and reviews. When we encountered unclear or incomplete data, the corresponding authors were consulted.
The following terms were applied to literature searching: “immune checkpoint inhibitor or immunotherapy”, “nivolumab or pembrolizumab or atezolizumab or ipilimumab”, “advanced or metastatic”, “non‐small‐cell lung cancer or NSCLC”, “PD‐1 or PD‐L1”, and “randomized controlled trial.”
Only clinical trials in the phase II and III level evaluating nivolumab, pembrolizumab, or atezolizumab for the treatment of previously untreated advanced NSCLC were included in this analysis. We included qualified studies that met the inclusion criteria: RCTs in advanced NSCLC; randomization of patients to either immunotherapy with ICI or chemotherapy; performing subgroup comparison of progression‐free survival (PFS) or overall survival (OS) by PD‐L1 expression level; and providing the hazard ration (HR) and its 95% confidence interval (CI).
Data extraction
All data were extracted from studies independently by two evaluators using standardized data extraction sheets, and all discrepancies were resolved by discussion with the third reviewer until a consensus was reached. The following information was extracted: baseline characteristics of each patient, such as age, sex, and description and dosages of the administered treatment; tumor histology; disease stage; PD‐L1 expression level; treatment primary end‐point measurements (PFS and OS, HR with 95% CI); objective response (including complete response and partial response); stable disease; progressive disease; and treatment‐related adverse events (AEs).
Quality assessment
We used the Cochrane risk of bias assessment to explore sources of bias in included randomized trials.24 This scale evaluates the following criteria: (i) randomized sequence generation; (ii) allocation concealment; (iii) blinding of participants, personnel, and outcome assessors; (iv) incomplete outcome data; (v) selective outcome reporting; and (vi) other sources of bias. Risk of bias was labeled as high, low, or unclear if any item of randomization or blinding was judged as high risk, then the trial had a high risk of bias. Single‐arm trials have a high risk of bias by their nature; therefore, they were not further assessed for bias.
Statistical analysis
Statistical analyses were undertaken using the methods described by the Cochrane Collaboration guidelines for meta‐analysis, using Review Manager (RevMan version 5.3; Oxford, UK). Statistical heterogeneity was evaluated with the Cochran χ2‐test and the I 2 statistics. A P‐value of <0.10 for χ2 was defined as showing the presence of heterogeneity. Statistical heterogeneity between studies was with the I 2 statistic, where I 2 values of 30–60% represented a moderate level of heterogeneity. We used a fixed‐effect model (Mantel–Haenszel method) to calculate the pooled HR if the heterogeneity was low in the analyses, and a random effects model (DerSimonian‐Laird method) was applied otherwise. Subgroup analysis was also carried out according to different PD‐1 inhibitors or PD‐L1 expression level. The final result was reported with odds ratio (OR), HRs and corresponding 95% CIs. All P‐values tests were two‐sided, and P < 0.05 was considered to show statistical significance.
Sensitivity analysis was carried out by excluding one study at a time and also by removing one study with the highest weightage, among the included data to examine the influence of bias on the deduced statistical significance and interpretation. Risk ratios were calculated for AEs at 95% CIs.
Results
Results of search
Using the search strategy, we originally retrieved 1015 records from our database search. Among these, 86 articles were excluded for duplication, and 884 articles were excluded by screening the title and abstract. After carefully reading the full texts of the remaining 45 articles, six eligible studies21, 25, 26, 27, 28, 29 met the inclusion criteria. Five abstracts30, 31, 32, 33, 34, 35 were included from the American Society of Clinical Oncology conference proceedings. The part publication of four American Society of Clinical Oncology abstracts were published after the literature search date and have been included instead. One additional study36 was identified through manual searches in 2018 AACR. Our selection process and reasons for study exclusion are shown in Figure 1.
Figure 1.
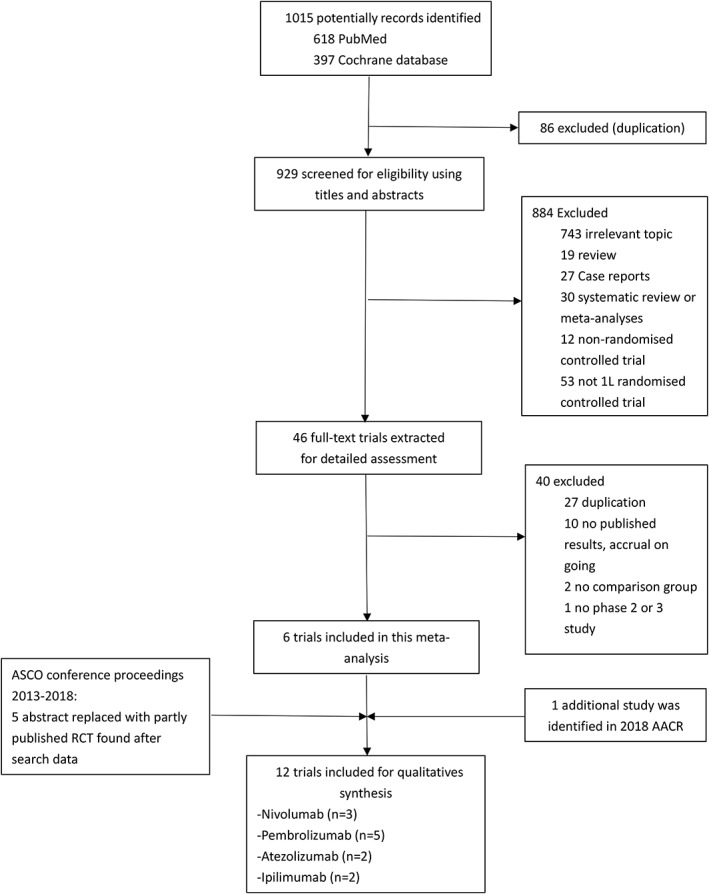
Study identification and selection process. AACR, American Association for Cancer Research; ASCO, American Society of Clinical Oncology.
Characteristics of the eligible studies
These 12 eligible studies were all published between 2010 and 2018. Of the 12 studies enrolled, three28, 30, 34 were carried out in patients with SQ NSCLC, three27, 29, 36 in those with non‐SQ NSCLC, and the other six21, 25, 26, 31, 32, 33, 35 were carried out in all subtypes of NSCLC. A total of 1021, 25, 28, 29, 30, 32, 33, 34, 35, 36 phase III and two26, 27 phase II randomized clinical trials were considered eligible for the meta‐analysis. A total of 8384 patients (ICIs: 3842; chemotherapy: 3120) were included in the analysis from five25, 27, 30, 33, 36 pembrolizumab trials, three21, 32, 35 nivolumab trials, two29, 34 atezolizumab trials, and two26, 28 ipilimumab trials. The detailed characteristics of the 12 studies are presented in Table 1.
Table 1.
Characteristics of the 12 randomized controlled trials comparing immune checkpoint inhibitors ± chemotherapy with chemotherapy ± placebo
| Study/year of publication | Study phase | PD‐1/D‐L1 inhibitors | Mean age (years) | Male (%) | Histology | PD‐L1 cut‐off | Treatment comparison | No. patients |
|---|---|---|---|---|---|---|---|---|
| Reck et al./201625 | Phase III | Pembrolizumab | 64.5 (33–90) vs. 66 (38–85) | 92,59.7% vs. 95,62.9% | Any | PD‐L1 ≥50% | Pembrolizumab 200 mg Q3W vs. chemotherapy | 305 |
| Lopes et al./201833 | Phase III | Pembrolizumab | 63 (25–89) vs. 63 (31–90) | 450, 70.6% vs. 452, 71.0% | Any | PD‐L1 ≥1% | Pembrolizumab 200 mg, Q3w vs. chemotherapy | 1274 |
| Gandhi et al./201836 | Phase III | Pembrolizumab | 65 (34–84) vs. 63.5 (34–84) | 254, 62% vs. 109, 52.9% | Non‐squamous | PD‐L1 ≥1% | Pembrolizumab 200 mg Q3W + chemo vs. chemo + placebo | 616 |
| Langer et al./201827, 31 | Phase II | Pembrolizumab | 62.5 (54–70) vs. 63.2 (58–70) | 22, 37% vs. 26, 41% | Non‐squamous | PD‐L1 ≥1% | Pembrolizumab 200 mg Q3W + PC vs. PC | 123 |
| Paz‐Ares et al./201830 | Phase III | Pembrolizumab | 65 (29–87) vs. 65 (36–88) | 220, 79.1% vs. 235, 83.6% | Squamous | Any | Pembrolizumab 200 mg Q3W + chemo vs. chemo | 560 |
| Carbone et al./201721 | Phase III | Nivolumab | 63 (32–89) vs. 65 (29–87) | 184, 68% vs. 148, 55% | Any | PD‐L1 ≥5% | Nivolumab 3 mg/kg Q2W + chemo vs. chemo + placebo | 541 |
| Hellmann et al./201835 | Phase III | Nivolumab Ipilimumab |
64 (41–87) vs. 64 (29–80) | 98, 70.5% vs. 106, 66.2% | Any | Any | Nivolumab 3 mg/kg Q2W + Ipilimumab 1 mg/kg Q6W vs. Nivolumab 240 mg Q2W + chemo vs. chemo | 1189 |
| Borghaei et al./201832 | Phase III | Nivolumab Ipilimumab |
64 vs. 64 | 27% vs. 33% | Any | PD‐L1 <1% | Nivolumab 3 mg/kg Q2W + ipilimumab 1 mg/kg Q6W vs. Nivolumab 360 mg Q3W + chemo vs. chemo | 550 |
| Jotte et al./201834 | Phase III | Atezolizumab | 66 (43–85) vs. 65 (23–83) vs. 65 (38–86) | 278, 82% vs. 279,81%, vs. 278, 82% | Squamous | Any | Ate + Carb + NAB‐pac vs. Ate + Carb + Pac vs. Carb + NAB‐pac | 1021 |
| Socinski et al./201829 | Phase III | Atezolizumab | 63 (31–89) vs. 63 (31–90) | 240, 60% vs. 239, 59.8% | Non‐squamous | Any | Atezo 1200 mg + PC + bevacizumab vs. PC + bevacizumab | 1045 |
| Govindan et al./201728 | Phase III | Ipilimumab | 64 (28–84) vs. 64 (28–85) | 326, 84% vs. 309,85% | Squamous | NA | Ipilimumab 10 mg/kg Q3W + chemo vs. chemo + placebo | 956 |
| Lynch et al./201226 | Phase II | Ipilimumab | 59 (36–82) vs. 61 (36–88) vs. 62 (36–88) | 53, 76% vs. 49,72%, vs. 49, 74% | Any | NA | Concurrent or phased ipilimumab 10 mg/kg Q3W + chemo vs. chemo + placebo | 204 |
Carb, carboplatin; Chemo, chemotherapy; NA, not available; NAB‐pac, nab‐paclitaxel; PC, paclitaxel plus carboplatin; PD‐L1, programmed death‐ligand 1; Q2W, two weeks using a time; Q3W, three weeks using a time; Q6W, six weeks using a time.
Quality of studies
All or most of the included randomized trials had a low risk of detection bias, reporting bias, and other bias, because most were open‐label, double‐blind and phase III trials. Most of the studies had a high risk of attrition bias, as some secondary end‐points were assessed in the as‐treated population, which included all patients who had undergone randomization and received at least one dose of the assigned combination therapy. However, selection bias and performance bias were not determined due to insufficient information (Fig. 2).
Figure 2.
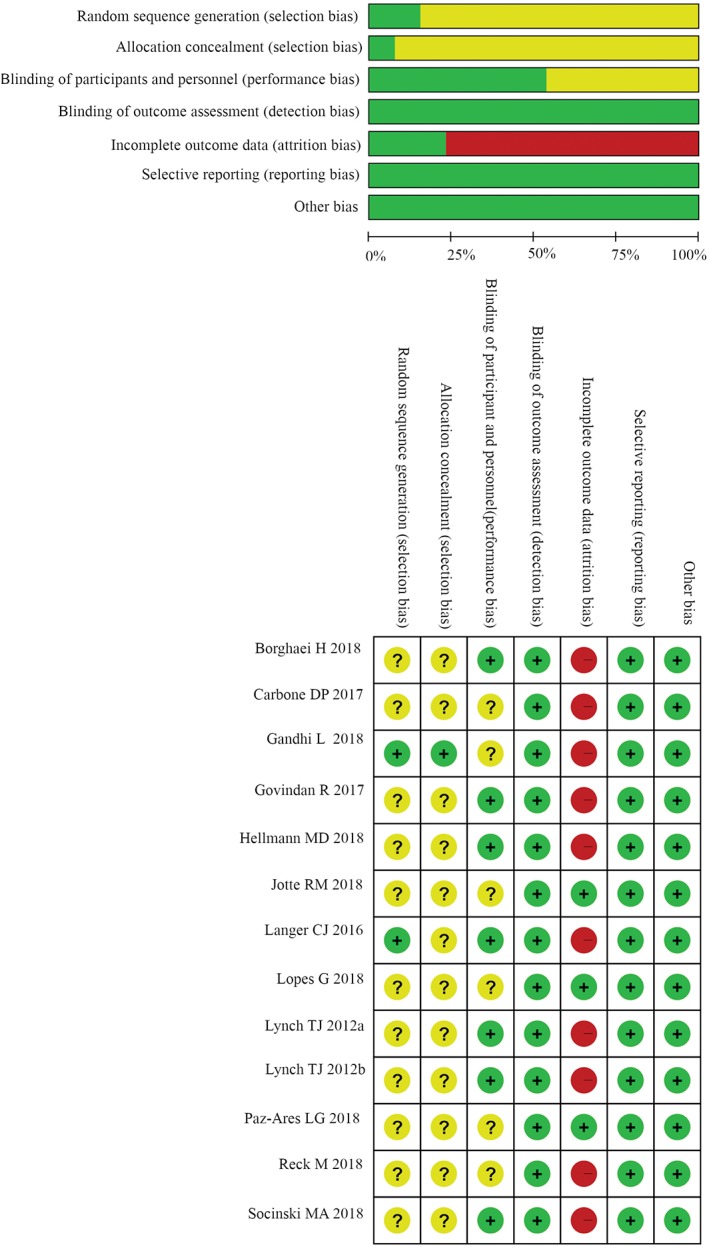
Quality of studies. ( ) Low risk of bias, (
) Low risk of bias, ( ) Unclear risk of bias, and (
) Unclear risk of bias, and ( ) High risk of bias
) High risk of bias
Effect of immunotherapy on OS, PFS, and overall response rate
All trials reported the OS data and the PFS data. The median OS, the PFS, and the 95% CI, HR, and 95% CI for the treatment group versus control group were retrieved from the published edition (Table 2). The pooled HRs with 95% CIs for OS were calculated using Review Manager 5.35.The pooled HR showed a significant improvement in OS for ICIs + chemotherapy over chemotherapy alone (Fig. 3b; HR 0.77, 95% CI 0.64–0.91, P = 0.003), whereas no significant difference in OS for ICIs alone over chemotherapy (Fig. 3a; HR 0.82, 95% CI 0.68–1.00, P = 0.06).
Table 2.
Overall survival and progression‐free survival in the 12 randomized controlled trials comparing immune checkpoint inhibitors ± chemotherapy with chemotherapy ± placebo
| Name of RCTs | Study arms | OS | PFS | ||||
|---|---|---|---|---|---|---|---|
| Months (95% CI) | Pooled HR (95% CI) | P‐value | Months (95% CI) | Pooled HR (95% CI) | P‐value | ||
| Reck et al.25 | Pembrolizumab | 30 | 0.63 (0.47–0.86) | 0.002 | 10.3 (6.7–NR) | 0.5 (0.37–0.68) | <0.001 |
| chemotherapy | 14.2 | 6(4.2–6.2) | |||||
| Lopes et al.33 | Pembrolizumab | 16.7 (13.9–19.7) | 0.81 (0.71–0.93) | 0.0018 | |||
| chemotherapy | 12.1 (11.3–13.3) | ||||||
| Langer et al.27, 31 | Pembrolizumab + PC | NR (22.8‐NR) | 0.59 (0.24–1.05) | 0.03 | 19 (8.5‐NR) | 0.54 (0.33–0.88) | 0.0067 |
| PC | 20.9 (14.9‐NR) | 8.9 (6.2–11.8) | |||||
| Gandhi et al.36 | Pembrolizumab + chemo | NR (NR‐NR) | 0.49 (0.38–0.64) | <0.001 | 8.8 (7.6–9.2) | 0.52 (0.43–0.64) | <0.00001 |
| Chemo + placebo | 11.3 (8.7–15.1) | 4.9 (4.7–5.5) | |||||
| Paz‐Ares et al.30 | Pembrolizumab + chemo | 15.9 (13.2‐NR) | 0.64 (0.49–0.85) | 0.0008 | 6.4 (6.2–8.3) | 0.56 (0.45–0.70) | <0.0001 |
| Chemo | 11.3 (9.5–14.8) | 4.8 (4.3–5.7) | |||||
| Carbone et al.21 | Nivolumab + chemo | 14.4 | 1.02 (0.8–1.3) | NR | 4.2 | 1.15 (0.91–1.45) | 0.25 |
| Chemo + placebo | 13.2 | 5.9 | |||||
| Hellmann et al.35 | Nivolumab + ipilimumab | 23 | 0.79 (0.56–1.10) | 7.2 | 0.58 (0.41–0.81) | 0.0002 | |
| Chemo | 16.4 | 5.4 | |||||
| Jotte et al. 34 | Ate + Carb + NAB‐pac | 14 (12.0–17.0) | 0.96 (0.78–1.18) | 0.6931 | 6.3 (5.7–7.1) | 0.71 (0.60–0.85) | 0.0001 |
| Carb + NAB‐pac | 13.9 (12.3–16.4) | 5.6 (5.5–5.7) | |||||
| Socinski et al.29 | Atezo + PC + bevacizumab | 19.2 (17–23.8) | 0.78 (0.64–0.96) | 0.016 | 8.3 (7.7–9.8) | 0.59 (0.50–0.70) | <0.0001 |
| PC + bevacizumab | 14.7 (13.3–16.9) | 6.8 (6.0–7.1) | |||||
| Govindan et al.28 | Ipilimumab + chemo | 13.4 (11.8–14.8) | 0.91 (0.77–1.07) | 0.25 | 5.6 (5.4–5.9) | 0.87 (0.75–1.01) | 0.07 |
| Chemo + placebo | 12.4 (11.6–13.6) | 5.6 (5.5–5.7) | |||||
| Lynch et al.26 | Concurrent ipilimumab + chemo | 9.7 (7.59–12.48) | 0.99 (0.67–1.46) | 0.48 | 4.1 (2.76–5.32) | 0.88 (0.61–1.27) | 0.25 |
| Phased ipilimumab + chemo | 12.2 (9.26–14.39) | 0.87 (0.59–1.28) | 0.23 | 5.1 (4.17–5.72) | 0.69 (0.48–1.00) | 0.02 | |
| Chemo + placebo | 8.3 (6.80–12.39) | 4.2 (2.76–5.32) | |||||
Ate/Atezo, Atezolizumab; Carb, carboplatin; Chemo, chemotherapy; CI, confidence interval; HR, hazard ratio; NAB‐pac, nab‐paclitaxel; NR, not reported; OS, overall survival; PC, paclitaxel plus carboplatin; PFS, progression‐free survival; RCTs, randomized controlled trials.
Figure 3.
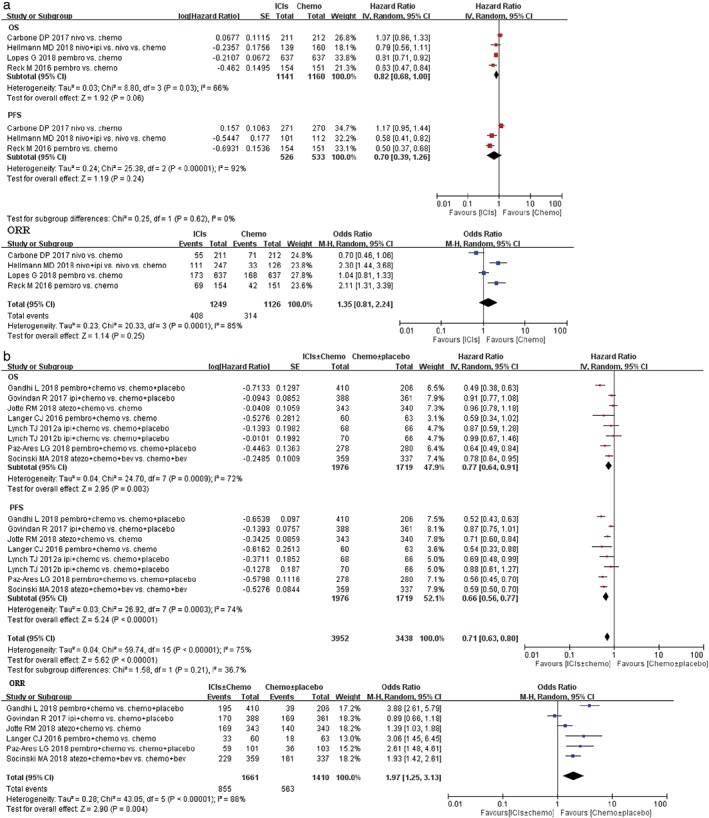
Forest plots of hazard ratio (HR) of overall survival (OS); HR of progression‐free survival (PFS); odds ratio (OR) of overall response rate (ORR) associated with (a) immune checkpoint inhibitors (ICIs) ± chemotherapy versus chemotherapy ± placebo or (b) ICIs versus chemotherapy in first‐line treatment of non‐small cell lung cancer (NSCLC) population with programmed death‐ligand 1 (PD‐L1) unselected. Chemo, chemotherapy; CI, confidence interval; Placbo, placebo.
The PFS remains controversial in several randomized clinical trials. In CheckMate‐02621 and Govindan R’s phase III28 studies, PFS was similar between the treatment groups in the intention‐to‐treat population. However, in the other studies, PFS was improved after anti‐PD1/PD‐L1 antibody treatment, which showed superior efficacy to chemotherapy. Thus, we calculated the pooled HRs for PFS in the present study. The pooled HRs showed a significant improvement in PFS for ICIs + chemotherapy compared with chemotherapy alone (Fig. 3b; HR 0.66, 95% CI 0.56–0.77, P < 0.00001), nevertheless. For anti‐PD‐1/PD‐L1 monotherapy, no positive result in PFS was obtained when compared with chemotherapy (Fig. 3a; HR 0.70, 95% CI 0.39–1.26, P = 0.24).
Many studies included in this meta‐analysis also reported the partial or complete overall response rate according to RECIST (version 1.1). We compared the overall response rate of ICIs therapy with chemotherapy for advanced NSCLC patients. The pooled OR for the overall response rate (ORR) in the ICIs arm over the chemotherapy arm had no significant differences (Fig. 3a in ICIs vs. chemotherapy: OR 1.35, 95% CI 0.81–2.24, P = 0.25); whereas the pooled OR for ORR between ICIs + chemotherapy and chemotherapy alone was 1.97 (95% CI 1.25–3.13, P = 0.004; Fig. 3b).
Indirect comparisons by PD‐L1 expression
PD‐L1 is a potential biomarker that is expressed on tumor cells and tumor infiltrating immune cells. The PD‐L1 expression level plays a critical role in the prognosis of cancer patients.37, 38 Therefore, we carried out a subgroup analysis to assess the impact of PD‐L1 expression level on the efficacy of anti‐PD‐1/PD‐L1 antibody therapy. To better analyze the importance of PD‐L1 expression, we redefined PD‐L1‐positive as >1% or TC1/2/3 or IC1/2/3 based on the included 11 RCTs, and analyzed the OS, PFS, and ORR in the subgroups, respectively. We also defined PD‐L1‐negative as <1% or TC0 and IC0.
The subgroup analysis according to PD‐L1 expression level showed that in the PD‐L1 ≥50% subgroup, anti‐PD‐1/PD‐L1 antibody‐containing therapy significantly improved the OS compared with control arms (Fig. 4a ICIs vs. chemotherapy, HR 0.71, 95% CI 0.60–0.84, P < 0.0001; Fig. 4b ICIs + chemotherapy vs. chemotherapy alone, HR 0.56, 95% CI 0.44–0.73, P < 0.0001). In addition, for the PD‐L1‐negative subgroup, OS was significantly improved in the combination arm (Fig. 4b HR 0.76, 95% CI 0.64–0.91, P = 0.002). However, for the PD‐L1 = 1–49% subgroup, ICIs monotherapy or ICIs + chemotherapy did not improve OS significantly ((Fig. 4a ICIs vs. chemotherapy, HR 0.92, 95% CI 0.77–1.10, P = 0.36; Fig. 4b ICIs + chemotherapy vs. chemotherapy alone, HR 0.78, 95% CI 0.51–1.19, P = 0.25). All predefined subgroups had superior PFS in the immunotherapy/chemotherapy arm. The subgroup analysis based on the PD‐L1 expression status showed that anti‐PD1/PD‐L1 antibody combined with chemotherapy treatment improved PFS in the PD‐L1 ≥ 50% subgroup and the PD‐L1 = 1–49% subgroup (Fig. 4b HR 0.38, 95% CI 0.31–0.47, P < 0.00001 in PD‐L1 ≥50% subgroup; and HR 0.60, 95% CI 0.51–0.71, P < 0.00001 in PD‐L1 = 1–49% subgroup, respectively), and also in the PD‐L1‐negative group when ICIs were compared with chemotherapy (Fig. 4a HR 0.48, 95% CI 0.27–0.85, P = 0.01). The results in other subgroups were not statistically significant (Fig. 4a HR 0.76, 95% CI 0.52–1.11, P = 0.15 in PD‐L1 ≥ 50% subgroup; Fig. 4b HR 0.85, 95% CI 0.70–1.03, P = 0.09 in the PD‐L1 negative subgroup, respectively).
Figure 4.
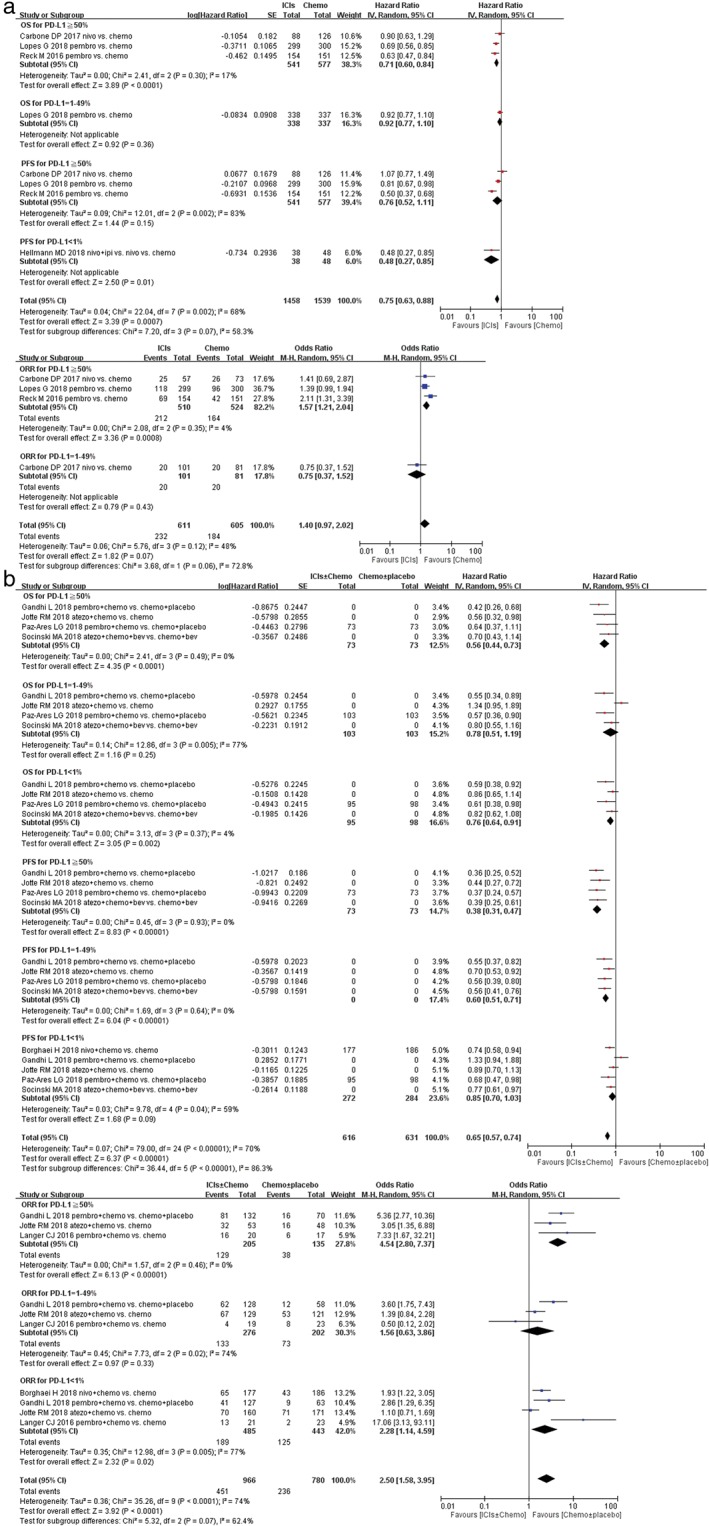
Forest plots of hazard ratio (HR) of overall survival (OS); HR of progression‐free survival (PFS); odds ratio (OR) of overall response rate (ORR) associated with (a) immune checkpoint inhibitors (ICIs) ± chemotherapy versus chemotherapy ± placebo or (b) ICIs versus chemotherapy in first‐line treatment of non‐small cell lung cancer (NSCLC) population with programmed death‐ligand 1 (PD‐L1) subgroups. Chemo, chemotherapy; CI, confidence interval; Placbo, placebo.
The objective response rate was better with the addition of immunotherapy in all three PD‐L1 TC categories. Subgroup analysis based on the PD‐L1 expression status for overall response rate was also interesting. For PD‐L1 ≥ 50% patients, the pooled ORs for the ORR were significantly different whether comparing ICIs with chemotherapy or ICIs + chemotherapy versus chemotherapy (Fig. 4a OR 1.57, 95% CI 1.21–2.04, P = 0.0008; Fig. 4b OR 4.54, 95% CI 2.80–7.37, P < 0.00001). For PD‐L1 <1% patients, the same result was also gained (Fig. 4b OR 2.28, 95% CI 1.14–4.59, P = 0.02), which suggested a statistically significantly response rate for chemotherapy than for ICIs in advanced NSCLC patients. However, the pooled OR, in the PD‐L1 = 1–49% subgroup, showed no significant improvement in ORR for both ICIs monotherapy and ICIs + chemotherapy (Fig. 4a OR 0.75, 95% CI 0.37–1.52, P = 0.43; Fig. 4b OR 1.56, 95% CI 0.63–3.86, P = 0.33).
Subgroup analysis by NSCLC tumor mutational burden
Two RCTs assessed the effect of the tumor mutational burden (TMB) on outcomes. In OS analysis, no statistically significant difference was detected in high TMB NSCLC subgroups. Yet, there was a trend to favor ICIs therapy than chemotherapy in the first‐line setting, although the P‐value did not reach a significance threshold (Fig. 5a HR 0.87, 95% CI 0.65–1.17, P = 0.36). In PFS analysis, ICIs monotherapy or combined with chemotherapy were associated with longer PFS benefit than chemotherapy in the high TMB NSCLC subgroups (Fig. 5a HR 0.59, 95% CI 0.45–0.79, P = 0.0003; Fig. 5b HR 0.56, 95% CI 0.35–0.90, P = 0.02).
Figure 5.
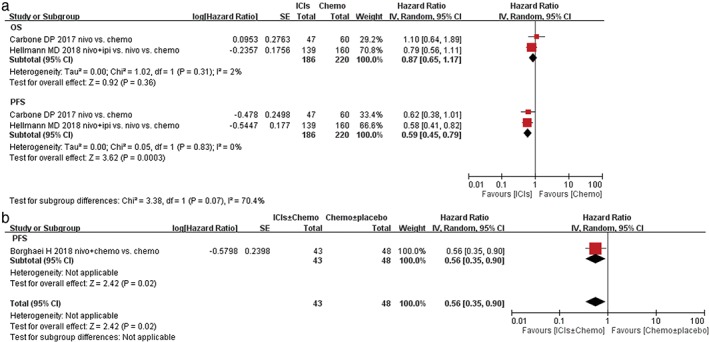
Forest plots of hazard ratio (HR) of overall survival (OS); HR of progression‐free survival (PFS) associated with (a) immune checkpoint inhibitors (ICIs) ± chemotherapy versus chemotherapy ± placebo or (b) ICIs versus chemotherapy in first line treatment of non‐small cell lung cancer (NSCLC) population with high tumor‐mutational burden (TMB). Chemo, chemotherapy; CI, confidence interval; ORR, overall response rate; Placbo, placebo.
Subgroup analysis by NSCLC histological type
Five studies with squamous NSCLC patients and five trials with non‐squamous NSCLC cases reported HRs and 95% CIs for OS. After the meta‐analysis, we found that ICIs monotherapy induced a 24% reduction of the death risk and 39% reduction of recurrence risk in patients with squamous NSCLC (OS: HR 0.76, 95% CI 0.63–0.93, P = 0.008; PFS: HR 0.61, 95% CI 0.40–0.95, P = 0.03; Fig. 6a). Nevertheless, it was noted that ICIs + chemotherapy also reduced risk of recurrence by 29%, but did not reduce the risk of death. (OS: HR 0.84, 95% CI 0.68–1.04, P = 0.11; PFS: HR 0.71,95% CI 0.56–0.90, P = 0.005; Fig. 6b). For patients with non‐squamous NSCLC, ICIs + chemotherapy also induced 38% reduction in the risk of death and 44% reduction in the risk of recurrence.(OS: HR 0.62, 95% CI 0.44–0.87, P = 0.006; PFS: HR 0.56, 95% CI 0.49–0.63, P < 0.00001; Fig. 6b), On the contrary, ICIs monotherapy did not improve OS and PFS in non‐squamous NSCLC (OS: HR 0.99, 95% CI 0.73–1.34, P = 0.95; PFS: HR 0.74, 95% CI 0.40–1.37, P = 0.34; Fig. 6a).
Figure 6.
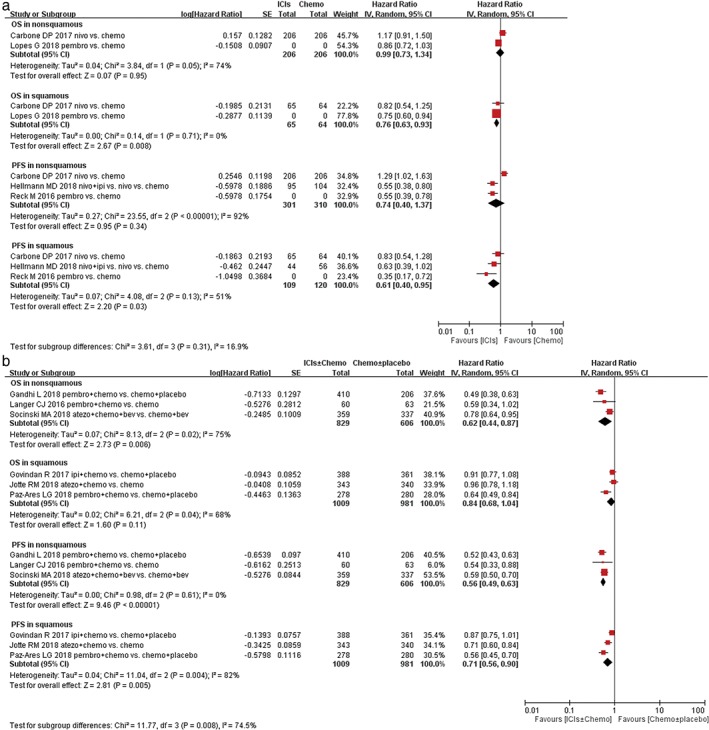
Forest plots of hazard ratio (HR) of overall survival (OS); HR of progression‐free survival (PFS) associated with (a) immune checkpoint inhibitors (ICIs) ± chemotherapy versus chemotherapy ± placebo or (b) ICIs versus chemotherapy in first‐line treatment of non‐small cell lung cancer (NSCLC) population with squamous (SQ) or non‐SQ histological type. Chemo, chemotherapy; CI, confidence interval; Placbo, placebo.
Effect of immunotherapies on treatment‐related AEs
In general, all studies included in this meta‐analysis reported treatment‐related AEs (Table 3), as well as treatment‐related high‐grade AEs according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. The incidence of any all‐grade (85.0% vs. 91.4%) or high‐grade (47.1% vs. 50.2%) AEs was lower in ICIs compared with chemotherapy (Fig. 7). Patients treated with ICIs stopped therapy for toxicity more frequently than control therapy (18.5% vs. 12.3%); the RR of treatment discontinuation due to AEs was 1.50 (P = 0.01). Deaths attributed to study treatment occurred in 73 patients in the ICIs group and 51 patients in the control group. There were no significant differences in the incidence of treatment‐related deaths (Fig. 7). Immune‐mediated AEs were also reported in both treatment arms, such as hypothyroidism, hyperthyroidism, pneumonitis, colitis, hypophysitis, hepatitis, and thyroiditis. The pooled RRs showed significantly higher rates of any grade immune‐associated AEs in the ICIs groups than in the chemotherapy groups, including hypothyroidism (RR 5.53, 95% CI 3.43–8.91), hyperthyroidism (RR 3.99, 95% CI 1.93–8.28), pneumonitis (RR 4.33, 95% CI 2.33–8.05), severe skin reaction (RR 3.35, 95% CI 1.25–9.26), colitis (RR 3.82, 95% CI 1.81–8.05), hypophysitis (RR 5.17, 95% CI 1.35–19.81), hepatitis (RR 11.49, 95% CI 2.74–48.26), and thyroiditis (RR 7.14, 95% CI 1.62–31.48). No significant differences, such as infusion reaction, nephritis, pancreatitis, diabetes mellitus, myositis, and adrenal insufficiency, were mentioned between two arms in this meta‐analysis (Table 4).
Table 3.
Incidence and response rate of summary toxicity end‐points, including 95% confidence interval and number of trials in each analysis
| Summary AE end‐points | No. trials | PD‐1/PD‐L1 inhibitor incidence (%) | Chemotherapy incidence (%) | RR (95% CI) | P‐value |
|---|---|---|---|---|---|
| Any all‐grade AEs | 10 | 85.0 | 91.4 | 0.97 (0.93–1.02) | 0.13 |
| Any high‐grade AEs | 10 | 47.1 | 50.2 | 0.85 (0.65–1.10) | 0.24 |
| Treatment discontinuation | 12 | 18.5 | 12.3 | 1.46 (1.01–2.11) | 0.01 |
| Treatment‐related deaths | 9 | 2.8 | 2.1 | 1.20 (0.79, 1.84) | 0.56 |
AE, adverse event; CI, confidence interval; PD‐1, programmed death receptor‐1; PD‐L1, programmed death‐ligand 1; RR, relative risk
Figure 7.
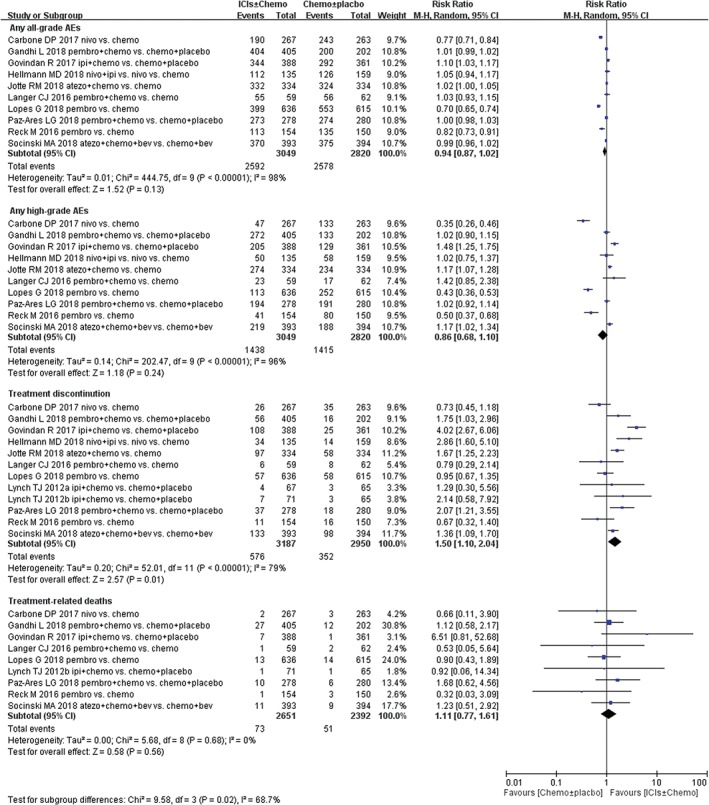
Forest plots of relative risk of immune‐related adverse events (AEs) associated with immune checkpoint inhibitors (ICIs) ± chemotherapy versus chemotherapy ± placebo in first‐line treatment of non‐small cell lung cancer (NSCLC) population. Chemo, chemotherapy; CI, confidence interval; Placbo, placebo.
Table 4.
Comparative immune‐mediated adverse events (any grade) of immune checkpoint inhibitors‐containing group versus chemotherapy group in 12 randomized controlled trials
| Adverse events | No. trials | I group events/pts | C group events/pts | Pooled RR (95%CI) | P‐value |
|---|---|---|---|---|---|
| Hypothyroidism | 9 | 274/2917 | 43/2870 | 5.53 (3.43,8.91) | P < 0.00001* |
| Hyperthyroidism | 6 | 80/1623 | 17/1422 | 3.99 (1.93,8.28) | P = 0.0002* |
| Pneumonitis | 8 | 141/2526 | 25/2300 | 4.33 (2.33,8.05) | P < 0.00001* |
| Infusion reaction | 7 | 50/2133 | 40/1906 | 1.57 (0.68,3.62) | P = 0.29 |
| Severe skin reaction | 7 | 44/2192 | 10/1966 | 3.35 (1.21,9.26) | P = 0.02* |
| Colitis | 7 | 42/2467 | 7/2238 | 3.82 (1.81,8.05) | P = 0.0004* |
| Hypophysitis | 5 | 13/1866 | 0/1641 | 5.17 (1.35,19.81) | P = 0.02* |
| Nephritis | 6 | 21/2133 | 20/1904 | 1.78 (0.42,7.48) | P = 0.43 |
| Pancreatitis | 4 | 10/1588 | 0/1361 | 4.43 (0.96,20.37) | P = 0.06 |
| diabetes mellitus | 4 | 7/1286 | 1/1080 | 2.94 (0.72,12.05) | P = 0.13 |
| Myositis | 3 | 6/952 | 1/746 | 2.68 (0.54,13.37) | P = 0.23 |
| Hepatitis | 4 | 26/1712 | 0/1491 | 11.49 (2.74,48.26) | P = 0.0009* |
| Thyroiditis | 4 | 18/1473 | 0/1247 | 7.14 (1.62,31.48) | P = 0.009* |
| Adrenal insufficiency | 3 | 6/1434 | 5/1211 | 0.99 (0.28,3.44) | P = 0.98 |
Significant difference.
C group, chemotherapy group; I group, immune checkpoint inhibitors‐containing group; pts, patients.
Sensitivity analysis
In order to assess the robustness and to eliminate bias in the results, we re‐analyzed the PFS and OS data by excluding individual trials with the highest or lowest weightage. Such analysis did not qualitatively change the obtained results and conclusions (data not elaborated). These conclusions are consistent with an earlier study,39 indicating that the benefits of immunotherapy are real and reproducible.
Discussion
Advanced NSCLC has been characterized by the presence of a multitude of driver mutations and, consequently, a multitude of molecularly‐guided therapeutics. This includes EGFR, ALK, BRAF and KRAS mutations.40 However, it should be realized that most NSCLC patients do not harbor these oncogenic drivers. For patients with WT EGFR tumors, the options were limited to cytotoxic chemotherapy in the first‐line setting, which are modest in extending survival.
Enhancing the immune system to eliminate cancer cells is an effective way to prolong survival and time to progression. In contrast to disease‐modifying agents, such as cytotoxic chemotherapy and mutation‐targeted drugs, PD‐1/PD‐L1 antibody unleashes suppressed T cell‐mediated antitumor responses of the host by disturbing the PD‐1 and PD‐L1 interaction, showing promising effects in second‐ and third‐line therapy in recent trials.8
PD‐1/PD‐L1 targeted therapeutics have gained remarkable attention because of their impressive results. Nevertheless, a question has remained about how to better tailor these treatments and choose the best candidates for such a therapy. PD‐L1 has emerged as the logical biomarker on which to guide molecular selection for NSCLC receiving PD‐1/PD‐L1 inhibitors. The present meta‐analysis showed that the combination of ICIs with chemotherapy significantly enhanced PFS and OS in PD‐L expression ≥50% of previously untreated advanced NSCLC patients, whereas no survival benefit was noted in the same patients comparing ICIs with chemotherapy. We found that PD‐L1 expression might be a very important prognostic factor for the efficacy of PD‐1/PD‐L1 inhibitors. Our analysis showed an improvement in PFS and OS in combination therapy (P < 0.05) for NSCLC patients with high PD‐L1‐expressing tumors (PD‐L1 ≥50%). However, no between‐group difference was noted with regard to ORR PFS in the PD‐L1=1‐49% subgroups of patients treated with either ICIs + chemotherapy or monotherapy ICIs, which might be explained in part by the imbalances of some RCTs between groups in the number of patients and the characteristics of the patients.
For patients with PD‐L1 = 1–49%, both ICIs and ICIs‐containing therapies were not associated with significantly longer OS and ORR than chemotherapy; PFS was significantly more enhanced in the ICIs + chemotherapy group than in the chemotherapy alone group.
For patients with PD‐L1 expression <1%, the survival benefit was associated with combination treatment, which would reduce mortality rates. PFS was only found to be significantly increased in the ICIs group, and not in the chemotherapy group (P = 0.01).
From our meta‐analysis, it can be seen that widespread detection of PD‐L1 expression as a predictive biomarker of response to PD‐1 pathway ICIs in NSCLC has been investigated in clinical practice. However, intratumoral heterogeneity of neoantigens,41 different methods (distinct immunohistochemistry antibody clones, staining methods, and scoring systems), and different cut‐off values in the clinical evaluation of PD‐L1 might have also led to discordant results. PD‐L1 assays have been further complicated by a lack of standardization in testing methods across agents. Nevertheless, at present, PD‐L1 immunohistochemistry remains an imperfect biomarker in NSCLC. Expression of PD‐L1 was neither prognostic nor predictive of clinic benefit. Given this lack of a reference standard for PD‐L1 testing, efforts are now ongoing to harmonize various PD‐L1 assays (e.g. International Association for the Study of Lung Cancer Blueprint Project).
Beyond PD‐L1 testing, TMB is another biomarker that is thought to be associated with the amount of neoantigen in the NSCLC and to have an important role in predicting the effect of ICIs. However, the relevance of TMB to prognosis is not yet fully understood. Preliminary data suggest that tumors harboring high levels of somatic mutations might be highly sensitive to ICIs.22, 23 Our meta‐analysis showed that ICIs represented an effective treatment regimen for patients with a high TMB, irrespective of PD‐L1 expression level. Meanwhile, our meta‐analysis contained a randomized phase III trial with stage IV or recurrent NSCLC; nivolumab as first‐line therapy was found not to be superior to chemotherapy in PFS or response rate in patients whose tumor had PD‐L1 expression of ≥5%. In this RCT, it was observed that tumor mutation burden on its own is a key factor for nivolumab efficacy and longer median PFS.21 Nevertheless, in another phase III trial with stage IV or recurrent NSCLC that was not previously treated with chemotherapy, TMB was found to be strongly associated with the efficacy of ICI combination therapy and was independent of PD‐L1 expression.35 High TMB predicted better objective response, durable benefit and longer PFS in patients treated with nivolumab plus ipilimumab when compared with chemotherapy, regardless of PD‐L1 expression.
To the best of our knowledge, in current studies, the method of TMB detection is genome analysis, including whole genome sequencing, whole exon sequencing, and selective gene sequencing (e.g. hybrid capture‐based next‐generation sequencing). Some studies reported that different sequencing combinations might affect TMB accuracy. There is no uniform standard for the cut‐off value of TMB and whether there are differences between various tumor species. Currently, it is generally accepted that <6 mutations/Mb is defined as low TMB, and >20 mutations/Mb is defined as high TMB.
Gene analysis found that TMB was more likely to be high in patients with lung cancer carrying the following genetic variants: RRM1, TP53, FANCE, NEIL1, POLE, POLG, FANCE, GEN1, and RPA1. In NSCLC, patients with identified drug‐therapeutic target mutations, such as eml4‐alk fusion, EGFR mutation, ROS1 rearrangement, BRAF fusion, and so on, usually have low TMB expression.
Indeed, higher non‐synonymous mutational burden in NSCLC, assessed by whole exome sequencing, is associated with an improved ORR, durable clinical benefit, and PFS in patients treated with anti‐PD‐1/PD‐L1 therapy.23 Despite the proven utility of whole exome sequencing in measuring TMB and predicting response to PD‐1/PD‐L1 blockade, it has many limitations. Whole exome sequencing is expensive, time‐consuming, and labor intensive, and, therefore, difficult to incorporate into clinical practice42.TMB, measured by hybrid‐based next‐generation sequencing, has been shown to correlate with response to PD‐1/PD‐L1 blockade in patients with NSCLC, as shown above. However, it is unknown whether TMB serves as a useful biomarker for predicting response to PD‐1/PD‐L1 blockade in lung cancer. Large‐scale studies are required to determine the relationship between PD‐L1 intensity and mutation burden.
However, it was noticed that squamous cell carcinoma and adenocarcinoma in NSCLC have different mutational profiles.22, 43, 44 We assumed that histological subtypes of NSCLC might influence the survival outcomes of ICIs. In this meta‐analysis, although OS in patients with non‐squamous NSCLC was significantly not prolonged by ICIs, PFS was extended in both squamous and non‐squamous NSCLC, compared with chemotherapy. The PFS benefit from ICIs regardless of histological subtypes in patients with advanced NSCLC might have several explanations. First, the difference in the mutational burden between squamous and non‐squamous NSCLC might not be significant. Second, other biomarkers, including PD‐L1 expression level, might interact to dilute the effect of the difference in the mutational load. Third, chemotherapy might influence the effect of the immunotherapy. It has been reported that chemotherapy changes the immune microenvironment of tumors in various ways,45 and dynamically alters the PD‐L1 expression on tumor cells.46, 47
The benefit of efficacy should be balanced against the risk of toxic effects. Conversely, an analysis of toxicity profile could not be carried out, as the data of adverse events from each study were not available. Even so, in our analysis of summary toxicity end‐points, any all‐ and high‐grade AEs in the ICIs arm occurred less frequently than in the control arm in randomized trials (85.0% vs. 91.4% and 47.1% vs. 50.2%, respectively). Though immune‐related adverse events, such as pneumonitis, hypothyroidism, hyperthyroidism, colitis, hepatitis, and thyroiditis can occur and might be severe, most events are low grade and can be improved/resolved with drug holding/immunosuppression.48 These results suggested that, for previously untreated NSCLC patients, ICIs could be a preferable treatment choice over chemotherapy.
Nevertheless, our study had some limitations. First, we extracted data from published articles without individual patient data, which might result in the bias of data analysis. Second, the appropriate cut‐off value with which to consider a tumor specimen as PD‐L1‐positive was variable among different clinical studies; with some studies using 1%, 5%, 10% or 25% as cut‐off values. For this reason, we formulated a uniform definition of PD‐L1 expression in patients within all these clinical trials. Therefore, the number of studies included in this meta‐analysis is small. Because of the aforementioned limitations in our study, further studies based on the information from ongoing trials are required to verify the efficacy and safety of anti‐PD1/PD‐L1 therapy versus chemotherapy in patients with advanced NSCLC.
This network meta analysis unanimously agreed that ICIs should be used first‐line in patients with PD‐L1‐positive (PD‐L1 expression ≥50%) metastatic NSCLC. In patients with non‐squamous cell NSCLC without EGFR, ALK, or ROS1 aberrations and PD‐L1 ≥50%, our meta‐analysis recommended ICIs monotherapy, but recognized that combination ICIs + platinum‐based chemotherapy can be appropriate in specific cases. For patients with non‐squamous, advanced NSCLC with PD‐L1 expression <50% and no actionable mutations, it was unanimously recommended that patients should receive first‐line ICIs + platinum‐containing chemotherapy.
Regarding treatment recommendations for patients with squamous histology, In all, ICIs monotherapy was recommended for patients with squamous cell NSCLC and PD‐L1 expression ≥50%; however, it also supported ICIs in combination with chemotherapy in specific cases based on keynote 407. For patients with squamous histology and PD‐L1 expression <50%, our meta‐analysis decided to prospectively consider combination ICIs with chemotherapy as an option for the treatment of patients.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
References
- 1. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non‐small‐cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015; 5: 2892–911. [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 3. Global Burden of Disease Cancer C , Fitzmaurice C, Allen C et al Global, regional, and National Cancer Incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017; 3: 524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang J, Zou ZH, Xia HL, He JX, Zhong NS, Tao AL. Strengths and weaknesses of immunotherapy for advanced non‐small‐cell lung cancer: A meta‐analysis of 12 randomized controlled trials. PLoS One 2012; 7: e32695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel JD, Socinski MA, Garon EB et al PointBreak: A randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2013; 31: 4349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dietrich K, Theobald M. Immunological tumor therapy. Internist (Berl) 2015; 56: 907–16 quiz 917. [DOI] [PubMed] [Google Scholar]
- 7. Chinai JM, Janakiram M, Chen F, Chen W, Kaplan M, Zang X. New immunotherapies targeting the PD‐1 pathway. Trends Pharmacol Sci 2015; 36: 587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen YM. Immune checkpoint inhibitors for nonsmall cell lung cancer treatment. J Chin Med Assoc 2017; 80: 7–14. [DOI] [PubMed] [Google Scholar]
- 9. Kreamer KM. Immune checkpoint blockade: A new paradigm in treating advanced cancer. J Adv Pract Oncol 2014; 5: 418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zou W, Chen L. Inhibitory B7‐family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8: 467–77. [DOI] [PubMed] [Google Scholar]
- 11. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013; 39: 74–88. [DOI] [PubMed] [Google Scholar]
- 12. Jure‐Kunkel M, Masters G, Girit E et al Synergy between chemotherapeutic agents and CTLA‐4 blockade in preclinical tumor models. Cancer Immunol Immunother 2013; 62: 1533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen DS, Mellman I. Oncology meets immunology: The cancer‐immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 14. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Velcheti V, Schalper KA, Carvajal DE et al Programmed death ligand‐1 expression in non‐small cell lung cancer. Lab Invest 2014; 94: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fehrenbacher L, Spira A, Ballinger M et al Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–46. [DOI] [PubMed] [Google Scholar]
- 17. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 18. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carbone DP, Reck M, Paz‐Ares L et al First‐line Nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med 2017; 376: 2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Champiat S, Ferte C, Lebel‐Binay S, Eggermont A, Soria JC. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology 2014; 3: e27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Altman DG, Gotzsche PC et al The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 26. Lynch TJ, Bondarenko I, Luft A et al Ipilimumab in combination with paclitaxel and carboplatin as first‐line treatment in stage IIIB/IV non‐small‐cell lung cancer: Results from a randomized, double‐blind, multicenter phase II study. J Clin Oncol 2012; 30: 2046–54. [DOI] [PubMed] [Google Scholar]
- 27. Langer CJ, Gadgeel SM, Borghaei H et al Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: A randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol 2016; 17: 1497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Govindan R, Szczesna A, Ahn MJ et al Phase III trial of Ipilimumab combined with paclitaxel and carboplatin in advanced squamous non‐small‐cell lung cancer. J Clin Oncol 2017; 35: 3449–57. [DOI] [PubMed] [Google Scholar]
- 29. Socinski MA, Jotte RM, Cappuzzo F et al Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–301. [DOI] [PubMed] [Google Scholar]
- 30. Paz‐Ares LG, Luft A, Tafreshi A et al Phase 3 study of carboplatin‐paclitaxel/nab‐paclitaxel (chemo) with or without pembrolizumab (Pembro) for patients (pts) with metastatic squamous (Sq) non‐small cell lung cancer (NSCLC). J Clin Oncol 2018; 36: 105–5. [Google Scholar]
- 31. Gentzler RD, Langer CJ, Borghaei H et al 24‐month overall survival from KEYNOTE‐021 cohort G: Pemetrexed‐carboplatin plus pembrolizumab as first‐line therapy for advanced nonsquamous NSCLC. J Clin Oncol 2018; 36: 9026–6. [Google Scholar]
- 32. Borghaei H, Hellmann MD, Paz‐Ares LG et al Nivolumab (Nivo) + platinum‐doublet chemotherapy (chemo) vs chemo as first‐line (1L) treatment (Tx) for advanced non‐small cell lung cancer (NSCLC) with <1% tumor PD‐L1 expression: Results from CheckMate 227. J Clin Oncol 2018; 36: 9001–1. [Google Scholar]
- 33. Lopes G, Wu Y‐L, Kudaba I et al Pembrolizumab (pembro) versus platinum‐based chemotherapy (chemo) as first‐line therapy for advanced/metastatic NSCLC with a PD‐L1 tumor proportion score (TPS) ≥ 1%: Open‐label, phase 3 KEYNOTE‐042 study. J Clin Oncol 2018; 36: LBA4–4. [Google Scholar]
- 34. Jotte RM, Cappuzzo F, Vynnychenko I et al IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab‐paclitaxel vs carboplatin + nab‐paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018; 36: LBA9000–0. [Google Scholar]
- 35. Hellmann MD, Ciuleanu TE, Pluzanski A et al Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gandhi L, Rodriguez‐Abreu D, Gadgeel S et al Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018; 378: 2078–92. [DOI] [PubMed] [Google Scholar]
- 37. Zhang T, Xie J, Arai S et al The efficacy and safety of anti‐PD‐1/PD‐L1 antibodies for treatment of advanced or refractory cancers: A meta‐analysis. Oncotarget 2016; 7: 73068–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar R, Collins D, Dolly S, McDonald F, O'Brien MER, Yap TA. Targeting the PD‐1/PD‐L1 axis in non‐small cell lung cancer. Curr Probl Cancer 2017; 41: 111–24. [DOI] [PubMed] [Google Scholar]
- 39. Zhou L, Wang XL, Deng QL, Du YQ, Zhao NQ. The efficacy and safety of immunotherapy in patients with advanced NSCLC: A systematic review and meta‐analysis. Sci Rep 2016; 6: 32020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abdel‐Rahman O, Elhalawani H. Risk of fatal pulmonary events in patients with advanced non‐small‐cell lung cancer treated with EGF receptor tyrosine kinase inhibitors: A comparative meta‐analysis. Future Oncol 2015; 11: 1109–22. [DOI] [PubMed] [Google Scholar]
- 41. McGranahan N, Furness AJ, Rosenthal R et al Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351: 1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johnson DB, Frampton GM, Rioth MJ et al Targeted next generation sequencing identifies markers of response to PD‐1 blockade. Cancer Immunol Res 2016; 4: 959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sozzi G, Carney D. Molecular biology of lung cancer. Curr Opin Pulm Med 1998; 4: 207–12. [DOI] [PubMed] [Google Scholar]
- 44. Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nat Rev Genet 2013; 14: 703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: Immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11: 215–33. [DOI] [PubMed] [Google Scholar]
- 46. Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death‐1‐ligand 1 (PD‐L1) surface expression in breast cancer cells and promote PD‐L1‐mediated T cell apoptosis. Mol Immunol 2008; 45: 1470–6. [DOI] [PubMed] [Google Scholar]
- 47. Sheng J, Fang W, Yu J et al Expression of programmed death ligand‐1 on tumor cells varies pre and post chemotherapy in non‐small cell lung cancer. Sci Rep 2016; 6: 20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Naidoo J, Wang X, Woo KM et al Pneumonitis in patients treated with anti‐programmed Death‐1/programmed death ligand 1 therapy. J Clin Oncol 2017; 35: 709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


