Summary
Enhanced glucose uptake and a switch to glycolysis are key traits of M1 macrophages, whereas enhanced fatty acid oxidation and oxidative phosphorylation are the main metabolic characteristics of M2 macrophages. Recent studies challenge this traditional view, indicating that glycolysis may also be critically important for M2 macrophage differentiation, based on experiments with 2-DG. Here we confirm the inhibitory effect of 2-DG on glycolysis but also demonstrate that 2-DG impairs oxidative phosphorylation, significantly reduces 13C-labeled Krebs cycle metabolites and intracellular ATP levels. These metabolic derangements were associated with reduced JAK-STAT6 pathway activity and M2 differentiation marker expression. While glucose deprivation and glucose substitution with galactose effectively suppressed glycolytic activity, there was no effective suppression of oxidative phosphorylation, intracellular ATP levels, STAT6 phosphorylation, and M2 differentiation marker expression. These data indicate that glycolytic stimulation is not required for M2 macrophage differentiation as long as oxidative phosphorylation remains active.
Graphical Abstract

In Brief (“eTOC blurb”)
XXX et al reassess the role of glycolysis in alternatively activated (M2) macrophages and find that glucose depletion, leaving OXPHOS intact, does not affect M2 activation. However, the glucose analogue 2-DG, which impairs both glycolysis and OXPHOS, and reduces intracellular ATP and JAK-STAT6 signaling, impairs M2 differentiation.
Introduction
A growing body of literature has emerged demonstrating the significance of metabolism, especially the “glycolytic switch”, for the differentiation and activity of immune cells including T cells (Gubser et al., 2013), dendritic cells (Everts et al., 2014), and macrophages (Tannahill et al., 2013; Mills et al., 2016; Jha et al., 2016; Vats et al., 2006). Two main subtypes of macrophages have been defined with characteristic metabolic profiles (Galván-Peña and O’Neill, 2014; Rodríguez-Prados et al., 2010; Jha et al., 2016). The classical pro-inflammatory M1 macrophage subtype is activated by lipopolysaccharide (LPS) and/or interferon gamma (IFN-γ) and is characterized by an increase in aerobic glycolysis and a decrease in oxidative phosphorylation (OXPHOS) (Tannahill et al., 2013; Mills et al., 2016). Conversely, the anti-inflammatory M2 macrophages subtype is activated by IL-4 and characterized by enhanced OXPHOS and fatty acid oxidation (Vats et al., 2006; Huang et al., 2014). The latter aspect seems to be crucial as promoting fatty acid metabolism enhances whereas inhibition of fatty acid oxidation reduces the anti-inflammatory effects of M2 macrophages (Johnson et al., 2016). However, recent studies indicated that glycolysis may also be critical for M2 macrophage activation (Covarrubias et al., 2016; Huang et al., 2016; Van den Bossche et al., 2017). The implied mechanistic link between glycolysis and M2 macrophage activation is the generation of pyruvate by glycolysis, which then feeds into the TCA cycle to promote Ac-CoA synthesis and histone acetylation (Covarrubias et al., 2016) or mitochondrial OXPHOS (Huang et al., 2016). Worth considering, these studies used the glucose analogue 2-DG to block glycolysis, which may have additional off target effects and may not be a reliable method to assess the role of glycolysis in M2 macrophage activation (Kurtoglu et al., 2007; Ralser et al., 2008).
In the current study, using advanced cellular metabolomics and stable isotope 13C technologies, we determined the effects of 2-DG on glycolysis and OXPHOS and the role of these processes in M2 macrophage differentiation. We demonstrate that 2-DG impairs both, glycolysis and OXPHOS as well as the activation of the JAK-STAT6 pathway by reducing intracellular ATP level, which is required for JAK activity. Depletion of glucose or substitution of glucose with galactose, which likewise suppressed glycolysis and reduced pyruvate production, left OXPHOS intact and had no effect on alternative (M2) macrophage activation. These findings indicated that M2 macrophages have broader metabolic flexibility and can support OXPHOS by pathways other than pyruvate production by glycolysis. 13C6-glucose and 13C5-glutamine labeling experiments suggest that glutamine is one such pathway to fuel the tricarboxylic acid (TCA) or Krebs cycle. Thus, we uncovered that glycolytic stimulation is not a requirement for M2 macrophage activation and that 2-DG-based studies must be interpreted cautiously not to overestimate the role of glycolysis in cellular processes.
Results
2-DG but not glucose depletion impairs alternative (M2) macrophage differentiation
M1 macrophages are characterized by increased aerobic glycolysis and decreased OXPHOS, while M2 macrophages have enhanced OXPHOS and fatty acid oxidation (Pearce et al., 2013; O’Neill et al., 2016). Recent studies found that longer stimulation of M2 macrophages with IL-4 increased glucose consumption and lactation production (Covarrubias et al., 2016; Huang et al., 2016). However, real-time measurements of metabolic changes of macrophages have remained limited. In this study we found that LPS stimulation triggered a rapid increase in extracellular acidification rate (ECAR, an indicator of aerobic glycolysis), while the oxygen consumption rate (OCR, an indicator of OXPHOS) remained unchanged in bone marrow-derived macrophages (BMDMs) (Figure 1a and Table S1). On the contrary, no change in either real-time ECAR or real-time OCR was observed in macrophages stimulated with IL-4 (Figure 1b and Table S2). In agreement, LPS stimulation increased intracellular lactate levels (Figure 1c) while IL-4 had no impact on lactate production compared with control conditions (Figure 1d). Furthermore, LPS but not IL-4 increased the level of glucose-labeled lactate (Figure 1c and 1d). In resting as well as M1 and M2 macrophages, 13C6-glucose labeled 70–90% of upstream glycolytic metabolites (Figure S1a–c and S1e–g) but only 20–40% of pyruvate ( Figure S1d and S1h) and lactate (Figure 1c and 1d). These data are consistent with the role of glucose in maintaining glycolysis and support the presence of pathways other than glycolysis for pyruvate and lactate generation as pointed out before (Hui et al., 2017).
Figure 1. LPS but not IL-4 induces a glycolytic switch and 2-DG but not glucose depletion inhibits M2 macrophage differentiation.
(a)Real-time measurement of ECAR and OCR of BMDMs left unstimulated (−) or stimulated with LPS. Vertical line indicates initial injection of the activator. Data are representative of three independent experiments (n=5, mean ± SEM)
(b) Real-time measurement of ECAR and OCR of BMDMs left unstimulated (−) or stimulated with IL-4, vertical line indicates initial injection of the activator. Data are representative of three independent experiments (n=5, mean ± SEM)
(c) and (d) BMDMs were incubated in the medium containing 11.1 mM 13C6-glucose for 1 hour, followed by stimulation with LPS (c) or IL-4 (d) for 2 hours, relative amount and 13C-labeled ratio of lactate were measured by GC/MS with natural abundance correction. * p<0.05, *** p<0.001, NS no significant difference. (n=3, mean ± SEM)
(e) Immunoblot analysis of IL-1β expression in BMDMs after 6 and 12 hour stimulation with LPS +/− 1 hour pre-treatment with 2-DG (10 mM). Data are representative of three independent experiments
(f) Immunoblot analysis of IL-1β expression in BMDMs after 24 and 48 hour stimulation with LPS in the medium with or without glucose. Data are representative of three independent experiments
(g) Flow cytometry analysis of PD-L2 and RELMα in BMDMs stimulated with IL-4 for 24 hours in medium containing glucose, glucose + 2-DG (10 mM) or glucose depletion (with FBS final glucose concentration 0.3 mM). Data are representative of three independent experiments
To test the role of glycolysis in macrophage differentiation, we treated BMDMs with the glucose analogue 2-DG prior to LPS or IL-4 stimulation. 2-DG blocked IL-1β production after LPS stimulation (Figure 1e) and the expression of the M2 macrophage differentiation markers PD-L2 and RELMα after Il-4 stimulation (Figure 1g), in agreement with previous studies (Covarrubias et al., 2016; Huang et al., 2016). However, unlike 2-DG treatment, glucose depletion (i.e. use of medium without any glucose, but with the addition of standard fetal bovine serum leading to a final glucose concentration of 0.3 mM) had no effect on these two M2 macrophage markers (Figure 1g) while it did reduce IL-1β production of M1 macrophages (Figure 1f) (Tannahill et al., 2013; Mills et al., 2016). These data indicate that the effects of 2-DG on M2 macrophages may not be attributed solely to its effects on glycolysis.
Glycolysis interference with galactose does not inhibit M2 macrophage differentiation
To further test if 2-DG had effects other than glycolysis inhibition, we used galactose, which must be metabolized by the Leloir pathway before entering glycolysis, leading to profound reduction in glycolytic flux and functionally effective inhibitory slow down (Bustamente et al., 1977; Weinberg et al., 2010; Chang et al., 2013). Previous studies concluded that pyruvate produced from glucose via glycolysis was required for supporting OXPHOS in alternatively activated macrophages as 2-DG significantly reduced OCR after IL-4 stimulation (Huang et al., 2016). Herein, we likewise found that 2-DG treatment reduced OCR in addition to ECAR (Figure 2a). However, while galactose did decrease ECAR, it did not result in a reduction of OCR (Figure 2a). Moreover, galactose and glucose depletion significantly decreased glycolytic metabolites in cells (G6P, DHAP, 3PG, and PEP) and culture medium (pyruvate and lactate). The cellular levels of DHAP and PEP and extracellular levels of pyruvate and lactate were even lower with these interventions than with 2-DG treatment (Figure 2b–2d and Figure S2a–2f). 2-DG treatment resulted in significantly increased production of 2-DG6p (>400-fold of G6p) (Figure 2e), which is consistent with the expectation that 2-DG cannot be metabolized further after being converted to 2-DG6p (Pelicano et al., 2006). Importantly, 2-DG, but not galactose or glucose depletion, blocked M2 macrophage differentiation markers (Figure 1g and 2f). These data further suggest that impairment of M2 macrophage differentiation by 2-DG cannot be attributed to glycolysis inhibition alone.
Figure 2. Galactose suppresses glycolytic throughout but not M2 differentiation.
(a) Real-time measurement of ECAR and OCR of BMDMs incubated in medium containing glucose, galactose or glucose + 10 mM 2-DG for 1 hour, left unstimulated (−) or stimulated with IL-4, vertical line indicates initiation injection of the activator. Data are representative of three independent experiments (n=5, mean ± SEM)
(b) - (e) BMDMs were incubated in the medium containing glucose, glucose + 2-DG (10 mM), galactose or glucose depletion (with FBS final glucose concentration 0.3 mM) for 1 hour, followed by stimulation with IL-4 for 2 hours. Intracellular levels of glucose (b), Glucose 6-phosphate (c), lactate (d) and 2-deoxyglucose-6-phosphate (e) were measured by GC/MS. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001, NS no significant difference. Data are representative of two independent experiments (n=5, mean ± SEM)
(f) Flow cytometry analysis of PD-L2 and RELMα in BMDMs stimulated with IL-4 for 24 hours in medium containing glucose, glucose + 2-DG (10 mM) or galactose. Data are representative of three independent experiments
M2 macrophages are metabolically flexible and can use sources other than glucose to fuel the TCA cycle
Labeling studies were performed to define the contribution of glucose to generation of TCA intermediate. We observed that approximately 10–25% of individual TCA cycle metabolites were labeled by 13C6-glucose in bone-marrow-derived macrophages (BMDMs) (Figure 3a–3d and Figure S3a–3d). Importantly, IL-4 stimulation did not change the relative enrichment level of 13C6-glucose -labeled TCA metabolites (Figure 3a–3d and Figure S3a–3d), while LPS stimulation increased the level of 13C6-glucose-labeled TCA metabolites (Figure 3e–3h and Figure S3e–3h). 2-DG but neither glucose depletion nor galactose led to a significant reduction in TCA cycle metabolites (Figure 3i–3m). Of further interest, 30–50% of individual TCA cycle metabolites were labeled by 13C5-glutamine in BMDMs under resting conditions (Figure 3n–3r). IL-4 stimulation increased 13C5-glutamine labeling of TCA metabolites (Figure 3n–3r). Intriguingly, 2-DG significantly reduced glutamine consumption ( Figure S2g) and the relative enrichment levels of 13C5-glutamine labeled TCA cycle metabolites, including glutamate, citrate, succinate, fumarate and malate (Figure 3n–3r, while galactose and glucose depletion increased the levels of 13C5-glutamine labeled TCA cycle metabolites (Figure 3n–3r). These data indicate that glutamine fuels the TCA cycle, especially in M2 macrophages under IL-4 stimulation and under conditions of reduced glycolytic flux. Macrophages stimulated with IL-4 are therefore metabolically flexible and are not dependent on glycolysis. Importantly, 2-DG not only blocks glycolysis but also reduces glutamine fueling of the TCA cycle in M2 macrophages.
Figure 3. Differential effect of LPS and IL-4 as well as 2-DG and galactose or glucose depletion on 13C6-glucose and 13C5-glutamine labeled TCA cycle metabolites.
(a)-(d) BMDMSs were incubated in medium containing 11.1 mM 13C6-glucose for 1 hour, followed by stimulation with IL-4 for 2 hours Relative enrichment of 13C-labeled ratio of TCA cycle metabolites was measured by GC/MS with natural abundance correction. NS no significant difference. Data are representative of two independent experiments (n=3, mean ± SEM)
(e)-(h) BMDMs were incubated in medium containing 11.1 mM 13C6-glucose for 1hour, followed by stimulation with LPS for 2 hours. Relative enrichment of 13C-labeled ratio of TCA cycle metabolites was measured by GC/MS with natural abundance correction. * p<0.05, *** p<0.001, **** p<0.0001. Data are representative of two independent experiments (n=3, mean ± SEM)
(i)-(m) BMDMs were incubated in medium containing glucose, glucose + 2-DG (10 mM), galactose or or glucose depletion (with FBS final glucose concentration 0.3 mM) for 1 hour, followed by stimulation with IL-4 for 2 hours. Intracellular levels of TCA cycle metabolites were measured by GC/MS. * p<0.05, *** p<0.001, **** p<0.0001, NS no significant difference. Data are representative of two independent experiments (n=5, mean ± SEM)
(n)-(r) BMDMs were incubated in the 13C5-glutamine-supplemented (2 mM) medium containing glucose, glucose + 2-DG (10 mM), galactose or or glucose depletion (with FBS final glucose concentration 0.3 mM) for 1 hour, followed by stimulation with IL-4 for 2 hours. Relative enrichment of 13C-labeled ratio of TCA cycle metabolites were measured by GC/MS. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. Data are representative of two independent experiments (n=5, mean ± SEM)
2-DG and OXPHOS inhibition differ in their inhibitory effects on M2 macrophages
Previous studies found that 2-DG blocked M2 macrophage differentiation and attributed this to a reduction of the production of pyruvate, which feeds into the TCA cycle to promote Ac-CoA synthesis and histone acetylation (Covarrubias et al., 2016) or mitochondrial OXPHOS (Huang et al., 2016). To test if the inhibitory effects of 2-DG can be attributed to OXPHOS inhibition, we treated cells with the fatty acid oxidation inhibitor etomoxir and the mitochondrial electron chain inhibitor oligomycin, both of which significantly reduced OXPHOS (Figure 4a and 4b). We found that both etomoxir and oligomycin inhibited M2 macrophage differentiation markers PD-L2 and RELMα (Figure 4c), confirming that OXPHOS is important for M2 macrophage differentiation (Vats et al., 2006; Huang et al., 2014). However, only 2-DG but not etomoxir or oligomycin decreased the expression of CD36, which is an important marker required for M2 macrophage differentiation (Figure 4d). These data indicate that the inhibition of M2 macrophage differentiation by 2-DG cannot be attributed to OXPHOS inhibition alone.
Figure 4. Etoxomir and oligomycin differ in their effects on M2 differentiation markers from 2-DG.
(a) BMDMs were incubated with or without etomoxir (200 µM) for 1 hour, followed by stimulation with IL-4 for 3 hours and OCR measurement. ** p<0.01. Data are representative of three independent experiments (n=5, mean ± SEM)
(b) Raw264.7 cells were treated with or without 2-DG (10 mM) or etomoxir (200 µM) for 1 hour, followed by stimulation with IL-4. OCR profiles were measured at baseline and after indicated injections (n=5)
(c) BMDMs were incubated with or without etomoxir (200µM) and oligomycin (1µM) for 1 hour, followed stimulated by IL-4 for 24 hours. PD-L2 and RELMα expression were analyzed by flow cytometry. Data are representative of three experiments
(d) 3D (left) and 2D (right) flow cytometry analysis of CD36 expression in BMDMs treated as (b). Data are representative of three experiments
2-DG but not inhibition of glycolysis or OXPHOS alone blocks JAK-STAT6 pathway activation after IL-4 stimulation
IL-4 stimulation triggers JAK-STAT6 activation and thereby the expression of genes, which play a key role in M2 macrophage differentiation (Vats et al., 2006; Covarrubias et al., 2016; Huang et al., 2016). As 2-DG, but not galactose or glucose depletion blocked alternative activation of macrophages, we next studied if JAK-STAT6 activation could be affected by 2-DG. Intriguingly, 2-DG treatment reduced STAT6 phosphorylation after IL-4 stimulation (Figure 5a and 5b and Figure S4a). However, neither galactose nor glucose depletion affected STAT6 phosphorylation in M2 macrophages from BMDMs (Figure 5f and Figure S4b and S4c). Likewise, inhibition of the last step of aerobic glycolysis catalyzed by lactate dehydrogenase A (LDHA) with FX-11, a NADH competitive inhibitor of LDHA that does not affect the activities of LDHB and glyceraldehyde-3-phosphate dehydrogenase even at higher concentrations (Le et al., 2010), did not reduce STAT6 phosphorylation (Figure 5c and Figure S4f). Finally, inhibition of the mitochondrial pyruvate transport by UK-5099 (Halestrap, 1976) or fatty acid oxidation by etomoxir (Vats et al., 2006; Huang et al., 2014; Covarrubias et al., 2016; Huang et al., 2016) had no impact on JAK-STAT6 activation (Figure 5d and 5e and Figure S4d and S4e)
Figure 5. STAT-6 signaling, PPARɣ, and CD36 expression is suppressed only by 2-DG but no other intervention including glucose depletion and galactose.
(a) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for indicated time +/− 1 hour pre-treatment with or without 2-DG (10 mM). Data are representative of three independent experiments
(b) Immunofluorescence staining of p-STAT6 in BMDMs after 3 hour stimulation with IL-4 in medium containing glucose, glucose + 2-DG, galactose or glucose depletion (with FBS final glucose concentration 0.3 mM). Data are representative of two independent experiments
(c) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for indicated times, after 1 hour pre-treatment with or without FX11 (80 µM). Data are representative of three independent experiments
(d) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for indicated times +/− 1 hour pre-treatment with UK-5099 (100 µM). Data are representative of three independent experiments
(e) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for indicated times, after 1 hour pre-treatment with or without etomoxir (200 uM). Data are representative of three independent experiments
(f) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for indicated times in medium containing glucose, galactose and glucose depletion (with FBS final glucose concentration 0.3 mM). Data are representative of three independent experiments
(g) Immunoblot analysis of PPAR-γ in BMDMs stimulated with IL-4 for 12 hours, after 1 hour pre-treatment with or without 2-DG (10 mM). Data are representative of three independent experiments
(h) Immunoblot analysis of PPAR-γ in BMDMs stimulated with IL-4 for 12 hours in the medium containing glucose or galactose. Data are representative of three independent experiments
(i) Immunoblot analysis of PPAR-γ in BMDMs stimulated with IL-4 for 12 hours in the medium with or without glucose (with FBS final glucose concentration 0.3 mM). Data are representative of three independent experiments
(j) 3D (left) and 2D (right) Flow cytometry analysis of CD36 in BMDMs stimulated with IL-4 in the medium containing glucose, glucose + 2-DG (10 mM), galactose or no glucose (with FBS final glucose concentration 0.3 mM) for 24 hours. Data are representative of three independent experiments
We next tested whether STAT6-dependent markers of M2 macrophage differentiation could be reduced by 2-DG. IL-4-mediated JAK activation and JAK-mediated phosphorylation of the transcription factor STAT6 leads to the expression of ligand-dependent nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) (Szanto et al., 2010). PPARγ in turn mediates the expression of scavenger receptor CD36 (Huang et al., 1999). These steps are critical for facilitating lipid uptake and fatty acid synthesis and oxidation (Vats et al., 2006; Odegaard et al., 2007; Huang et al., 2014). PPARγ expression was reduced by 2-DG (Figure 5g and Figure S5a); however, glucose depletion, galactose or etomoxir had no effect (Figure 5h and 5i and Figure S5a–5c). Furthermore, STAT6-dependent scavenger receptor CD36 expression was blocked by 2-DG, but remained unaffected by galactose, glucose depletion, or etomoxir treatment (Figure 5j and Figure S5d and 5e). Thus, 2-DG impaired M2 activation by blocking JAK-STAT6 pathway activation and its dependent gene expression. This may relate to its combined effect on glycolysis and OXPHOS, as inhibition of these metabolic pathways individually did not suffice to reduce JAK-STAT6 pathway activation and M2 macrophage differentiation.
Reduced ATP production by 2-DG is responsible for impaired JAK-STAT6 activation
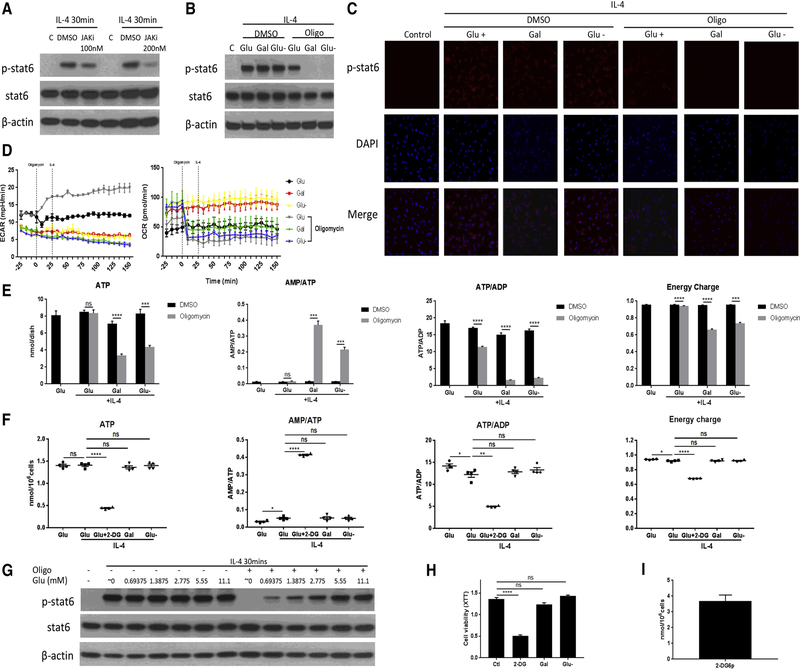
Following IL-4 stimulation, JAK undergoes autophosphorylation as well as conformational changes to phosphorylate STAT6 by catalyzing the transfer of the γ-phosphate from ATP to STAT6 (Shuai and Liu, 2003). JAK kinases are well known as ATP-dependent kinases and can be blocked by ATP-competitive inhibitors (Hammarén et al., 2015). Indeed, STAT6 phosphorylation can be blocked by an ATP competitive inhibitor of JAK (Figure 6a). Alterations in intracellular ATP levels may thus influence the activation of JAK and the JAK-STAT6 pathway. To test this hypothesis, we treated cells with the mitochondrial ATP synthase inhibitor oligomycin in combination with glucose, galactose, or neither. Glucose depletion or galactose treatment had no impact on STAT6 phosphorylation after IL-4 stimulation (Figure 5f and Figure 6b and Figure S6a and 6b). However, STAT6 phosphorylation was completely blocked when oligomycin was combined with glucose depletion or galactose substitution (Figure 6b and 6c and Figure S6a–6c), while cell viability was still maintained (Figure S6d). As expected, cell viability could not be maintained at later stages of this combined inhibitory approach (Figure S7a and S7b). Glycolysis may thus take a compensatory role when OXPHOS is blocked by oligomycin, but only as long as glucose is present (Figure 6d). In keeping with the above, ATP production was significantly reduced by oligomycin under glucose depletion and galactose conditions, while it remained unchanged when glucose was presented (Figure 6e).
Figure 6. 2-DG decreases intracellular ATP content and STAT-6 phosphorylation similar to the combined action of oligomycin with either glucose depletion or galactose.
(a) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for 30min following 1 hour pre-treatment with or without ATP competitive inhibitor of JAK (100 or 200 nM). Data are representative of three independent experiments
(b) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for 30min in the medium containing glucose, galactose or glucose depletion (Glu-, with the use of standard FBS final glucose concentration 0.3 mM) +/− 30 minutes of pre-treatment with oligomycin A (1 µM). Data are representative of three independent experiments
(c) Immunofluorescence staining of p-STAT6 in BMDMs after 30 min stimulation with IL-4 in culture medium with glucose, galactose, or glucose depletion (Glu-, with the use of standard FBS final glucose concentration 0.3 mM) without or with 30 minutes of pre-treatment with oligomycin A (1 µM). Data are representative of two independent experiments
(d) Real-time measurement of ECAR and OCR of BMDMs incubated in the medium containing glucose, galactose or glucose depletion (Glu-, with the use of standard FBS final glucose concentration 0.3 mM) for 1 hour, left unstimulated or stimulated with oligomycin (1 µM) and IL-4, vertical line indicates initiation injection of the activator. Data are representative of three independent experiments (n=3–4, mean ± SEM)
(e) ATP, AMP/ATP, ATP/ADP and energy charge of RAW264.7 cells stimulated with IL-4 for 30 min in the medium containing glucose, galactose, or glucose depletion (Glu-, with the use of standard FBS final glucose concentration 0.3 mM) +/− 30 minutes of pre-treatment with oligomycin A (1 µM). *** p<0.001, **** p<0.0001, NS no significant difference (n=4, mean ± SEM)
(f) Intracellular ATP, AMP/ATP, ATP/ADP and energy charge of BMDMs stimulated with IL-4 for 3 hours in the medium containing glucose, glucose + 2-DG (10 mM), galactose, or glucose depletion (Glu-, with the use of standard FBS final glucose concentration 0.3 mM). * p<0.05, ** p<0.01, **** p<0.0001, NS no significant difference (n=4, mean ± SEM)
(g) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for 30 min in culture medium with different glucose concentrations (with the use of standard FBS, minimal glucose concentration 0.3 mM) +/− 30 minutes of pre-treatment with oligomycin A (1 µM). Data are representative of three independent experiments
(h) Cell viability of BMDMs cells after stimulation with IL-4 for 12 hours in the medium containing glucose, glucose + 2-DG, galactose, or glucose depletion (with the use of standard FBS, minimal glucose concentration 0.3 mM). **** p<0.0001, NS no significant difference. Data are representative of three independent experiments (n=4, mean ± SEM)
(i) BMDMs were incubated in the medium containing 2-DG (10 mM) 1 hour, followed by stimulation with IL-4 for 2 hours. The absolute amount of 2-DG6p was quantified (n=4, mean ± SEM)
Thus, either cytosolic or mitochondrial ATP production is sufficient to support cell survival and JAK-STAT6 activation, and glycolysis can compensate for ATP production under circumstances of OXPHOS inhibition. This compensatory response is abolished by 2-DG (Figure S6e). Importantly, glucose, in a dose-dependent manner, can restore STAT6 phosphorylation in oligomycin-treated cells (Figure 6g and Figure S6f). It can be assumed that under such conditions any ATP produced must be derived from glycolysis and that the higher the glucose concentration provided, the higher the glycolytic activity, the greater the ATP production, and the higher the level of p-STAT6. As such, these data support the hypothesis that intracellular ATP is critical for JAK-STAT6 activation and that glycolysis suffices to maintain the activity of this pathway.
To further evaluate ATP production as the mechanistic explanation for why 2-DG rather than glycolysis inhibition reduces JAK-STAT6 activation, we measured intracellular ATP, ADP, and AMP levels. 2-DG decreased ATP levels and the ATP/ADP ratio but increased the AMP/ATP ratio (Figure 6f and Figure S7c–7f), along with a reduction in viability (Figure 6h). On the contrary, galactose and glucose depletion did not influence ATP production, ATP/ADP or AMP/ATP ratio (Figure 6f), and neither did they impair M2 macrophage viability (Figure 6h and Figure S7g). This is consistent with the real-time ECAR and OCR data (Figure 2a) showing that both, glycolysis and OXPHOS, are impaired by 2-DG whereas OXPHOS remains intact for ATP production after glycolysis inhibition by glucose depletion or galactose treatment. Moreover, unlike glucose metabolism, which has a net gain of 2 molecular ATP via glycolysis, 2-DG has a net consumption of ATP and net production of ADP and AMP. This is illustrated in the phosphorylation of 2-DG, which requires ATP and was in the order of 3.68 nmol per million cells over 3 hours (Figure 6i), leaving an average remaining ATP content of only 0.5 nmol ATP per million cells (Figure 6f).
2-DG is not an ideal reagent to differentiate between the role of glycolysis and OXPHOS
To test if lower concentrations of 2-DG can be used to differentiate between the role of glycolysis and OXPHOS in M2 macrophage differentiation, we exposed BMDMs to different concentrations of 2-DG. As shown in Figure 7a, we found that 2-DG reduced glycolysis in a dose-dependent manner, starting at doses as low as 0.156 mM. Intriguingly, 2-DG treatment also affected OXPHOS in a differential manner, increasing it at lower doses (<1.25 mM) but decreasing it at higher doses (10 mM) (Figure 7a). Moreover, 2-DG reduced ATP production and M2 macrophage differentiation at concentrations as low as 0.3 mM (Figure 7b–7d). We therefore identified a dose range of 2-DG, at which there is reduction in ECAR but no change in ATP production and M2 macrophage differentiation, in further support of the view that glycolysis is not mandatory for M2 macrophages. Furthermore, in glucose dose experiments (using dialyzed serum, which allowed for experiments with complete lack of any measurable glucose content) we confirmed glycolytic flux as a function of extracellular glucose levels (Figure 7e). However, there was no difference in the expression of M2 differentiation markers across the spectrum of different glucose concentrations (Figure 7f and 7h). Moreover, even complete glucose depletion with the use of dialyzed serum instead of standard FBS had no impact on JAK-STAT6 activation and PPAR-γ expression (Figure 7i and 7j). Importantly, ATP production and OXPHOS of M2 macrophages remained unchanged in the presence of different levels of glycolytic flux (Figure 7g).
Figure 7. Dose-dependent effects of 2-DG and glucose on metabolism, intracellular ATP content and differentiation of M2 macrophages.
(a) BMDMs were incubated in the medium containing different concentrations of 2-DG for 1 hour, real-time changes in ECAR (a) and OCR (b) were measured in BMDMs stimulated with IL-4, injection indicated by the vertical line. Data are representative of three independent experiments (n=3–4, mean ± SEM)
(b) Flow cytometry analysis of PD-L2 and RELMα in BMDMs stimulated with IL-4 for 24 hours in the medium containing different concentrations of 2-DG. Data are representative of three experiments
(c) 3D (left) and 2D (right) Flow cytometry analysis of CD36 in BMDMs stimulated with IL-4 for 24 hours in the medium containing different concentrations of 2-DG. Data are representative of three experiments
(d) Intracellular ATP, AMP/ATP, ATP/ADP and energy charge of BMDMs stimulated with IL-4 for 3 hours in the medium containing different concentrations of 2-DG. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 as compared to 0 mM. Data are representative of two independent experiments (n=4, mean ± SEM)
(e) BMDMs were incubated in the medium without glucose (in distinction to the other experiments, 10% dialyzed FBS was used, which resulted in no detectable glucose, i.e. glucose concentration 0 mM) for 1 hour, real-time changes in ECAR (a) and OCR (b) were measured in BMDMs injected with different concentrations of glucose, followed stimulated with IL-4, and vertical line indicates initiation injection of the glucose and IL-4. Data are representative of three independent experiments (n=5, mean ± SEM)
(f) Flow cytometry analysis of PD-L2 and RELMα in BMDMs stimulated with IL-4 for 24 hours in the medium containing different concentrations of glucose (with the use of 10% dialyzed FBS resulting in no detectable glucose, i.e. minimal glucose concentration of 0 mM). Data are representative of three experiments
(g) 3D (left) and 2D (right) Flow cytometry analysis of CD36 in BMDMs stimulated with IL-4 for 24 hours in the medium containing different concentrations of glucose (with the use of 10% dialyzed FBS resulting in no detectable glucose, i.e. minimal glucose concentration of 0 mM).. Data are representative of three experiments
(h) Intracellular ATP, AMP/ATP, ATP/ADP and energy charge of BMDMs stimulated with IL-4 for 3 hours in the medium containing different concentrations of glucose (with the use of 10% dialyzed FBS resulting in no detectable glucose, i.e. minimal glucose concentration of 0 mM). * p<0.05, ** p<0.01, *** p<0.001 compared with 11.1 mM. Data are representative of two independent experiments (n=4, mean ± SEM).
(i) Immunoblot analysis of STAT6 phosphorylation in BMDMs stimulated with IL-4 for indicated times in medium with or without glucose plus 10% dialyzed FBS (resulting in no detectable glucose, i.e. glucose concentration 0 mM). Data are representative of three independent experiments
(j) Immunoblot analysis of PPAR-γ in BMDMs stimulated with IL-4 for 12 hours in the medium with or without glucose plus 10% dialyzed FBS (resulting in no detectable glucose, i.e. minimal glucose concentration 0 mM). Data are representative of three independent experiments
Discussion
The role of metabolism in alternative macrophage differentiation and activation is still controversial. M2 macrophages were found to have enhanced fatty acid oxidation about one decade ago (Vats et al., 2006), and inhibition of fatty acid oxidation by etomoxir reduced M2 macrophage-associated gene expression (Vats et al., 2006; Huang et al., 2014). On the contrary, genetic depletion of carnitine palmitoyltransferase (CPT) 2, which facilitates the transport of long-chain fatty acids into the mitochondrial matrix, inhibited fatty acid oxidation but had no effect on M2 macrophage activation (Nomura et al., 2016). More recently, studies found that macrophages increase glucose consumption after 16–24 hours of stimulation with IL-4 (Covarrubias et al., 2016; Huang et al., 2016). Covarrubias et al. found that AKT-mTORC1, parallel to the canonical JAK-STAT6 pathway, is responsible for the increased glucose consumption after stimulation with IL-4 (Covarrubias et al., 2016). A subsequent study showed that mTORC2 but not mTORC1 enhanced glucose utilization (Huang et al., 2016). Both studies concluded that glycolysis is important for M2 macrophage activation, as glycolysis inhibition with 2-DG significantly impaired the expression of the M2 markers PD-L1 and RELMα (Covarrubias et al., 2016; Huang et al., 2016). This was attributed to glycolysis-driven generation of pyruvate, which would then support the TCA cycle and promote Ac-CoA synthesis (Covarrubias et al., 2016) and/or mitochondrial OXPHOS (Huang et al., 2016).
In principle, two functions of glycolysis need to be differentiated: a regulatory and an obligatory function. The latter relates to the preservation of cell survival, the former to the alteration of cell function and/or phenotype. It is intuitively impossible to conclude on the regulatory role under conditions that challenge the obligatory role. Herein we show that 2-DG at doses that have been commonly used in prior studies to define the regulatory function of glycolysis operate at a level when the obligatory role of glycolysis is needed as 2-DG affects both glycolysis and OXPHOS. In dose response experiments we were able to define that 0.156 mM 2-DG reduced glycolysis significantly but had no impact on ATP production and M2 differentiation. At two and four times this dose (0.3125 mM and 0.625 nM, respectively) there was a significant reduction in energy charge although OXPHOS was not yet reduced compared with untreated controls. At a dose of 10 mM 2-DG, which is commonly used, OXPHOS was also suppressed leading to a marked drop in intracellular ATP content and energy charge that compromised cell viability. Thus, there is a very narrow dose range for 2-DG that allows for a differentiation of regulatory and obligatory functions of glycolysis. Glycolysis does not take a mandatory regulatory role in M2 differentiation, but assumes an obligatory role once OXPHOS is compromised, and suppression of glycolysis and OXPHOS at higher 2-DG doses suppresses intracellular ATP content and M2 differentiation.
The view that glycolysis is not mandatory for M2 differentiation is supported by experiments with different glucose concentrations. While glycolytic flux is a function of the extracellular glucose concentration, M2 differentiation is not and remains completely unaffected by the extracellular glucose milieu. In fact, even complete glucose depletion using dialyzed serum did not affect M2 differentiation. Similarly, using galactose, which has to be metabolized via the Leloir pathway and thereby rendering glycolysis functionally ineffective, had no influence on M2 differentiation over the observed time period. Both interventions, glucose depletion and galactose, did reduce glycolytic flux significantly, but left TCA cycle, OXPHOS, and intracellular ATP levels intact. This is an intriguing observation given the role attributed to pyruvate, considered to be mainly derived from glycolysis. Indeed, blocking mitochondrial pyruvate transport with UK-5099 (Huang et al., 2016) or genetic depletion of pyruvate dehydrogenase kinase 1 (PDK1) (Tan et al., 2015) has been shown to impair M2 activation. 13C6-glucose labeling experiments in the current study, however, show that only 40% of pyruvate is derived from glucose. These results suggest that there are major sources of pyruvate other than glucose breakdown as pointed out before (Hui et al, 2017). Furthermore, 13C5-glutamine labeling experiments suggest that glutamine can fuel the TCA cycle under glucose depletion and galactose conditions, further decreasing the reliance on pyruvate. This is in agreement with previous studies, showing that cells utilize more glutamine when cultured in galactose versus glucose conditions (Reitzer et al., 1979; Chang et al., 2013). In addition, M2 macrophages increase glutamine but not glucose incorporation into the TCA cycle under IL-4 stimulation, underscoring the significance of glutamine metabolism for the metabolic flexibility of this cell population.
One of the cardinal metabolic differences between 2-DG and glucose depletion or galactose is the reduction in intracellular ATP levels. It is known that cellular ATP is used to phosphorylate 2-DG leading to nucleotide imbalance and loss through adenylate kinase and 5’-nucleotidase reactions, which along with an inorganic phosphate trap (2-DG-p) could compromise metabolic enzymes and mitochondrial functions (Chen et al., 1996). Our study, indeed, confirms that this step consumes a significant amount of ATP, which might explain the shift in the intracellular nucleotide balance at lower doses of 2-DG, i.e. even before OXPHOS is compromised. Inhibition of OXPHOS, e.g. with oligomycin, renders macrophages dependent on glycolysis for ATP production and provides a unique opportunity to test glycolytic capacity. In the current study, this approach not only confirmed that under conditions of glucose depletion or glucose substitution with galactose, the glycolytic response is impaired but also provided new insight on the differentiation pathway. STAT6 phosphorylation was nearly absent when IL-4 stimulated macrophages were treated with oligomycin and no glucose was provided. However, STAT6 phosphorylation could be restored with relatively small concentrations of glucose. With JAK being an ATP-dependent kinase, ATP generation as a function of glycolysis under OXPHOS inhibition conditions likely exerts this modulating effect, which translates in the expression of M2 differentiation markers.
PPAR-γ and the scavenger receptor CD36 are well-known products of JAK-STAT6-dependent gene expression whereas PD-L2 and RELMα are less so (Szanto et al., 2010; Huang et al., 1999). CD36 plays a critical role in M2 macrophage activation by facilitating lipid uptake for fatty acid synthesis in support of fatty acid oxidation (Huang et al., 2014). A previous study showed that CD36 was unaffected by inhibition of mTORC1, and this was responsible for the increased glucose consumption (Covarrubias et al., 2016). However, CD36 expression could be blocked by 2-DG, further indicating that 2-DG affects alternative macrophage activation independent of glycolysis inhibition. While mTORC2 inhibition has been shown to reduce glucose utilization (Huang et al., 2016), mTORC2 also promotes fatty acid synthesis independent of glucose flux (Hagiwara et al., 2012), which might explain the inhibition of M2 macrophage activation by mTORC2 suppression. Herein we found that, indeed, 2-DG decreased PPAR-γ and CD36 expression, whereas interference with either OXPHOS or glycolysis failed to do so.
In conclusion, this study uncovered that M2 macrophage differentiation/activation via the JAK-STAT6 pathway requires a threshold level of intracellular ATP, which can be supported by either glycolysis or OXPHOS pathways. Glycolysis for this reason is not mandatory for alternative activation of macrophages as long as OXPHOS is intact. Moreover, glutamine can fuel the TCA cycle under conditions of reduced glycolysis as show in 13C-glucose and 13C-glutamine labeling experiments. 2-DG at commonly used doses impairs glycolysis and OXPHOS, thereby suppressing ATP production, JAK-STAT6 pathway activation and the M2 phenotype. At least in these cells, 2-DG cannot be confirmed as a specific glycolysis inhibitor.
Limitations of the study
Although extensive experimentation supports conclusions regarding the role of glycolysis in M2 macrophage differentiation, there are still several limitations.
Genetic knockout strategies would be desirable to show that a macrophage incapable of glycolysis could still become a functional M2 macrophage. The use of dialyzed serum, however, allows for studies with no glucose available for uptake and glycolysis.
Single time point labelling experiments may be insufficient for steady state conditions. However, based on the real-time ECAR and OCR measurements, a solid plateau was noted at the time of these measurements supporting the notion that cells approached steady state conditions.
Modulation of intracellular ATP levels can have multiple effects, one of which is the activity of ATP-dependent kinases like JAK. It is yet to be shown that providing ATP can overcome the suppressive effect of 2-DG in M2 macrophages. In a prior study in M1 macrophages, however, we were able to demonstrate that restoring (“rescuing”) the intracellular ATP pool under suppressive 2-DG conditions can restore JAK-STAT signaling and cell differentiation/activation (Wang et al., 2018).
Future studies are required to define how 2-DG interferes with glutamine metabolism and the compensatory glutamine supply to the TCA cycle when glycolysis and pyruvate flux from glycolysis is blocked. Indeed, further work on the metabolic pathways for pyruvate and glutamine feeding into the TCA cycle other than glycolysis is needed.
Methods
STAR★Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Joerg Herrmann, MD (herrmann.joerg@mayo.edu)
Experimental Model and Subject Details
Mouse Strains
Female wild-type C57BL/6J mice (8–12 weeks of age) were used for bone marrow-derived macrophages (BMDMs) isolation in this study. The mice were bred and maintained under pathogen-free conditions. This study was approved by the Mayo Clinic Institutional Animal Use and Care Committee (IACUC).
BMDMs isolation
BMDMs were isolated from C57BL/6 as previously described (Zhang et al., 2008). Briefly, the tibia and femora were isolated and pulverized using a pestle and mortar to obtain bone marrow cells. These cells were suspended and grown in RPMI-1640 (10% heat inactivated FBS and 1% penicillin and streptomycin) containing 10 ng/ml M-CSF. On day 3, half volume of fresh grow medium was added. After 7 days in culture, macrophages were harvested and plated for further experimentation. All animal experiments were performed with IACUC approval.
Raw264.7 culture
Mouse leukemic monocyte/macrophage cell line (Raw264.7) was purchased from ATCC and cultured in high glucose DMEM containing 10% FBS and 1% penicillin and streptomycin. Cells were passaged every 3 days.
Method Details
Macrophage differentiation
BMDMs and Raw264.7 cells were seeded at 1×106/ml for experiments. The cells were stimulated with LPS (100 ng/ml) or IL-4 (20 ng/ml) for indicated times to induce classical or alternative activation, respectively.
Metabolism assay
Real-time changes in ECAR and OCR of BMDMs were analyzed with an XF-24 Extracellular Flux Analyzer (Seahorse Bioscience) as described (Chang et al., 2013; Everts et al., 2014). Briefly, BMDMs were seeded in XF24 Cell Culture Microplates for 24 hours before the experiments. Cells were then switched to Seahorse media with or without glucose, galactose or glucose plus different concentrations of 2-DG and incubated for 1 hour. ECAR and OCR were measured 3–4 times at baseline and kinetics were recorded for the indicated times after injection with different concentrations of glucose, stimulators (LPS and IL-4) or inhibitors (Oligomycin, FCCP, Rotenone and Antimycin A).
Intracellular metabolites were measured by GC/MS. Briefly, 6 million BMDMs were incubated in the media with or without glucose, galactose or glucose plus different concentrations of 10 mM 2-DG for 1 hour. After stimulation with LPS or IL-4 for an additional 3 hours, culture medium was quickly removed and cells were washed 3 times by PBS, quenched with −20℃ 1:1 water:methanol, and flash frozen in liquid nitrogen. Cells were collected, frozen in liquid nitrogen and thawed on ice for 20 minutes. After vortexing, samples were centrifuged at 10,000 g for 10 minutes at 4 °C. The supernatant was then collected. The extraction procedure was repeated, the supernatant was combined and completely dried in a SpeedVac concentrator, subsequently methoximated using 10 µL of MOX™ Regent at 30°C for 90 min and then derivatized using 40 µL of MSTFA+1% TMCS (N-methyl-N-trimethylsilyltrifluoroacetamide with 1% trimethylchlorosilane) at 37°C for 30 min. Metabolite levels were determined using GC-MS (Hewlett-Packard, HP 5980B) with DB5-MS column. GC-MS spectra were deconvoluted using AMDIS software, after that SpectConnect software was used to create metabolite peaks matrix. The Agilent Fiehn GC/MS Metabolomics RTL Library was used for metabolite identifications. Ion count peak area was used for analysis of the relative abundance of the metabolites.
Metabolite levels in the culture medium were analyzed by NMR spectroscopy. Two million Raw264.7 cells in 2 ml culture medium were seeded in the 60 mm petri dishes. These cells were stimulated with IL-4 in the medium containing glucose, glucose+2-DG (10 mM), galactose or no glucose for 3 hours. Original and cell culture media were collected and deproteinized before NMR analysis. In 150 µL of media samples 450 µL of cold methanol were added. The samples were spun down at 10,000 g for 10 min, supernatants were collected and dried down in centrifugal vacuum evaporator for 12 h. In dried samples, 500 µL of 0.1M phosphate buffer and 50 µL of 1 mM TSP-d4 solution in D2O were added. Samples were vortexed for 20 seconds and transferred to 5 mm NMR tubes. NMR spectra were acquired on a Bruker 500 MHz Avance III HD spectrometer equipped with a BBO cryoprobe and SampleCase auto sampler (Bruker Biospin, Rheinstetten, Germany). 1H-NMR spectra were recorded using 1D noesy pulse sequence with presaturation (noesygppr1d), with 90 degree pulse (~13 µs), 4.68 seconds acquisition time, and 4 seconds relaxation delay. Spectra were phase and baseline corrected using the Topspin 3.5 software. Metabolites were identified and quantified using the software program Chenomx NMR Suite 8.2, by fitting the spectral lines of library compounds into the recorded NMR spectrum of the cell extracts. The quantification was based on peak area of TSP-d4 signal, and metabolite concentrations were reported as µM in medium.
13C6-glucose and 13C5-glutamine tracing experiments
Metabolic tracing analysis of U-13C-glucose and glutamine in BMDMs and Raw264.7 stimulated with LPS or IL-4 was determined by GC/MS. Briefly, 6 million cells were incubated in glucose-deficient (but not 100% glucose-void as dialyzed serum was not used yielding a final glucose concentration of 0.3 mM) RPMI-1640 medium containing 11.1 mM 13C6-glucose or 2 mM 13C5-glutamine for 1 hour. After stimulated with LPS or IL-4 for an additional 2 hours, samples were collected and processed as described in intracellular metabolites measurement. Metabolites were quantified on the basis of total ion count peak area of specific mass ions. To determine 13C-labeling, mass information for known fragments of labeled metabolites was retrieved. These fragments contained either the whole or partial carbon skeleton of the metabolite. For each fragment, the retrieved data comprised mass intensities for the lightest isotopomer (without any heavy isotopes, M+0), and isotopomers with increasing unit mass (up to M+6) relative to M0. These mass distributions were normalized by dividing by the sum of M0 to M6, and corrected for the natural abundance of heavy isotopes, using matrix-based probabilistic methods as described (Lee et al., 1991) implemented in Microsoft Excel. 13C-labeling data are expressed as fractional abundance of each isotopologue of a measured metabolite pool, or relevant enrichment of each metabolite. Data were from four biological replicates.
Flow cytometry
BMDMs and Raw264.7 cells were seeded in 24-well plate overnight, incubated overnight, and then switched to medium containing different concentrations of glucose, galactose, or glucose with inhibitors (i.e, 0–10 mM 2-DG, UK-5099, oligomycin or etomoxir) for 1 hour. Cells were then simulated with IL-4 for 24 hours. These activated cells were collected by pipetting in PBS containing 1 mM EDTA. For the surface staining of PD-L2 and CD36, cells were incubated with antibodies on ice for 15–20 min in the dark. After two times washing, cells were resuspended in staining buffer and further stained with 7-AAD to exclude dead cells. For acellular staining of PD-L2 and RELMα, cells were fixed and permeabilized and incubated with antibodies for 20 min in the dark at room temperature. After two washes, cells were stained with secondary antibodies. After additional washings, cells were analyzed by flow cytometry immediately. To exclude the effect of dead cells, we also performed additional experiments by staining with 7-AAD after CD36 staining.
p-STAT6 nuclear translocation
BMDMs were seeded in chamber slides overnight, and then incubated in medium containing with or without glucose, galactose, no glucose or glucose plus different reagents (2-DG or oligomycin) for 1 hour. Cells were then stimulated with IL-4 for indicated durations, washed with PBS, and fixed with 1:1 methanol:acetone at −20°C for 15 min. Blocking steps included 15min incubation with Avidin D solution and 15min incubation with Biotin solution, followed by incubation with 5% normal horse serum in PB-ST for 30 minutes before staining. Primary antibody p-STAT6 was added in PB-ST with 5% normal horse serum and incubated at 4 °C overnight. The fluorochrome-conjugated secondary antibody was incubated for 1 hour. DAPI was used for nucleus staining. Images were analyzed using a laser scanning confocal microscope.
Nucleotide measurements
Intracellular nucleotides were measured by HPLC. Briefly, after culture medium was quickly removed, cells were rinsed with PBS, quenched with 6% ice-cold HClO4, and flash frozen in liquid nitrogen. Cells were collected, frozen in liquid nitrogen and thawed on ice for 20 minutes. After vortexing, samples were centrifuged at 10,000 g for 10 minutes at 4 °C. The supernatant was then collected and neutralized to pH 7.4 with 2 M KHCO3. After another centrifuging at 10,000 g for 10 minutes at 4 °C, nucleotides in the supernatant were separated on a reversed-phase Discovery C18 column (SIGMA, St. Louis, MO) with the Hewlett-Packard series 1100 HPLC system (Agilent, Santa Clara, CA), as described previously (Dzeja et al., 2004). Briefly, a phosphate buffer with tetrabutylammonium sulfate and methanol mixture was used as mobile phase at 0.7 ml/min flow rate. Gradient elution was applied and the separation of nucleotides completed in 20 minutes. The nucleotide levels were normalized by cell number.
Cell viability
Cell viability was tested by using the XTT assay. BMDMs were seeded in 96-well plates overnight and incubated in medium containing glucose, galactose, no glucose or glucose plus 10 mM 2-DG or 1µM oligomycin for 0.5–1 hour. Cells were then stimulated with IL-4 for indicated times. Absorbance was read at 450 nm after 1–3 hours incubation with 50 ul XTT detection solution at 37℃.
Western blotting
After different treatment and stimulation, cells were lysed in RIPA buffer supplemented with Complete Mini EDTA-Free protease inhibitor cocktail and phosphatase inhibitor cocktail (Roche). Cell lysate was boiled in SDS sample buffer for 5 min at 95–100℃, separated by SDS-PAGE, and transferred to PVDF membranes. The membranes were blocked for 1 hour in TBS plus 5% nonfat dry milk and then incubated with primary antibodies overnight. After washing three times, the membranes were incubated with secondary antibodies for 1 hour in TBS-T plus 5% nonfat dry milk and washed three times. ECL western blotting chemiluminescent substrates were added for 5 minutes. Bands of interest were developed by using an autoradiographic film.
Statistical Analysis
Results were presented as means+/−SEM. Unpaired Student’s t-test was used to test the differences between two groups, based on the assessment of variance of the data. All data were analyzed by GraphPad Prism software (version 7). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. Statistical values can be found in the figure legends.
Supplementary Material
Table S1: Dataset of real-time ECAR and OCR measurements of bone marrow-derived macrophages stimulated with LPS. Related to Figure 1a.
Table S2: Dataset of real-time ECAR and OCR measurements of bone marrow-derived macrophages stimulated with IL-4. Related to Figure 1b.
Key Resources Table
| REAGENT or Material | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-Phospho-STAT6 | Cell Signaling Technology | Cat# 9361 |
| Rabbit monoclonal anti-STAT6 | Cell Signaling Technology | Cat# 5397 |
| Rabbit monoclonal anti-PPARγ | Cell Signaling Technology | Cat# 2435 |
| Goat polyclonal anti-mouse IL-1β /IL-1F2 | R&D Systems | Cat# AF-401 |
| Rabbit polyclonal anti- β Actin | Abcam | Cat# ab8227 |
| Anti-Murine RELMα | Peprotech | Cat# 500-P214 |
| PE Anti-Murine Monoclonal PD-L2 antibody | ThermoFisher | Cat# 12–5986-81 |
| PE/Cy7 anti-mouse CD36 Antibody | BioLegend | Cat# 102615 |
| Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 647) | Abcam | Cat# ab150075 |
| Reagents, Peptides, and Recombinant Proteins | ||
| Lipopolysaccharides from Escherichia coli O127:B8 | Sigma-Aldrich | Cat# L4516 |
| Recombinant Mouse IL-4 Protein | R&D Systems | Cat# 404-ML |
| Recombinant Murine M-CSF | PeproTech | Cat# 315–02 |
| 2-Deoxy-D-glucose | Sigma-Aldrich | Cat# D8375 |
| Lactate Dehydrogenase A Inhibitor, FX11 | EMD Millipore | Cat# 427218 |
| D-(+)-Galactose | Sigma-Aldrich | Cat# G0750 |
| D-(+)-Glucose | Sigma-Aldrich | Cat# G8644 |
| D-GLUCOSE U-13C6 | Cambridge Isotope Laboratories | Cat# CLM-1396 |
| L-Glutamine-13C5 | Sigma-Aldrich | Cat# 605166 |
| Etomoxir sodium salt hydrate | Sigma-Aldrich | Cat# E1905 |
| UK-5099 | Sigma-Aldrich | Cat# PZ0160 |
| ATP Solution | Thermo Fisher Scientific | Cat# R0441 |
| Oligomycin A | Sigma-Aldrich | Cat# 75351 |
| Oligomycin | Sigma-Aldrich | Cat# O4876 |
| FCCP | Sigma-Aldrich | Cat# C2920 |
| Antimycin A | Sigma-Aldrich | Cat# A8674 |
| Rotenone | Sigma-Aldrich | Cat# R8875 |
| JAK Inhibitor I | EMD Millipore | Cat# 420097 |
| MOXTM Regent | Thermo Fisher Scientific | Cat# TS-45950 |
| MSTFA+1% TMCS | Thermo Fisher Scientific | Cat# TS-48915 |
| Methanol CHROMASOLV®, for HPLC | Sigma-Aldrich | Cat# 34860 |
| 7-AAD Viability Staining Solution | BioLegend | Cat# 420403 |
| Fluoroshield Mounting Medium With DAPI | Abcam | Cat# ab104139 |
| RIPA Buffer | Cell Signaling Technology | Cat# 9806 |
| Protease Inhibitor Cocktail | Roche | Cat# 11873580001 |
| Phosphatase inhibitor cocktail | Roche | Cat# 04906845001 |
| Dialyzed Fetal Bovine Serum | ThermoFisher | Cat# A3382001 |
| Critical Commercial Assays | ||
| Avidin/Biotin Blocking Kit | Vector Laboratories | Cat# SP-2001 |
| XTT Cell Viability Kit | Cell Signaling Technology | Cat# 9095 |
| Deposited Data | ||
| NMR spectral data | ||
| Experimental Models: Cell Lines | ||
| RAW 264.7 | ATCC | Cat# ATCC® TIB-71™ |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | The Jackson Laboratory | Cat# 000664 |
| Software and Algorithms | ||
| GraphPad Prism | GraphPad Software ( version 7) | https://www.graphpad.com/scientific-software/prism/ |
| BD CellQuest™ Pro (Version 6.0) | BD Biosciences | https://www.bdbiosciences.com/documents/15_cellquest_prosoft_analysis.pdf |
Highlights.
M2 differentiation via the JAK-STAT6 pathway requires a threshold level of ATP
Glycolysis is not required for M2 differentiation as long as OXPHOS remains intact
When glycolysis is reduced, glutamine can fuel the Krebs cycle in M2 macrophages
2-DG impairs not only glycolysis but all of the above
Acknowledgements
This work was supported by the National Institute of Health/National Heart Lung Blood Institute (HL116952-05 and HL 85744-09, HL 121079-02), and U24DK100469 (Mayo Clinic Metabolomics Resource Core), a fellowship grant of the Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA, and an endowment by the Sachs family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
None for any author.
References
- Akira S, Uematsu S, and Takeuchi O (2006). Pathogen recognition and innate immunity. Cell 124, 783–801. [DOI] [PubMed] [Google Scholar]
- Biswas SK and Mantovani A (2010). Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunology 11, 889–896. [DOI] [PubMed] [Google Scholar]
- Burelle Y, Lamoureux MC, Péronnet F, Massicotte D, Lavoie C. (2006) Comparison of exogenous glucose, fructose and galactose oxidation during exercise using 13C-labelling. Br J Nutr 96:56–61. [DOI] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB, Faubert B, Villarino AV, O’Sullivan D, Huang SCC, van der Windt GJ, Blagih J, Qiu J et al. (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Hoerter J, Guéron M (1996) A comparison of AMP degradation in the perfused rat heart during 2-deoxy-D-glucose perfusion and anoxia. Part I: The release of adenosine and inosine. J Mol Cell Cardiol 28:2163–74. [DOI] [PubMed] [Google Scholar]
- Chinetti-Gbaguidi G, Colin S, and Staels B (2015). Macrophage subsets in atherosclerosis. Nature Reviews Cardiology 12, 10–17. [DOI] [PubMed] [Google Scholar]
- Covarrubias AJ, Aksoylar HI, Yu J, Snyder NW, Worth AJ, Iyer SS, Wang J, Ben-Sahra I, Byles V, Polynne-Stapornkul., et al. (2016). Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. eLife 5, e11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B, Amiel E, Huang SCC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJ, et al. (2014). TLR-driven early glycolytic reprogramming via the kinases TBK1-IKK ɛ supports the anabolic demands of dendritic cell activation. Nature Immunology 15, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván-Peña S and O’Neill LA (2014). Metabolic reprograming in macrophage polarization. Frontiers in Immunology 5, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S and Martinez FO (2010). Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604. [DOI] [PubMed] [Google Scholar]
- Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C (2013). Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nature immunology 14, 1064–1072. [DOI] [PubMed] [Google Scholar]
- Halestrap AP (1976). The mechanism of the inhibition of the mitochondrial pyruvate transportater by alpha-cyanocinnamate derivatives. Biochem J 156, 181–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Rüegg MA and Hall MN (2012). Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metabolism 15, 725–738. [DOI] [PubMed] [Google Scholar]
- Hammarén HM, Ungureanu D, Grisouard J, Skoda RC, Hubbard SR, and Silvennoinen O (2015). ATP binding to the pseudokinase domain of JAK2 is critical for pathogenic activation. Proceedings of the National Academy of Sciences 112, 4642–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JT, Welch JS, Ricote M and Binder CJ, (1999). Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 400, 378–382. [DOI] [PubMed] [Google Scholar]
- Huang SCC, Everts B, Ivanova Y, O’sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O’Neill LA et al. (2014). Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nature Immunology 15, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SCC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD and Pearce EJ (2016). Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity 45, 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Zhan Le, Yanxiang Guo J, White E, Rabinowitz JD (2017). Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Huang SCC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B et al. (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Qin Y, Cozzo AJ, Freemerman AJ, Huang MJ, Zhao L, Sampey BP, Milner JJ, Beck MA, Damania B, et al. (2016). Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Molecular Metabolism 5, 506–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtoglu M, Gao N, Shang J, Maher JC, Lehrman MA, Wangpaichitr M, Savaraj N, Lane AN and Lampidis TJ (2007) Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Molecular Cancer Therapeutics 6, 3049–3058. [DOI] [PubMed] [Google Scholar]
- Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV (2010). Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proceedings of the National Academy of Sciences 107, 2037–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WN, Byerley LO, Bergner EA, Edmond J. (1991). Mass isotopomer analysis: theoretical and practical considerations. Biol Mass Spectrom 20, 451–8. [DOI] [PubMed] [Google Scholar]
- Mills EL, Kelly B, Logan A, Costa AS, Varma M, Bryant CE, Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I et al. (2016) Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ and Wynn TA (2011). Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology 11, 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Liu J, Rovira II, Gonzalez-Hurtado E, Lee J, Wolfgang MJ and Finkel T (2016). Fatty acid oxidation in macrophage polarization. Nature Immunology 17, 216–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, et al. (2007). Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Kishton RJ and Rathmell J (2016). A guide to immunometabolism for immunologists. Nature Reviews Immunology 16, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL and Pearce EJ (2013). Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RA and Huang P (2006). Glycolysis inhibition for anticancer treatment. Oncogene 25, 4633–4646. [DOI] [PubMed] [Google Scholar]
- Ralser M, Wamelink MM, Struys EA, Joppich C, Krobitsch S, Jakobs C and Lehrach H (2008). A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth. Proceedings of the National Academy of Sciences 105, 17807–17811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer LJ, Wice BM and Kennell D (1979). Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. Journal of Biological Chemistry 254, 2669–2676. [PubMed] [Google Scholar]
- Rodríguez-Prados JC, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, Cascante M, Boscá L, et al. (2010). Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. Journal of Immunology 185, 605–614. [DOI] [PubMed] [Google Scholar]
- Shuai K, and Liu B (2003). Regulation of JAK–STAT signalling in the immune system. Nature Reviews Immunology 3, 900–911. [DOI] [PubMed] [Google Scholar]
- Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A, Szeles L, Poliska S, Oros M, Evans RM, et al. (2010). STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ-regulated gene expression in macrophages and dendritic cells. Immunity 33, 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ and Liu G (2015). Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. Journal of Immunology 194, 6082–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. (2013). Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J, O’Neill LA and Menon D (2017). Macrophage immunometabolism: where are we (going)? Trends in Immunology 38, 395–406. [DOI] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Murray PJ and Chawla A (2006). Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metabolism 4, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhang S, Jeon R, Vuckovic I, Jiang X, Lerman A, Folmes CD, Dzeja PD, Herrmann J (2018). Interferon Gamma Induces Reversible Metabolic Reprogramming of M1 Macrophages to Sustain Cell Viability and Pro-Inflammatory Activity. EBioMedicine 30, 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, Cho HC, Popescu NI, Coggeshall KM, Arditi M, et al. (2016). Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166, 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Dataset of real-time ECAR and OCR measurements of bone marrow-derived macrophages stimulated with LPS. Related to Figure 1a.
Table S2: Dataset of real-time ECAR and OCR measurements of bone marrow-derived macrophages stimulated with IL-4. Related to Figure 1b.