Abstract
Background
The systemic immune‐inflammation index (SII) is correlated with patient survival in various types of solid tumors. However, only a few studies have focused on the prognostic value of the SII in patients with surgically resected non‐small cell lung cancer (NSCLC).
Methods
This study was a single center retrospective analysis of 569 NSCLC patients who underwent curative lobectomy at the Department of Thoracic Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between 2006 and 2012. A receiver operating characteristic curve was plotted to compare the discriminatory ability of the SII for overall survival (OS). A Cox proportional hazards regression model was used to perform univariate and multivariate analyses.
Results
The SII, neutrophil‐lymphocyte ratio (NLR), and platelet‐lymphocyte ratio (PLR) all correlated with OS in NSCLC patients, and the SII was an independent prognostic factor for OS (hazard ratio 1.256, 95% confidence interval 1.018–1.551; P = 0.034). The area under the receiver operating characteristic curve of the SII (0.547) was larger than the NLR (0.541) and PLR (0.531). Furthermore, the SII retained prognostic significance in the lung adenocarcinoma subgroup.
Conclusion
The SII is a promising prognostic predictor for patients with surgically resected NSCLC and retained prognostic significance in the lung adenocarcinoma subgroup. The prognostic value of the SII is superior to the NLR and PLR.
Keywords: Neutrophil‐lymphocyte ratio, non‐small cell lung cancer, platelet‐lymphocyte ratio, prognosis, systemic immune‐inflammation index
Introduction
Lung cancer is the most common and serious type of cancer worldwide.1, 2 There are two main types of primary lung cancer, non‐small cell lung cancer (NSCLC) and small cell lung cancer (SCLC).3, 4 NSCLC accounts for approximately 85% of all new lung cancer diagnoses.3 Currently, the clinical treatment decisions and prognostic prediction for NSCLC are based on tumor node metastasis (TNM) staging. However, because of the late and rapid clinical manifestations of occult symptoms of the disease, prognosis is extremely poor.5 In addition, accurate TNM staging is obtained postoperatively, and preoperative prediction remains difficult. Although molecular profiling has shown great potential to guide patient management strategies, the tools are complex and expensive at present. Therefore, more potential and propagable biomarkers should be included in clinical practice to improve prognostic prediction of NSCLC.
Recently some inflammation‐based parameters, such as the neutrophil‐lymphocyte ratio (NLR) and platelet‐lymphocyte ratio (PLR), have been reported to be associated with patient survival for several types of solid tumors, such as lung,6, 7 breast,8 melanoma,9 colorectal,10 gastric,11 and esophageal.12 However, these two inflammatory indicators only integrate two types of inflammatory cells. The systemic immune‐inflammation index (SII), a parameter that integrates three types of inflammatory cells (lymphocytes, neutrophils, and platelets), has been shown to be more promising than the NLR or PLR.13, 14, 15, 16 Three studies have reported the prognostic significance of the SII in NSCLC.17, 18, 19 Although these studies showed that the SII was an independent prognostic predictor for NSCLC patients, their cohorts included a relatively small number of patients. Thus, we conducted the present study to investigate and verify the prognostic value of the SII in a larger cohort of patients with surgically resected NSCLC.
Methods
Patients
Medical records of 569 NSCLC patients who underwent curative lobectomy with R0 resection between July 2006 and May 2012 at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College were retrospectively reviewed. The inclusion criteria were: histologically confirmed NSCLC with complete clinical information, laboratory data, and follow‐up. The exclusion criteria were: administration of neoadjuvant chemoradiotherapy; in‐hospital death; and clinical evidence of chronic inflammatory, hematological, or autoimmune diseases. Patients were followed up regularly in the outpatient clinic every three to six months for the first two two years after surgery and then annually thereafter. Follow‐up concluded on 27 September 2018.
The study followed the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Informed consent was exempted for this retrospective study.
Clinicopathological parameters
Patient clinicopathological parameters, including age, gender, smoking history, pathological diagnosis, tumor differentiation grade, tumor size, lymph node metastasis, TNM stage, operation duration, intraoperative blood loss, and routine blood results, were obtained from medical records. Tumor staging was assessed according to the eighth edition of the American Joint Committee on Cancer TNM staging system.20
Blood sample analysis and systemic immune‐inflammation index (SII), neutrophil‐lymphocyte ratio (NLR), and platelet‐lymphocyte ratio (PLR) evaluations
Complete blood count data were analyzed in the general laboratory of our hospital within one week before surgery. We calculated the SII, NLR, and PLR as follows: SII = platelet count × neutrophil count/lymphocyte count, NLR = neutrophil count/lymphocyte count, and PLR = platelet count/lymphocyte count.
Statistical analysis
SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses. The Pearson chi‐square test was used to compare categorical variables. Multiple linear regression analyses were used to determine the factors associated with the SII, NLR, and PLR. We constructed receiver operating characteristic (ROC) curves to determine the optional cutoff values for the SII, NLR, and PLR that yielded the maximum joint sensitivity and specificity. The Kaplan–Meier method was used to perform survival analyses, and the differences between the survival curves were compared by the log‐rank test. We used the Cox proportional hazard model for univariate and multivariate analyses, and hazard ratios (HRs) with 95% confidence intervals (CIs) were used to quantify the prognostic value of the predictors. A two‐sided value of P < 0.05 was considered statistically significant.
Results
Patient characteristics
The 569 patients included in this study consisted of 425 (74.7%) men and 144 (25.3%) women (Table 1). The median age was 60 (range: 27–80) years. The distribution of pathological stages was as follows: stage I, 147 patients (25.8%); stage II, 177 patients (31.1%); and stage III, 245 patients (43.1%). The mean follow‐up duration was 60.3 (range: 0.9–146.7) months. At the final follow‐up, 345 (60.6%) patients had died.
Table 1.
Characteristics of the NSCLC patients grouped by SII values
| SII | ||||
|---|---|---|---|---|
| Characteristic | Patients (n, %) | Low | High | P |
| Gender | 0.016 * | |||
| Male | 425 (74.7) | 183 | 242 | |
| Female | 144 (25.3) | 79 | 65 | |
| Age (years) | 0.401 | |||
| ≤ 60 | 286(50.3) | 137 | 149 | |
| > 60 | 283 (49.7) | 125 | 158 | |
| Smoking | 0.377 | |||
| Ever | 372 (65.4) | 166 | 206 | |
| Never | 197 (34.6) | 96 | 101 | |
| Histological type | 0.014 * | |||
| LUAD | 295 (51.8) | 153 | 142 | |
| LUSC | 225 (39.5) | 88 | 137 | |
| Others | 49 (8.6) | 21 | 28 | |
| Tumor size | 0.001 * | |||
| ≤ 4 | 277 (48.7) | 165 | 112 | |
| > 4 | 292 (51.3) | 97 | 195 | |
| Differentiation | 0.800 | |||
| Well | 182 (32.0) | 83 | 99 | |
| Moderately | 195 (34.3) | 87 | 108 | |
| Poorly | 192 (33.7) | 92 | 100 | |
| T stage | 0.000 * | |||
| T1 | 144 (25.3) | 89 | 55 | |
| T2 | 284 (49.9) | 132 | 152 | |
| T3 | 107 (18.8) | 33 | 74 | |
| T4 | 34 (6.0) | 8 | 26 | |
| N stage | 0.356 | |||
| N0 | 223 (39.2) | 112 | 111 | |
| N1 | 142 (25.0) | 61 | 81 | |
| N2 | 201 (35.3) | 87 | 114 | |
| N3 | 3 (0.5) | 2 | 1 | |
| Lymph node metastasis | 0.121 | |||
| Negative | 223 (39.2) | 54 | 20 | |
| Positive | 346 (60.8) | 86 | 40 | |
| TNM stage | 0.001 * | |||
| I | 147 (25.8) | 87 | 60 | |
| II | 177 (31.1) | 77 | 100 | |
| III | 245 (43.1) | 98 | 147 | |
| Operation duration (minutes) | 0.933 | |||
| < 150 | 302(53.1) | 140 | 162 | |
| ≥ 150 | 267 (46.9) | 122 | 145 | |
| Intraoperative blood loss (mL) | ||||
| < 150 | 170 (29.9) | 73 | 97 | 0.359 |
| ≥ 150 | 399 (70.1) | 189 | 210 | |
| NLR | 0.001 * | |||
| Low | 200 (35.1) | 179 | 21 | |
| High | 369 (64.9) | 83 | 286 | |
| PLR | 0.001 * | |||
| Low | 144 (25.3) | 132 | 12 | |
| High | 425 (74.7) | 130 | 295 | |
P < 0.05 is considered significant. LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NLR, neutrophil‐lymphocyte ratio; NSCLC, non‐small cell lung cancer; PLR, platelet‐lymphocyte ratio; SII, systemic immune‐inflammation index; TNM, tumor node metastasis.
At baseline, the median preoperative SII, NLR, and PLR values were 594.7 (range: 66.0–3394.4), 2.47 (range: 0.36–16.65), and 127.5 (range: 32.0–1088.2), respectively.
Selection of optimal cutoff values for the SII, NLR, and PLR
Previous studies have suggested different cutoff values when analyzing the prognostic value of the SII, NLR, and PLR. In the present study, we constructed ROC curves to determine the optional cutoff values. As shown in Figure 1, the areas under the ROC curves (AUCs) were 0.547, 0.541, and 0.531 for the SII, NLR, and PLR, respectively. The optimal cutoff values for the prediction of survival were 419.6 for the SII, 1.74 for the NLR, and 88.7 for the PLR. Consequently, we separated the patients into two groups according to the optimal cutoff values. Three hundred seven patients (54.0%) had SII values ≥ 419.6, 369 patients (64.8%) had NLRs ≥ 1.74, and 425 patients (73.8%) had PLRs ≥ 88.7.
Figure 1.
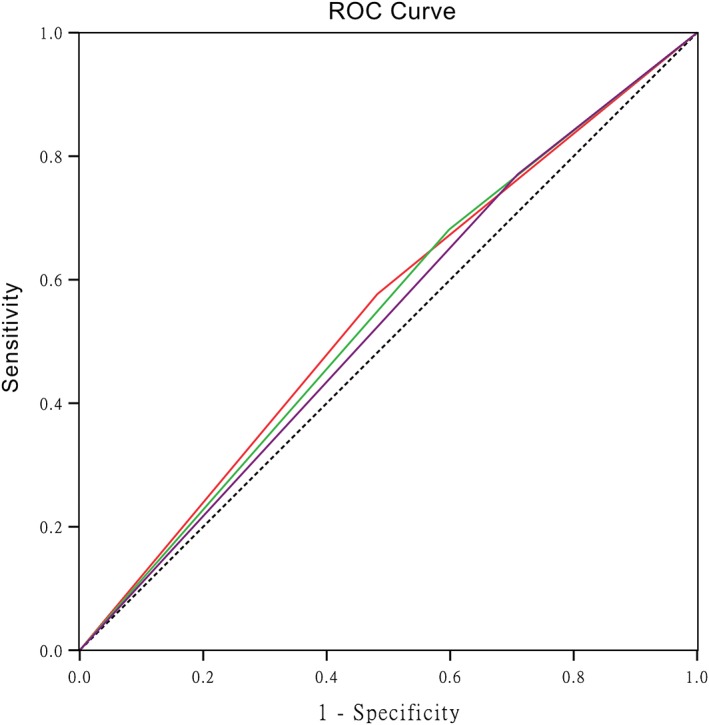
Receiver operating characteristic (ROC) curve analysis of the optimal cutoff value of the systemic immune‐inflammation index (SII), neutrophil‐lymphocyte ratio (NLR), and platelet‐lymphocyte ratio (PLR). The areas under the curve for overall survival were 0.547, 0.541, and 0.531 for the SII, NLR, and PLR, respectively. ( ) SII, (
) SII, ( ) NLR, (
) NLR, ( ) PLR, and (
) PLR, and ( ) Reference Line.
) Reference Line.
Correlation between clinicopathological parameters and the SII, NLR, and PLR
Correlations between the clinicopathological parameters and the SII are shown in Table 1. The preoperative SII was associated with gender (P = 0.016), histological type (P = 0.014), tumor length (P < 0.001), T stage (P < 0.001), TNM stage (P < 0.001), NLR (P < 0.001), and PLR (P < 0.001). No other significant differences were found between the groups.
Correlations between the clinicopathological parameters and the NLR and PLR are shown in Table 2. The preoperative NLR was associated with gender (P < 0.001), smoking history (P = 0.007), histological type (P = 0.009), tumor length (P < 0.001), and T stage (P < 0.001). The preoperative PLR was associated with gender (P = 0.001), smoking history (P = 0.001), tumor length (P < 0.001), and intraoperative blood loss (P = 0.036). No other significant differences were found between the groups.
Table 2.
Characteristics of the NSCLC patients grouped by NLR and PLR values
| NLR | PLR | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cases (n, %) | Low | High | P | Low | High | P |
| Gender | 0.001 * | 0.001 * | |||||
| Male | 425 (74.7) | 121 | 304 | 122 | 303 | ||
| Female | 144 (25.3) | 79 | 65 | 22 | 122 | ||
| Age (years) | 0.380 | 0.773 | |||||
| ≤ 60 | 286 (50.3) | 106 | 180 | 74 | 212 | ||
| > 60 | 283 (49.7) | 94 | 189 | 70 | 213 | ||
| Smoking | 0.007 * | 0.001 * | |||||
| Ever | 372 (46.0) | 116 | 256 | 111 | 261 | ||
| Never | 197 (54.0) | 84 | 133 | 33 | 164 | ||
| Histological type | 0.009 * | 0.642 | |||||
| LUAD | 295 (51.8) | 121 | 174 | 70 | 225 | ||
| LUSC | 225 (39.5) | 64 | 161 | 60 | 165 | ||
| Others | 49 (8.6) | 15 | 34 | 14 | 35 | ||
| Tumor length | 0.001 * | 0.001 * | |||||
| ≤ 4 | 277 (48.7) | 124 | 153 | 89 | 188 | ||
| > 4 | 292 (51.3) | 76 | 216 | 55 | 237 | ||
| Differentiation | 0.342 | 0.913 | |||||
| Well | 182 (32.0) | 57 | 125 | 48 | 134 | ||
| Moderately | 195 (34.3) | 69 | 126 | 49 | 146 | ||
| Poorly | 192 (33.7) | 74 | 118 | 47 | 145 | ||
| T stage | 0.001 * | 0.072 | |||||
| T1 | 144 (25.3) | 70 | 74 | 46 | 98 | ||
| T2 | 284 (49.9) | 97 | 187 | 68 | 216 | ||
| T3 | 107 (18.8) | 27 | 80 | 26 | 81 | ||
| T4 | 34 (6.0) | 6 | 34 | 4 | 30 | ||
| N stage | 0.235 | 0.249 | |||||
| N0 | 223 (39.2) | 82 | 141 | 61 | 162 | ||
| N1 | 142 (25.0) | 52 | 90 | 41 | 101 | ||
| N2 | 201 (35.3) | 65 | 136 | 41 | 160 | ||
| N3 | 3 (0.5) | 1 | 2 | 1 | 2 | ||
| Lymph node metastasis | 0.530 | 0.376 | |||||
| Negative | 223 (39.2) | 36 | 38 | 61 | 162 | ||
| Positive | 346 (60.8) | 54 | 72 | 83 | 263 | ||
| TNM stage | 0.107 | 0.099 | |||||
| I | 147 (25.8) | 60 | 87 | 43 | 104 | ||
| II | 177 (31.1) | 65 | 112 | 50 | 127 | ||
| III | 245 (43.1) | 75 | 170 | 51 | 194 | ||
| Operation duration (minutes) | 0.187 | 0.334 | |||||
| < 150 | 302(53.1) | 114 | 118 | 71 | 231 | ||
| ≥ 150 | 267 (46.9) | 86 | 181 | 72 | 194 | ||
| Intraoperative blood loss (mL) | 0.848 | 0.036 * | |||||
| < 150 | 170 (29.9) | 61 | 109 | 33 | 137 | ||
| ≥ 150 | 399 (70.1) | 139 | 260 | 111 | 288 | ||
P < 0.05 is considered significant. LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NLR, neutrophil‐lymphocyte ratio; NSCLC, non‐small cell lung cancer; PLR, platelet‐lymphocyte ratio; TNM, tumor node metastasis.
Prognostic values of the SII, NLR, and PLR
We used the Kaplan–Meier method to plot the overall survival (OS) curves and compared them using the log‐rank test. A high SII, NLR, and PLR were correlated with poor OS (P = 0.001, P = 0.07, and P = 0.023, respectively) (Fig 2a–c).
Figure 2.
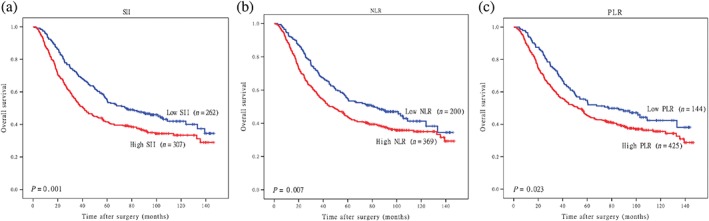
Kaplan–Meier overall survival curves according to the (a) systemic immune‐inflammation index (SII), (b) neutrophil‐lymphocyte ratio (NLR), and (c) platelet‐lymphocyte ratio (PLR).
Moreover, we also investigated the prognostic value of the SII separately in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) subgroups. LUAD patients with higher SII values had worse OS (P = 0.001) (Fig 3a); however, the correlation in the LUSC subgroup was not statistically significant (P = 0.116) (Fig 3b).
Figure 3.
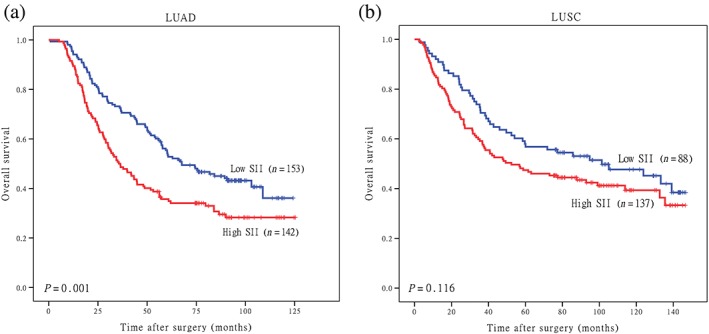
Kaplan–Meier overall survival (OS) curves of patients with high and low platelet‐lymphocyte ratios (PLRs) stratified by histological type. OS of patients with (a) lung adenocarcinoma (LUAD) and (b) lung squamous cell carcinoma (LUSC). SII, systemic immune‐inflammation index.
Univariate and multivariate analyses
Univariate analyses demonstrated that age, tumor length, T stage, lymph node metastasis, TNM stage, operation duration, intraoperative blood loss, SII, NLR, and PLR were significant risk factors for poor OS (Table 3). When conducting multivariate analyses, we used three separate models to avoid multicollinearity. In each test, only one immune‐inflammatory indicator (SII, NLR, or PLR) was included. The results revealed that age (P < 0.001), lymphatic metastasis (P = 0.026), TNM stage (P < 0.001), intraoperative blood loss (P = 0.039), and high SII values (P = 0.034) were independently associated with poor OS (Table 4).
Table 3.
Univariate analysis of OS in NSCLC patients
| OS | |||
|---|---|---|---|
| Characteristic | P | HR | 95% CI |
| Gender (male, female) | 0.242 | 0.862 | 0.673–1.105 |
| Age (≤60, > 60 years) | 0.001 * | 1.564 | 1.263–1.937 |
| Smoking (ever, never) | 0.248 | 1.142 | 0.911–1.431 |
| Tumor length (≤ 4, > 4) | 0.001 * | 1.545 | 1.248–1.913 |
| Differentiation (well/moderately, poorly) | 0.137 | 1.181 | 0.948–1.471 |
| T stage (T1/T2, T3/T4) | 0.001 * | 1.682 | 1.344–2.105 |
| Lymph node metastasis (negative, positive) | 0.001 * | 2.050 | 1.623–2.588 |
| TNM stage (I/II, III) | 0.001 * | 2.270 | 1.834–2.810 |
| Operation duration (minutes) (≤ 150, > 150) |
0.008 * | 1.333 | 1.079–1.647 |
| Intraoperative blood loss (mL) (≤ 150, > 150) | 0.034 * | 1.299 | 1.020–1.655 |
| SII (< 419.6, ≥ 419.6) | 0.001 * | 1.433 | 1.157–1.774 |
| NLR (< 1.74, ≥ 1.74) | 0.007 * | 1.364 | 1.087–1.710 |
| PLR (< 88.7, ≥ 88.7) | 0.024 * | 1.337 | 1.040–1.719 |
P < 0.05 is considered significant. CI, confidence interval; HR, hazard ratio; NLR, neutrophil‐lymphocyte ratio; NSCLC, non‐small cell lung cancer; OS, overall survival; PLR, platelet‐lymphocyte ratio; SII, systemic immune‐inflammation index; TNM, tumor node metastasis.
Table 4.
Multivariate analysis of OS in NSCLC patients
| OS | |||
|---|---|---|---|
| Characteristic | P | HR | 95% CI |
| Age (≤ 60, > 60 years) | 0.001 * | 1.672 | 1.349–2.071 |
| Tumor length (≤ 4, > 4) | 0.217 | 1.155 | 0.919–1.451 |
| T stage (T1/T2, T3/T4) | 0.326 | 1.139 | 0.878–1.479 |
| Lymph node metastasis (negative, positive) | 0.026 * | 1.401 | 1.041–1.885 |
| TNM stage (I/II, III) | 0.001 * | 1.850 | 1.410–2.428 |
| Operation duration (minutes) (≤ 150, > 150) |
0.340 | 1.109 | 0.897–1.372 |
| Intraoperative blood loss (mL) (≤ 150, > 150) | 0.039 * | 1.291 | 1.085–1.667 |
| SII (< 419.6, ≥ 419.6) | 0.034 * | 1.256 | 1.018–1.551 |
| NLR (< 1.74, ≥ 1.74) | 0.119 | 1.202 | 0.954–1.515 |
| PLR (< 87.83, ≥ 87.83) | 0.092 | 1.247 | 0.965–1.613 |
P < 0.05 is considered significant. CI, confidence interval; HR, hazard ratio; NLR, neutrophil‐lymphocyte ratio; NSCLC, non‐small‐cell lung cancer; OS, overall survival; PLR, platelet‐lymphocyte ratio; SII, systemic immune‐inflammation index; TNM, tumor node metastasis.
Univariate and multivariate analyses were also conducted in the LUAD and LUSC subgroups. In the LUAD subgroup, univariate analyses showed that gender, age, smoking history, tumor length, tumor differentiation, T stage, lymph node metastasis, TNM stage, SII, NLR, and PLR were significant risk factors for poor OS (Table S1). The results of multivariate analyses revealed that age (P < 0.001), tumor length (P = 0.001), lymphatic metastasis (P < 0.001), high SII values (P = 0.014), and high NLR values (P = 0.003) were independently associated with poor OS (Table S2). In the LUSC subgroup, the results of univariate analyses showed that tumor differentiation, T stage, lymph node metastasis, TNM stage, and operation duration were significant risk factors for poor OS (Table S3). Multivariate analyses revealed that tumor differentiation (P = 0.032) and TNM stage (P = 0.023) were independently associated with poor OS (Table S4).
Discussion
To the best of our knowledge, compared to previous studies, our sample included the largest sample of NSCLC patients. Our study focused exclusively on the prognostic value of the SII in patients with surgically resected NSCLC. We found that the SII was associated with gender, histological type, tumor length, T stage, TNM stage, NLR, and PLR. Furthermore, the preoperative SII was an independent prognostic biomarker for OS in patients with surgically resected NSCLC, and it retained prognostic significance in the LUAD subgroup. Comparison of the AUCs showed that the prognostic ability of the SII was superior to the NLR and PLR.
Cancer‐related inflammation is influential in the tumor microenvironment, and inflammatory cells in the circulation may also play important roles in tumor progression.21 In recent years a growing body of evidence has revealed that systemic inflammation is correlated with cancer development.22 Systemic inflammatory responses could induce tumor behavior and are associated with poor clinical outcomes in patients with various types of solid tumors.23, 24, 25, 26 The NLR and the PLR are two systemic inflammatory indicators; high NLR and PLR values correlate with a poor prognosis.6, 9, 11, 12, 27 However, these two inflammatory indicators only integrate two cell types. The SII, which is based on neutrophils, platelets, and lymphocytes, seems to be a stronger prognostic predictor in a variety of solid tumors,10, 13, 14 including NSCLC.17, 18, 19
The mechanism by which a high SII value contributes to poor survival for cancer patients remains unclear. Several theories have been proposed to explain this phenomenon. Neutrophils can activate both endothelial and parenchymal cells to enhance circulating tumor cell adhesion, promoting distant metastasis.28 Meanwhile, circulating vascular endothelial growth factor is contained in granulocytes, particularly in neutrophils, which could be important for tumor angiogenesis.29 Platelets can act as protective “cloaks” to shield circulating tumor cells from immune destruction, induce epithelial‐mesenchymal transition, and promote distant metastasis of tumor cells.30 Lymphocytes play an important role in immune surveillance and immune defence.31 Meanwhile, a high density of tumor‐infiltrating lymphocytes is associated with better clinical outcomes in solid tumors, and tumor‐infiltrating lymphocytes are correlated with lymphocytes circulating in the peripheral blood.32, 33 Based on this information, a higher SII may be associated with tumor angiogenesis, invasion, and metastasis, thus leading to poor survival. Therefore, an elevated SII is correlated with poor survival in cancer patients.
The results of our study should be interpreted with caution. Our study was a single center retrospective study, thus selection bias was inevitable. Collaborative, multicenter, prospective studies are warranted to confirm our results.
The SII is an independent prognostic predictor in patients with surgically resected NSCLC, and the SII retained prognostic significance in the LUAD subgroup. The SII showed better prognostic ability than the NLR and PLR.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Univariate analysis of overall survival (OS) in 295 patients with lung adenocarcinoma (LUAD).
Table S2. Multivariate analysis of overall survival (OS) in 295 patients with lung adenocarcinoma (LUAD).
Table S3. Univariate analysis of overall survival (OS) in 225 patients with lung squamous cell carcinoma (LUSC).
Table S4. Multivariate analysis with of overall survival (OS) in 225 patients with lung squamous cell carcinoma (LUSC).
Acknowledgments
This study was supported by the National Key R&D Programme of China (2016YFC0905400, 2017YFC1308702); the Beijing Municipal Science & Technology Commission (Z151100004015188, Z181100001918002); the CAMS Initiative for Innovative Medicine (2017‐I2M‐1‐005); and Institutional Fundamental Research Funds (NCC2017YKY‐03, 2017PT32001).
We thank Ms. Meihua Xiong, Fang Zhou, and the staff in Dr. Jie He’s laboratory in the Department of Thoracic Surgery for their support during the study.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Goldstraw P, Ball D, Jett JR et al Non‐small‐cell lung cancer. Lancet 2011; 378: 1727–40. [DOI] [PubMed] [Google Scholar]
- 4. Jackman DM, Johnson BE. Small‐cell lung cancer. Lancet 2005; 366: 1385–96. [DOI] [PubMed] [Google Scholar]
- 5. Vansteenkiste J, Crinò L, Dooms C et al 2nd ESMO consensus conference on lung cancer: Early‐stage non‐small‐cell lung cancer consensus on diagnosis, treatment and follow‐up. Ann Oncol 2014; 25: 1462–74. [DOI] [PubMed] [Google Scholar]
- 6. Deng M, Ma X, Liang X, Zhu C, Wang M. Are pretreatment neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio useful in predicting the outcomes of patients with small‐cell lung cancer? Oncotarget 2017; 8: 37200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu G, Yao Y, Bai C et al Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non‐small cell lung cancer patients. Thoracic Cancer 2015; 6: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takeuchi H, Kawanaka H, Fukuyama S, Kubo N, Hiroshige S, Yano T. Comparison of the prognostic values of preoperative inflammation‐based parameters in patients with breast cancer. PLoS One 2017; 12: e0177137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma J, Kuzman J, Ray A et al Neutrophil‐to‐lymphocyte ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci Rep 2018; 8: 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pine JK, Morris E, Hutchins G et al Systemic neutrophil‐to‐lymphocyte ratio in colorectal cancer: The relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer 2015; 113: 204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo J, Chen S, Chen Y, Li S, Xu D. Combination of CRP and NLR: A better predictor of postoperative survival in patients with gastric cancer. Cancer Manage Res 2018; 10: 315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng J‐F, Huang Y, Chen Q‐X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014; 12: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J‐H, Zhai E‐T, Yuan Y‐J et al Systemic immune‐inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017; 23: 6261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu H, Zheng J, Cai J et al Systemic immune‐inflammation index (SII) is useful to predict survival outcomes in patients after liver transplantation for hepatocellular carcinoma within Hangzhou criteria. Cell Physiol Biochem 2018; 47: 293–301. [DOI] [PubMed] [Google Scholar]
- 15. Wang L, Wang C, Wang J, Huang X, Cheng Y. A novel systemic immune‐inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2017; 143: 2077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu B, Yang X‐R, Xu Y, et al Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014; 20: 6212–22. [DOI] [PubMed] [Google Scholar]
- 17. Tomita M, Ayabe T, Maeda R, Nakamura K. Systemic immune‐inflammation index predicts survival of patients after curative resection for non‐small cell lung cancer. In Vivo 2018; 32: 663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tong YS, Tan J, Zhou XL, Song Y‐Q, Song YJ. Systemic immune‐inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non‐small cell lung cancer. J Transl Med 2017; 15: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune‐inflammation index predicts poor prognosis in patients with operable non‐small cell lung cancer. Clin Chim Acta 2018; 484: 272–7. [DOI] [PubMed] [Google Scholar]
- 20. Detterbeck FC. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Cardiovasc Surg 2018; 155: 356–9. [DOI] [PubMed] [Google Scholar]
- 21. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation‐induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013; 13: 759–71. [DOI] [PubMed] [Google Scholar]
- 23. Templeton AJ, Ace O, McNamara MG et al Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta‐analysis. Cancer Epidemiol Biomarkers Prev 2014; 23: 1204–12. [DOI] [PubMed] [Google Scholar]
- 24. Peng HX, Lin K, He BS et al Platelet‐to‐lymphocyte ratio could be a promising prognostic biomarker for survival of colorectal cancer: A systematic review and meta‐analysis. FEBS Open Bio 2016; 6: 742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsai P‐L, Su W‐J, Leung W‐H, Lai C‐T, Liu C‐K. Neutrophil–lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: A systematic review and meta‐analysis. J Cancer Res Ther 2016; 12: 582–9. [DOI] [PubMed] [Google Scholar]
- 26. Zhou X, Du Y, Huang Z et al Prognostic value of PLR in various cancers: A meta‐analysis. PLoS One 2014; 9: e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo H, Ge H, Cui Y et al Systemic inflammation biomarkers predict survival in patients of early stage non‐small cell lung cancer treated with stereotactic ablative radiotherapy ‐ A single center experience. J Cancer 2018; 9: 182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin‐8. Clin Cancer Res 2004; 10: 4895–900. [DOI] [PubMed] [Google Scholar]
- 29. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003; 6: 283–7. [DOI] [PubMed] [Google Scholar]
- 30. Stanger BZ, Kahn ML. Platelets and tumor cells: A new form of border control. Cancer Cell 2013; 24: 9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008; 454: 436–44. [DOI] [PubMed] [Google Scholar]
- 32. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour‐infiltrating lymphocytes in cancer: A systematic review with meta‐analysis. Br J Cancer 2011; 105: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liyanage UK, Moore TT, Joo H‐G et al Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002; 169: 2756–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate analysis of overall survival (OS) in 295 patients with lung adenocarcinoma (LUAD).
Table S2. Multivariate analysis of overall survival (OS) in 295 patients with lung adenocarcinoma (LUAD).
Table S3. Univariate analysis of overall survival (OS) in 225 patients with lung squamous cell carcinoma (LUSC).
Table S4. Multivariate analysis with of overall survival (OS) in 225 patients with lung squamous cell carcinoma (LUSC).


