Abstract
Background
The study was conducted to evaluate the clinical and computed tomography (CT) findings of non‐small cell lung cancer (NSCLC) patients to distinguish between ALK gene rearrangement, EGFR mutation, and non‐ALK/EGFR (no genetic abnormalities).
Methods
We enrolled 201 patients with primary NSCLC who had undergone molecular testing for both ALK gene rearrangement and EGFR mutation. The clinical features and CT findings of the main lesion and associated pulmonary abnormalities were investigated.
Results
Female gender (P = 0.0043 vs. non‐ALK/EGFR), young age (P = 0.0156 vs. EGFR), and a light or never smoking history (P = 0.0039 vs. non‐ALK/EGFR) were significant clinical characteristics of NSCLC with ALK gene rearrangement. The significant CT characteristics compared to NSCLC with EGFR mutation were a large mass (P = 0.0155), solid mass (P = 0.0048), and no air bronchogram (P = 0.0148). A central location (P = 0.0322) and lymphadenopathy (P = 0.0353) were also more frequently observed. Coexisting emphysema was significantly less frequent in NSCLC patients with ALK gene rearrangement (P = 0.0135) than non‐ALK/EGFR.
Conclusions
NSCLC with ALK gene rearrangement was more likely to develop in younger women with a light or never smoking history. The characteristic CT findings of NSCLC with ALK gene rearrangement were a large solid mass, less air bronchogram, a central location, and lymphadenopathy.
Keywords: Anaplastic lymphoma kinase, computed tomography, epidermal growth factor receptor, histological subtype, non‐small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide. Recently, lung cancer treatment has undergone remarkable changes. Some genetic abnormalities of non‐small cell lung cancer (NSCLC) can be diagnosed from tissue samples,1, 2, 3 and effective small‐molecule tyrosine kinase inhibitors (TKIs) have enabled individualized treatment for subgroups that are sensitive to ALK and EGFR inhibitors.4, 5, 6 These genetic abnormalities can be determined by specific analyses of histological samples obtained by invasive procedures, including biopsy or surgical resection. In general, ALK gene rearrangement and EGFR mutation are mutually exclusive: they are not present at the same time.7, 8, 9 Therefore, it would be beneficial to clarify the radiological features of each molecular subtype to determine when such invasive diagnostic procedures are warranted.
ALK rearrangements occur in 5% of NSCLC cases in East Asian countries.6 They typically occur in younger patients with a history of light or never smoking.6 ALK‐positive NSCLC is common among adenocarcinomas, and is associated with the solid histological subtypes. Therefore, these tumors tend to present on thin‐section computed tomography (CT) as a solid mass with little or no ground‐glass opacity (GGO) components.8, 10 In addition, according to previous studies, tumors with ALK gene rearrangement tend to be centrally located, relatively large masses without a cavity or pleural indentation.11, 12, 13 They may have a high incidence of associated pleural effusion, carcinomatous lymphangiosis, and lymphadenopathy.12, 14, 15, 16 However, there has been no consensus on imaging findings for ALK‐positive NSCLC.
The aim of this study was to evaluate the clinical and CT findings of NSCLC with ALK gene rearrangement that may enable distinction from NSCLC with EGFR mutation or with neither genetic abnormality.
Methods
Study population
We retrospectively searched our cancer database for patients who were diagnosed with primary NSCLC and had undergone molecular testing for both ALK gene rearrangement and EGFR mutation from June 2012 to December 2014 in our two institutions. The institutional review board approved this retrospective study and waived the need for written informed consent. The review committee of our two institutions approved the study in accordance with the declaration of Helsinki.
In total, 267 cases were identified from our database. Sixty‐six cases were excluded: re‐biopsy taken for recurrence (n = 28); extremely advanced cases in which the main tumor could not be evaluated (n = 14); multifocal primary tumors (n = 10); CT images taken before medication or surgery were not available (n = 7); tumors were located mainly in the mediastinum (n = 4); and complicated by severe infection (n = 2) and severe organizing pneumonia (n = 1). Finally, a total of 201 patients were included (Fig. 1). Of these cases, 91 underwent surgical resection and the remaining 110 underwent a biopsy of the primary tumor or metastatic lesions.
Figure 1.
The selection process for the study cohort. CT, computed tomography.
Data on age, gender, and smoking status were extracted from each patient's medical record. All patients were ethnically Asian, and the mean age was 67.5 (range: 30–90) years. Almost half of the patients were female (102/201, 50.7%) and non‐smokers (95/183, 51.9%); smoking history was not available for 18 cases.
Molecular testing and histological examination
Molecular analysis was performed for all tumor samples to determine the mutation status of EGFR exons 18, 19, 20, and 21 via real‐time PCR or PCR‐invader method, and ALK gene rearrangement via fluorescence in situ hybridization (FISH).1 , 2 The 201 patients were divided into three subgroups: ALK (21 patients), EGFR (124 patients), and non‐ALK/EGFR (56 patients).
The histological predominant subtypes of the 91 surgically resected patients (ALK group, 8 patients; EGFR group, 66 patients; and non‐ALK/EGFR group, 17 patients) were evaluated according to the 2015 World Health Organization (WHO) classification.17
Computed tomography (CT) analysis
CT examinations were performed using one of four CT systems (Aquilion ONE and Aquilion 64, Toshiba, Nasu, Japan; Somatom Definition Flash and Definition, Siemens, Erlangen, Germany). The CT parameters were as follows: detector collimation, 0.5–0.6 mm; beam pitch, 0.6–1.2; rotation time, 0.5 seconds; tube voltage, 120 kVp; tube current adjusted automatically with CT‐automatic exposure control; and a reconstruction kernel with a high‐frequency algorithm. The reconstruction thicknesses and intervals were 1.0–2.0 mm and 1.0–2.0 mm on the pulmonary window setting (WS) (width, 1500 HU; level, −600 Hounsfield units [HU]), and 3.0–4.0 mm and 2.4–4.0 mm on the mediastinal WS (width, 300 HU; level, 25 HU), respectively. Nonionic contrast medium (100 mL of Omnipaque 240, Daiichi‐Sankyo, Tokyo, Japan; 100 mL of Oypalomin 300 and 65 mL of Oypalomin 370, Konica Minolta, Tokyo, Japan) at a dose of 520–600 mgI/kg was used for CT examination in 137 patients (68.2%).
Two of three experienced radiologists independently evaluated the main tumor in terms of its size (the maximum axial diameter on pulmonary WS), type (solid, part‐solid GGN, or pure GGN based on the presence of GGO), air bronchogram, cavity, pleural indentation, margin (smooth/irregular, lobulated, or spiculated), site of the lesion (right upper lobe, middle lobe, right lower lobe, left upper lobe, or left lower lobe), location of the lesion (central [involving segmental or more proximal bronchi] or peripheral), and contrast‐enhanced characteristics (homogeneous, heterogeneous, or ring‐enhancement pattern, and presence or absence of inner vessels on CT). In addition, coexisting pulmonary emphysema (low attenuation areas compared to normal lung parenchyma), fibrosis (reticular opacities and honeycombing), pleural effusion, pulmonary metastasis (lung nodules < 5 mm [military] and ≥ 5 mm [scattered]) or lymphangitic metastasis, and lymphadenopathy (lymph nodes with a short axis ≥ 10 mm on the mediastinal WS) were also evaluated. When there was interobserver disagreement, a conclusion was reached by consensus.
Statistical analysis
All statistical analyses in this study were performed using the SAS version 9.2. Comparisons between ALK‐positive and EGFR‐positive cases, ALK‐positive and non‐ALK/EGFR cases, and ALK‐positive and non‐ALK cases (including both EGFR‐positive and non‐ALK/EGFR cases) were performed using Fisher's exact test for categorical variables and the Student's t‐test for continuous variables. A multivariate logistic regression model was applied with factors that showed a significant difference in univariate analysis. Variables that showed a significant difference in multivariate analysis were selected using a backward elimination method. The dependent variable was mutation status, and the independent variables were clinical and CT characteristics. The Akaike information criterion was used to select the most informative variables for a single parsimonious model.
Interobserver agreement was assessed by computing the κ coefficient and its 95% confidence interval (CI): κ 0.21–0.40 indicated fair agreement, κ 0.41–0.60 indicated moderate agreement, κ 0.61–0.80 indicated substantial agreement, and κ 0.81–1.00 indicated almost perfect agreement.18
Results
Clinical and histological characteristics
The clinical characteristics of the patients are shown in Table 1. Results of the t‐test indicated that ALK‐positive patients were younger than EGFR‐positive patients (mean age 63 ± 13 vs. 68 ± 9 years, respectively; P = 0.0156) and non‐ALK cases (P = 0.0187). There was a significantly higher proportion of women in the ALK group than in the non‐ALK/EGFR group (13/21, 62%, and 15/56, 27%, respectively; P = 0.0043), and the proportion of patients who had a history of light or never smoking was lower in the ALK group than in the non‐ALK/EGFR group (P = 0.0039).
Table 1.
Clinical patient characteristics
| P | ||||||
|---|---|---|---|---|---|---|
| Characteristic | ALK (n = 21) | EGFR (n = 124) | Non‐ALK/EGFR (n = 56) | ALK versus EGFR | ALK versus non‐ALK/EGFR | ALK versus non‐ALK |
| Age† | 30–80 (63 ± 13) | 46–88 (68 ± 9) | 45–90 (67 ± 10) | 0.0156 | 0.0979 | 0.0187 |
| Gender, N (%) | — | — | — | 0.8472 | 0.0043 | 0.2798 |
| Male | 8 (38) | 50 (40) | 41 (73) | — | — | — |
| Female | 13 (62) | 74 (60) | 15 (27) | — | — | — |
| Smoking history‡ | 0 (0, 23) | 0 (0, 21) | 38 (7, 56) | 0.6736 | 0.0039 | 0.4729 |
Age (years), range (mean ± SD).
Smoking history (pack‐year), median (the first quartile, the third quartile).
Values in bold indicate a statistically significant result.
The histological predominant subtypes of the surgically resected cases are shown in Table 2. The most common was invasive adenocarcinoma with a predominantly papillary subtype (28/91, 30.8%). EGFR‐positive cases were associated with a high frequency of papillary, lepidic, and minimally invasive adenocarcinoma as the predominant subtypes (23, 15, and 14 of 66; 35%, 23%, and 21%, respectively). In contrast, these subtypes were less frequent in the ALK‐positive cases, where the most predominant subtype was acinar (3/8, 38%). The non‐ALK/EGFR group showed various predominant patterns, with the solid pattern being the most frequent (5/17, 29%).
Table 2.
Histological predominant subtypes among surgically resected cases
| Predominant subtype | ALK (n = 8) | EGFR (n = 66) | Non‐ALK/EGFR (n = 17) |
|---|---|---|---|
| Solid, N (%) | 2 (25) | 5 (8) | 5 (29) |
| Papillary, N (%) | 1 (13) | 23 (35) | 4 (24) |
| Micropapillary, N (%) | 1 (13) | 3 (5) | 1 (6) |
| Acinar, N (%) | 3 (38) | 6 (9) | 1 (6) |
| Lepidic, N (%) | 0 (0) | 15 (23) | 1 (6) |
| Minimally invasive adenocarcinoma, N (%) | 0 (0) | 14 (21) | 1 (6) |
| Other, N (%) | 1 (13) | 0 (0) | 4 (24) |
CT characteristics
The interobserver agreement was fair to almost perfect (κ coefficient range: 0.379–0.910). The CT characteristics of the ALK group that significantly differed from the EGFR group were: a large mass (P = 0.0155), a solid mass (P = 0.0048), no air bronchogram (P = 0.0148), central location (P = 0.0322), and lymphadenopathy (P = 0.0353). Coexisting emphysema was significantly less frequent in the ALK group than in the non‐ALK/EGFR group (24% vs. 55%; P = 0.0135) (Table 3, Figs 2, 3).
Table 3.
Computed tomography characteristics of the main mass and coexisting lung abnormalities
| P | ||||||
|---|---|---|---|---|---|---|
| Characteristic | ALK (n = 21) | EGFR (n = 124) | Non‐ALK/EGFR (n = 56) | ALK versus EGFR | ALK versus non‐ALK/EGFR | ALK versus non‐ALK |
| Size† | 14–78 (39 ± 19) | 9–68 (31 ± 13) | 9–115 (44 ± 23) | 0.0155 | 0.3548 | 0.3423 |
| Type, N (%) | — | — | — | 0.0048 | 0.1183 | 0.0206 |
| Solid | 21 (100) | 80 (65) | 59 (89) | — | — | — |
| Part‐solid GGN | 0 (0) | 40 (32) | 6 (11) | — | — | — |
| Pure GGN | 0 (0) | 4 (3) | 0 (0) | — | — | — |
| Margin, N (%) | — | — | — | 0.1783 | 0.3698 | 0.5930 |
| Irregular | 14 (67) | 99 (80) | 31 (55) | — | — | — |
| Smooth | 7 (33) | 25 (20) | 25 (45) | — | — | — |
| Spiculation, N (%) | 6 (29) | 57 (46) | 15 (27) | 0.1369 | 0.8755 | 0.3091 |
| Lobulation, N (%) | 8 (38) | 30 (24) | 19 (34) | 0.1804 | 0.7329 | 0.2955 |
| Air bronchogram, N (%) | 6 (29) | 71 (57) | 16 (29) | 0.0148 | 1.0000 | 0.0857 |
| Cavity, N (%) | 2 (10) | 10 (8) | 7 (13) | 0.8224 | 0.7173 | 0.9906 |
| Pleural indentation, N (%) | 8 (38) | 64 (52) | 19 (34) | 0.2519 | 0.7329 | 0.4850 |
| Site of the lesion, N (%) | — | — | — | 0.7055 | 0.9730 | 0.8230 |
| RUL | 5 (24) | 40 (32) | 16 (29) | — | — | — |
| ML | 1 (5) | 13 (10) | 3 (5) | — | — | — |
| RLL | 6 (29) | 22 (18) | 13 (23) | — | — | — |
| LUL | 6 (29) | 33 (27) | 14 (25) | — | — | — |
| LLL | 3 (14) | 16 (13) | 10 (18) | — | — | — |
| Location, N (%) | — | — | — | 0.0332 | 0.1916 | 0.0486 |
| Central | 8 (38) | 22 (18) | 13 (23) | — | — | — |
| Peripheral | 13 (62) | 102 (82) | 43 (77) | — | — | — |
| Contrast enhancement pattern, N (%) | — | — | — | 0.7631 | 0.8535 | 0.8866 |
| Homogeneous | 3 (21) | 25 (29) | 6 (16) | — | — | — |
| Heterogeneous | 10 (71) | 52 (61) | 28 (74) | — | — | — |
| Ringed | 1 (7) | 8 (9) | 4 (11) | — | — | — |
| Inner vessel, N (%) | 4 (27) | 9 (10) | 7 (18) | 0.0800 | 0.5049 | 0.1470 |
| Lung metastasis pattern, N (%) | — | — | — | — | — | — |
| Miliary | 3 (14) | 17 (14) | 2 (4) | 0.9436 | 0.0893 | 0.6044 |
| Scattered | 3 (14) | 15 (12) | 5 (9) | 0.7785 | 0.4926 | 0.6654 |
| Lymphangitic | 4 (19) | 9 (7) | 10 (18) | 0.0803 | 0.9040 | 0.2473 |
| Lymphadenopathy, N (%) | 14 (67) | 52 (42) | 32 (57) | 0.0353 | 0.4479 | 0.0827 |
| Emphysema, N (%) | 5 (24) | 13 (10) | 31 (55) | 0.0868 | 0.0135 | 0.9489 |
| Fibrosis, N (%) | 2 (10) | 7 (6) | 9 (16) | 0.4957 | 0.4646 | 0.9232 |
| Pleural effusion, N (%) | 3 (14) | 18 (15) | 12 (21) | 0.9779 | 0.4809 | 0.7805 |
Size (mm), range (mean ± standard deviation). Values in bold indicate a statistically significant result.
GGO, ground glass opacity; LLL, left lower lobe; LUL, left upper lobe; ML, middle lobe; RLL, right lower lobe; RUL, right upper lobe.
Figure 2.
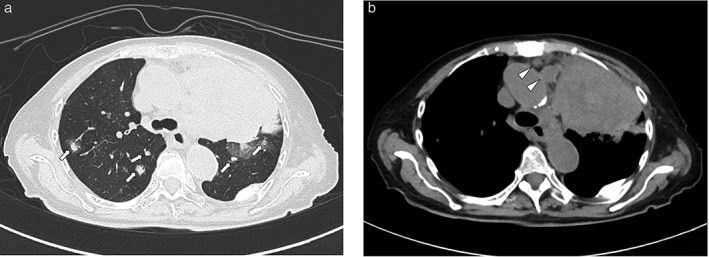
Computed tomography images of an 80‐year‐old woman with adenocarcinoma with ALK gene rearrangement. (a) Lung window image showing a large solid mass in the central area of the left upper lobe. Multiple scattered nodules suggest lung metastasis (arrows). (b) Mediastinal window image showing lymphadenopathy (arrowheads).
Figure 3.
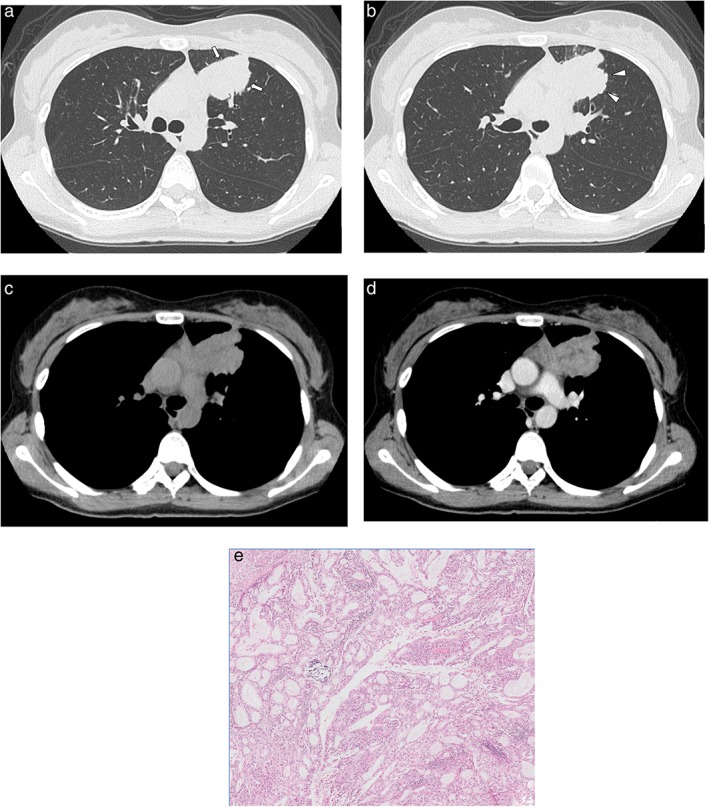
Computed tomography images of a 30‐year‐old woman with adenocarcinoma with ALK gene rearrangement. (a,b) Lung window images show a solid mass with a spiculated (arrow) and lobulated (arrowhead) margin in the periphery of the left upper lobe. (c,d) Mediastinal window images show the heterogeneous enhancement pattern of the mass. (e) High‐power photomicrograph of the tumor show the acinar predominant subtype (original magnification ×50; hematoxylin and eosin staining).
Multivariate analysis using the Akaike information criterion was performed including all clinical and CT variables; the results are summarized in Table 4. Among the variables analyzed, air bronchogram, emphysema, and a central location were independent predictive factors of ALK gene rearrangement status.
Table 4.
Multivariable logistic regression analyses
| Characteristic | Multivariate odds ratio (95% CI) | P |
|---|---|---|
| ALK vs. EGFR | ||
| Air bronchogram (−) | 3.508 (1.256–9.800) | 0.0166 |
| Emphysema (+) | 2.933 (0.880–9.780) | 0.0799 |
| ALK vs. non‐ALK/EGFR | ||
| Emphysema (−) | 3.968 (1.277–12.332) | 0.0172 |
| ALK vs. non‐ALK | ||
| Air bronchogram (−) | 2.339 (0.868–6.299) | 0.0929 |
| Central location | 2.550 (0.981–6.625) | 0.0547 |
CI, confidence interval.
Discussion
In this study, we investigated the characteristics of clinical and CT findings of NSCLC with ALK gene rearrangement. We found that a relatively large solid mass without air bronchogram was a significant characteristic CT finding for NSCLC with ALK gene rearrangement. NSCLC with ALK gene rearrangement was also more often associated with a central location and lymphadenopathy when compared to NSCLC with EGFR mutation; it was also more often associated with pulmonary emphysema when compared to non‐ALK/EGFR NSCLC patients. Patients who were younger, female, or had a light or no smoking history were more likely to have NSCLC with ALK gene rearrangement. These results are consistent with those of a previous clinical study.6 None of the ALK‐positive NSCLC cases in this study showed a lepidic growth pattern as the predominant histologic subtype.
The genetic abnormalities involved in NSCLC can be determined by specific analyses of histological samples obtained by invasive procedures. However, Isaka et al. reported that only approximately 50% of transbronchial biopsy specimens contain sufficient amounts of DNA for amplicon‐based massively parallel sequencing.19 Therefore, it would be beneficial to understand the clinical and radiological features that might help distinguish the different molecular subtypes in advance before invasive diagnostic procedures are performed. Moreover, ALK gene rearrangement and EGFR mutation are generally mutually exclusive,7, 8, 9 and ALK gene rearrangement is less common.6 It is important to distinguish the clinical and radiological findings of NSCLC with ALK gene rearrangement from other types of NSCLC, because it may enable medical oncologists to evaluate the consistency between these findings and molecular testing results, and patients could then receive potentially beneficial TKI therapy.
In the current study, pulmonary emphysema was less commonly observed in patients with ALK gene rearrangement than in those with non‐ALK/EGFR NSCLC. This result seems to reflect the fact that most of these patients had a history of light or never smoking. However, coexisting emphysema was an independent predictive factor of ALK gene rearrangement status when compared to EGFR mutation. A plausible reason for this is that EGFR mutation status has a stronger association with non‐emphysema status.
There has been no consensus on the imaging findings of ALK‐positive NSCLC. Wang et al. reported that a relatively large solid mass without “bubble‐like lucency” was more likely to be ALK‐positive than EGFR‐positive NSCLC.16 Ko et al. reported that ALK‐positive tumors were larger and had a solid proportion when compared to non‐ALK tumors.11 Consistent with these findings, our results indicate that a relatively large solid mass was a CT characteristic of NSCLC with ALK gene rearrangement. We found that air bronchogram occurred significantly less frequently in NSCLC with ALK gene rearrangement. According to previous studies, a more lobulated margin,14, 20 a less spiculated margin,13, 14 or less pleural indentation12, 13 are characteristics of the main lesion. However, few articles have reported the significant inner or margin characteristics of the main mass; thus, no consensus on the significance of these observations has been reached.
In this study, the main lesions of ALK‐positive NSCLC were more commonly located in the central region compared to EGFR‐positive or non‐ALK NSCLC. Yamamoto et al. similarly reported that ALK‐positive tumors were more commonly located in the central region, and that there were fewer operable cases in ALK‐positive than in non‐ALK NSCLC (8/47 and 44/123 cases, respectively).12 In our study, only 8 of the 21 cases underwent surgery. Similar to our results, several articles have reported that coexisting lymphadenopathy, which suggests lymph node metastasis, is more often observed in patients with ALK‐positive NSCLC.14, 16, 21 This finding seems to reflect the fact that patients with ALK‐positive NSCLC have a poor prognosis.
Histological examination is important for analyzing the main tumor. Previous studies have shown that ALK‐positive NSCLC is associated with the solid predominant subtype.10, 16, 22 Inamura et al. reported a relationship between ALK‐positive NSCLC and the papillary or acinar subtypes.8 No consensus on the significance of these histological characteristics has been reached; however, it seems that lepidic predominant adenocarcinomas (adenocarcinoma in situ, minimally invasive adenocarcinoma, and lepidic predominant invasive adenocarcinoma) are less likely to develop ALK gene rearrangement. None of the ALK‐positive NSCLC cases in this study showed a lepidic growth pattern as the predominant histological subtype.
This study has several limitations. First, this was a retrospective study. Second, there were fewer NSCLC patients in our sample with ALK gene rearrangement than with EGFR‐positive tumors; however, the prevalence of ALK gene rearrangement in NSCLC patients is approximately 5%,6 while that of EGFR mutation is 40–80%.1 The results of multivariate regression analysis in our study require further verification because of the small number of ALK‐positive NSCLC patients in our sample. Selection bias may be an issue, as we did not perform molecular testing in all NSCLC patients. Our sample may also have included more advanced cases that were considered for chemotherapy than would be found in the general NSCLC population. However, we believe that these limitations did not affect the main results of this study.
NSCLC with ALK gene rearrangement is more likely to develop in younger women with a history of light or never smoking. The characteristic CT findings of NSCLC with ALK gene rearrangement in comparison to EGFR mutation were a large solid mass, less air bronchogram, a central location, and lymphadenopathy. Combining these results may assist clinicians to assess the likelihood of NSCLC with ALK gene rearrangement.
Disclosure
No authors report any conflict of interest.
References
- 1. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. [DOI] [PubMed] [Google Scholar]
- 2. Paez JG, Janne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–500. [DOI] [PubMed] [Google Scholar]
- 3. Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature 2007; 448: 561–6. [DOI] [PubMed] [Google Scholar]
- 4. Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 5. Mitsudomi T, Morita S, Yatabe Y et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 6. Solomon BJ, Mok T, Kim DW et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014; 371: 2167–77. [DOI] [PubMed] [Google Scholar]
- 7. Fukui T, Yatabe Y, Kobayashi Y et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4‐ALK fusion oncogene. Lung Cancer 2012; 77: 319–25. [DOI] [PubMed] [Google Scholar]
- 8. Inamura K, Takeuchi K, Togashi Y et al. EML4‐ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008; 3: 13–7. [DOI] [PubMed] [Google Scholar]
- 9. Wong DW, Leung EL, So KK et al. The EML4‐ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild‐type EGFR and KRAS. Cancer 2009; 115: 1723–33. [DOI] [PubMed] [Google Scholar]
- 10. Kim H, Chung JH. Overview of clinicopathologic features of ALK‐rearranged lung adenocarcinoma and current diagnostic testing for ALK rearrangement. Transl Lung Cancer Res 2015; 4: 149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ko SJ, Lee YJ, Park JS et al. Epidermal growth factor receptor mutations and anaplastic lymphoma kinase rearrangements in lung cancer with nodular ground‐glass opacity. BMC Cancer 2014; 14: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto S, Korn RL, Oklu R et al. ALK molecular phenotype in non–small cell lung cancer: CT radiogenomic characterization. Radiology 2014; 272: 568–76. [DOI] [PubMed] [Google Scholar]
- 13. Zhou JY, Zheng J, Yu ZF et al. Comparative analysis of clinicoradiologic characteristics of lung adenocarcinomas with ALK rearrangements or EGFR mutations. Eur Radiol 2015; 25: 1257–66. [DOI] [PubMed] [Google Scholar]
- 14. Choi C‐M, Kim MY, Hwang HJ, Lee JB, Kim WS. Advanced adenocarcinoma of the lung: Comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology 2015; 275: 272–9. [DOI] [PubMed] [Google Scholar]
- 15. Rizzo S, Petrella F, Buscarino V et al. CT radiogenomic characterization of EGFR, K‐RAS, and ALK mutations in non‐small cell lung cancer. Eur Radiol 2016; 26: 32–42. [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Schabath MB, Liu Y et al. Clinical and CT characteristics of surgically resected lung adenocarcinomas harboring ALK rearrangements or EGFR mutations. Eur J Radiol 2016; 85: 1934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Travis WD, Brambilla E, Nicholson AG et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–60. [DOI] [PubMed] [Google Scholar]
- 18. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- 19. Isaka M, Serizawa M, Kenmotsu H et al. Comparison of clinically relevant mutation profiles between preoperative biopsy and corresponding surgically resected specimens in Japanese patients with non‐small‐cell lung cancer by amplicon‐based massively parallel sequencing. Clin Lung Cancer 2017; 18: 519–26 e1. [DOI] [PubMed] [Google Scholar]
- 20. Kim TJ, Lee CT, Jheon SH, Park JS, Chung JH. Radiologic characteristics of surgically resected non‐small cell lung cancer with ALK rearrangement or EGFR mutations. Ann Thorac Surg 2016; 101: 473–80. [DOI] [PubMed] [Google Scholar]
- 21. Halpenny DF, Riely GJ, Hayes S et al. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements? Lung Cancer 2014; 86: 190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong YJ, Cai YR, Zhou LJ et al. Association between the histological subtype of lung adenocarcinoma, EGFR/KRAS mutation status and the ALK rearrangement according to the novel IASLC/ATS/ERS classification. Oncol Lett 2016; 11: 2552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


